Abstract
Objective:
To address three controversial components in the Centers for Medicare and Medicaid Service’s sepsis bundle for performance measure (SEP-1): antibiotics within 3 hours, a 30 mL/kg fluid infusion for all hypotensive patients, and repeat lactate measurements within 6 hours if initially elevated. We hypothesized that antibiotic- and fluid-focused bundles like SEP-1 would probably show benefit, but evidence supporting specific antibiotic timing, fluid dosing, or serial lactate requirements would not be concordant. Therefore, we performed a meta-analysis of studies of sepsis bundles like SEP-1.
Data Sources:
PubMed, Embase, ClinicalTrials.gov through March 15, 2018.
Study Selection:
Studies comparing survival in septic adults receiving versus not receiving antibiotic- and fluid-focused bundles.
Data Extraction:
Two investigators (D.J.P., P.Q.E.).
Data Synthesis:
Seventeen observational studies (11,303 controls and 4,977 bundle subjects) met inclusion criteria. Bundles were associated with increased odds ratios of survival (odds ratio [95% CI]) in 15 studies with substantial heterogeneity (I2 = 61%; p < 0.01). Survival benefits were consistent in the five largest (1,697–12,486 patients per study) (1.20 [1.11–1.30]; I2 = 0%) and six medium-sized studies (167–1,029) (2.03 [1.52–2.71]; I2 = 8%) but not the six smallest (64–137) (1.25 [0.42–3.66]; I2 = 57%). Bundles were associated with similarly increased survival benefits whether requiring antibiotics within 1 hour (n = 7 studies) versus 3 hours (n = 8) versus no specified time (n = 2); or 30 mL/kg fluid (n = 7) versus another volume (≥ 2 L, n = 1; ≥ 20 mL/kg, n = 2; 1.5–2 L or 500 mL, n = 1 each; none specified, n = 4) (p = 0.19 for each comparison). In the only study employing serial lactate measurements, survival was not increased versus others. No study had a low risk of bias or assessed potential adverse bundle effects.
Conclusions:
Available studies support the notion that antibiotic- and fluid-focused sepsis bundles like SEP-1 improve survival but do not demonstrate the superiority of any specific antibiotic time or fluid volume or of serial lactate measurements. Until strong reproducible evidence demonstrates the safety and benefit of any fixed requirement for these interventions, the present findings support the revision of SEP-1 to allow flexibility in treatment according to physician judgment.
Keywords: bundle, management, performance measure, sepsis, septic shock, treatment
The Centers for Medicare and Medicaid Services (CMS) develops performance measures (PMs) to enhance the recognition and management of clinical problems and thereby improve outcomes (1). Despite these goals, the complexity of CMS’s original sepsis bundle PM (SEP-1) raised concerns that it hindered management, so it was simplified in 2017 (2–8) (Appendix Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). However, questions persist regarding the benefit of SEP-1’s continuing requirement for all patients to receive antibiotics within 3 hours, for all hypotensive patients to receive a 30 mL/kg fluid infusion, and for obtaining a second lactate measurement if initially elevated (7, 9–12).
Compliance with SEP-1 requires all components be completed, and hospitals must report their compliance through CMS’s In-hospital Quality Reporting program (4). CMS now publicly compares hospitals’ SEP-1 compliance, influencing where consumers spend healthcare dollars (13, 14). Although CMS does not presently base hospital reimbursement directly on SEP-1 compliance, this could occur in the future (15). As an increasingly mandated PM, SEP-1 should conform to National Quality Forum (NQF, which endorses PMs for CMS) and CMS standards and only include proven therapies because pressure for hospitals to demonstrate compliance with it carries risks (16, 17). For example, injudicious antibiotic use to meet SEP-1’s time requirements in patients with noninfectious etiologies may produce antibiotic-associated toxicities (e.g., renal toxicity or Clostridium difficile or resistant nosocomial infection) (7, 9, 18–20). Requiring fixed fluid volumes without adjusting for patients’ comorbidities and volume status risks under- or overtreatment (21–24). Finally, serial lactate measurements to guide resuscitation are of unproven benefit and safety (8, 25, 26).
We previously examined and found that the quality of evidence supporting individual hemodynamic components in the original version of SEP-1, including a fixed 30 mL/kg volume infusion, serial lactate measurements, and bedside cardiac ultrasound and fluid responsiveness testing was low (8). We now hypothesize that bundles with elements like the revised SEP-1 and including early antibiotics and fluid administration would likely show a survival benefit, but there would be no concordant data from these bundles supporting when or how these two therapies should be delivered or that serial lactate measurements be obtained. To test this hypothesis, a systematic review of sepsis bundles similar to SEP-1 was performed, and different from our prior study, we examined the overall effect of these bundle components on mortality as well as whether variation in the administration of the components altered the bundles’ outcome.
METHODS
This systematic review was registered on International Prospective Register of Systematic Reviews (PROSPERO) December 18, 2017. A completed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist and complete methods are provided in the supplementary methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E730) (27).
Data Sources and Searches
We searched PubMed, Embase, Scopus, Web of Science, and ClinicalTrials.gov (Appendix Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E730; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/E731) through March 15, 2018, using terms previously described to examine SEP-1 (8).
Study Selection
We included studies that compared mortality between subjects receiving versus not receiving a focused sepsis bundle that included antibiotic and fluid administration, with or without vasopressors. Studies evaluating prior SEP-1 interventions no longer required in the revised 2018 version were excluded (2–4).
| Bundle Group | Control Group | |||
|---|---|---|---|---|
| Fluids | Other Interventions | |||
| Timing | Volume | Adjunctive Aids | Other | |
| NS | NS | Yes | — | No sepsis alert |
| ≤ 1 hr | NS | Yes | O2 therapy ≤ 1 hr; measure urine output ≤ 1 hr | No bundle |
| ≤ 30 min | ≥ 20 mL/kg bolus | Yes | — | No bundle |
| ≤ 1 hr | ≥ 20 mL/kg | Yes | VP/I | No bundle |
| NS | 30 mL/kg | Yes | — | No bundle |
| ≤ 30 min | 500 mL | No | Hemoglobin of 7–9 g/dL; VP/I | Bundle uncompleted |
| ≤ 2 hr | ≥ 2 L | Yes | — | No bundle |
| NS | 30 mL/kg bolus | Yes | — | No bundle |
| ≤ 1 hr | NS | Yes | — | No bundle |
| ≤ 30 min | 30 mL/kg bolus | Yes | — | Bundle uncompleted |
| ≤ 30 min | 30 mL/kg bolus | Yes | — | Bundle uncompleted |
| ≤ 30 min | 30 mL/kg bolus | Yes | — | Bundle uncompleted |
| ≤ 3 hr | 30 mL/kg | Yes | — | No bundle |
| NS | NS | No | VP/I | Bundle uncompleted |
| ≤ 2 hr | 1.5–2 L | No | — | Bundle uncompleted |
| NS | 30 mL/kg bolus | No | VP/I | Bundle uncompleted |
| NS | 500–1000 mL bolus, titrate up to 30 mL/kg | Yes | VP/I | No bundle |
Endpoints
The overall survival effects of bundles were examined, followed by whether survival effects differed for bundles stipulating: differing antibiotic treatment times, 30 mL/kg fluid volumes versus other volumes, or obtaining versus not obtaining serial lactate measurements. The effects of bundles on antibiotic and fluid administration were assessed.
Data Extraction and Quality Assessment
Data extracted (Appendix Table 2, Supplemental Digital Content 1, http://links.lww.com/CCM/E730) included the following: study design, baseline characteristics, bundle requirements, proportion of subjects receiving interventions within stipulated time goals, amount of fluids infused, proportion of subjects with infection sensitive to administered antibiotics, and adverse effects related to bundle use. Bundles were defined as either stipulating 30 mL/kg fluid, another volume, or no specific volume. Bundle groups were considered favored if subjects were younger, had fewer comorbidities or decreased illness severity, or had fewer pulmonary or abdominal infections. Adjunctive aid use was examined and risk of bias assessed (28, 29).
Data Synthesis and Analysis
For survival or proportion of patients receiving interventions, odds ratio (OR) and 95% CIs were calculated. Reported median and interquartile range (IQR) values were converted to mean difference and ses (30). Outcome summary estimates used random-effects models (31).
RESULTS
Literature Search
Fifteen publications encompassing 17 studies measuring survival for a sepsis bundle versus control (32–46) were identified (Appendix Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E730; and legend, Supplemental Digital Content 2, http://links.lww.com/CCM/E731). One report analyzed three cohorts representing three individual studies designated here by each cohort’s final enrollment year (i.e., Leisman et al [41], 2012; Leisman et al [41], 2014; and Leisman et al [41], 2015).
Study Characteristics
Table 1 and Appendix Tables 2 and 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/E730) summarize study characteristics. All studies used observational designs, including 10 before/after cohort control studies (7,515 preintervention control and 8,568 postintervention bundle subjects) and seven concurrent cohort control studies (11,303 control and 4,977 intervention bundle subjects). Thirteen studies retrospectively enrolled control or control and bundle subjects, respectively. Three before/after studies employed International Classification of Diseases (ICD) codes to identify septic subjects, one employed an administrative data set, and six were quality improvement programs. Only four studies provided adjusted survival estimates, and in four studies, significant baseline differences in severity of illness favored bundle subjects (i.e., higher systolic blood pressure or reduced organ injury).
TABLE 1.
Summary of the Patient Numbers in Control and Bundle Groups, Study Designs, Sepsis Types, Location of Enrolled Patients, Country of Study, and the Number of Study Sites in the 17 Studies
| Reference | No. of Patients | Study Design | Sepsis Type | Location of Patients | Study Country | No. of Study Sites | |||
|---|---|---|---|---|---|---|---|---|---|
| Control Bundle | Control | Bundle | |||||||
| Austrian et al (32) | 838 | 1,306 | BACCa,b | Retrospective | Retrospective | SS/SSh | ED | United States | 1 |
| Bhat et al (33) | 67 | 54 | BACCb | Retrospective | Retrospective | Sepsis | Hospital | United Kingdom | 1 |
| Bruce et al (34) | 62 | 75 | BACC | Retrospective | Retrospective | SS/SSh | ED | United States | 2 |
| De Miguel-Yanes et al (35) | 53 | 50 | BACCb | Retrospective | Prospective | Sepsis/SS/SSh | ED | Spain | 1 |
| Ferreras Amez et al (36) | 222 | 222 | BACCa,b | Retrospective | Prospective | Sepsis/SSh | ED | Spain | 6 |
| Gao et al (37) | 49 | 52 | CCC | Prospective | Prospective | SS/SSh | ED + wards | United Kingdom | 2 |
| Gatewood et al (38) | 137 | 83 | BACCa,b | Retrospective | Prospective | SS/SSh | ED | United States | 1 |
| Hayden et al (39) | 108 | 130 | BACC | Retrospective | Retrospective | Sepsis/SSh | ED | United States | 1 |
| Kumar et al (40) | 55 | 71 | BACC | Retrospective | Prospective | Sepsis | ED | New Zealand | 1 |
| Leisman et al (41) (2012)c | 4,769 | 1,050 | CCCb | Prospective | Prospective | SS/SSh | ED + ICU | United States | 11 |
| Leisman et al (41) (2014)c | 958 | 739 | CCCb | Prospective | Prospective | SS/SSh | ED + ICU | United States | 1 |
| Leisman et al (41) (2015)c | 5,124 | 2,115 | CCCb | Prospective | Prospective | SS/SSh | ED + ICU | United States | 9 |
| Liu et al (42) | 5,942 | 6,544 | BACCa,b | Retrospective | Prospective | Sepsis | ED + ICU | United States | 21 |
| Prasad et al (43) | 287 | 742 | CCCb | Retrospective | Retrospective | SS/SSh | ED + ICU | United States | 1 |
| Ruangchan et al (44) | 70 | 158 | CCC | Retrospective | Retrospective | SS | Hospital | Thailand | 1 |
| Teles et al (45) | 46 | 121 | CCC | Retrospective | Retrospective | Sepsis | ICU + wards | Brazil | 1 |
| Tse et al (46) | 31 | 33 | BACC | Retrospective | Prospective | SS | ED | Hong Kong | 2 |
BACC = before-after cohort control, CCC = concurrent cohort control, ED = emergency department, SS = severe sepsis, SSh = septic shock.
Employed International Classification of Diseases, 9th Edition, codes or administrative data sets to identify septic patients.
Study described as part of a program to improve patient care quality (i.e., quality improvement).
One publication included comparisons of control and intervention patients from each of three distinct patient populations admitted to study hospitals over periods ending in either 2012, 2014, or 2016, and each group and comparison was analyzed as a single study here.
Summary of Bundles Studied
Antibiotics were required within 1 hour in seven studies, 3 hours in seven, within 3 hours in the emergency department, or 1 hour for inpatients in one (considered ≤ 3 hr in analysis), and two studies did not report times (Table 2; and Appendix Tables 4–6, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). The fluid volume required was 30 mL/kg in seven studies, greater than or equal to 2 L in one, greater than or equal to 20 mL/kg in two, and 1.5–2 L or 500 mL in one study each. Four studies did not stipulate a volume. Only one study required serial lactate measurements. Table 2 shows control treatments.
TABLE 2.
Bundle Components to Be Administered in Bundle Groups and Management in the Control Groups of the 17 Studies
| References | Overall Bundle Time | Bundle Group | ||||
|---|---|---|---|---|---|---|
| Reported Time Zero | Laboratory Data | Antibiotics | ||||
| Lactate | Blood Culture | Timing | Type | |||
| Austrian et al (32) | NS | NR | Yes | Yes | NS | Appropriate |
| Bhat et al (33) | 1 hr | Sepsis diagnosis | Yes, ≤ 1 hr | Yes, ≤ 1 hr | ≤ 1 hr | NS |
| Bruce et al (34) | 3 hr | Sepsis diagnosis | Yes | Yes | NS | BS |
| De Miguel-Yanes et al (35) | 3 hr | Sepsis diagnosis | Yes | Yes | ≤ 3 hr | NS |
| Ferreras Amez et al (36) | 3 hr | ED arrival | Yes | Yes | ≤ 1 hr | NS |
| Gao et al (37) | 6 hr | Sepsis criteria meta | — | Yes | ≤ 1 hr | NS |
| Gatewood et al (38) | 3 hr | ED triage | Yes | Yes | ≤ 3 hr | NS |
| Hayden et al (39) | NS | ED arrival | Yes | Yes | NS | BS |
| Kumar et al (40) | 1 hr | Initial nursing assessment | Yes, ≤ 1 hr | Yes, ≤ 1 hr | ≤ 1 hr | NS |
| Leisman et al (41) (2012)b | 3 hr | Sepsis criteria metc | Yes | Yes | ≤ 3 hr | BS |
| Leisman et al (41) 2014b | 3 hr | Sepsis criteria metc | Yes | Yes | ≤ 3 hr | BS |
| Leisman et al (41) 2015b | 3 hr | Sepsis criteria metc | Yes | Yes | ≤ 3 hr | BS |
| Liu et al (42) | 3 hr | Initial lactate test results obtained | Yesd | NS | ≤ 3 hr | NS |
| Prasad et al (43) | 3 hr | Sepsis criteria mete | Yes | Yes | ≤ 3 hr in ED; ≤ 1 hr inpatient | NS |
| Ruangchan et al (44) | NS | Sepsis diagnosis | — | Yes | ≤ 1 hr | Empiric |
| Teles et al (45) | 3 hr | Sepsis diagnosis | Yes | Yes | ≤ 1 hr | BS |
| Tse et al (46) | NS | ED registration | Yes | Yes | ≤ 1 hr | NS |
BS = broad-spectrum antibiotics, ED = emergency department, lactate = initial lactate, NR = not reported, NS = not stated, VP/I = vasopressor and/or inotrope for persistent hypotension.
All the following fulfilled: signs and symptoms of infection, documented source of infection, and ≥1 organ dysfunction.
See text for description of these three cohorts.
Time of laboratory result or time of vital sign measurement causing the patient to meet sepsis criteria.
Obtained second lactate value.
Time at which two systemic inflammatory response syndrome criteria and one sign of organ failure in the presence of known or suspected infection met.
Effect of Bundle Treatment on Survival
Appendix Table 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/E730) shows mortality endpoints of studies. Compared with controls, bundle treatment was associated with increased ORs of survival in 15 of 17 studies (statistically significant in nine), but there was substantial heterogeneity overall (I2 = 61%; p < 0.01). Categorizing studies by enrolled numbers isolated this heterogeneity to smaller trials (Fig. 1). Stratified into terciles based on enrollment numbers, the OR increased consistently in the five largest (1,697–12,486 subjects per study) (1.20 [1.11–1.30]; I2 = 0%; p = 0.65) and six medium-sized studies (167–1,029) (2.03 [1.52–2.71]; I2 = 8%; p = 0.36), but with significant heterogeneity in the six smallest studies (64–137) (1.25 [0.42–3.66]; I2 = 57%; p = 0.04). Bundle survival effects were fairly consistent in before/after studies (n = 10 studies) (1.20 [1.02–1.40]; I2 = 21%; p = 0.25), but not cohort-controlled ones (n = 7) (1.73 [1.21–2.48]; I2 = 76%; p < 0.01) in which three large and three small studies increased heterogeneity. A funnel plot using Egger regression analysis could not rule out publication bias (p = 0.10) (Appendix Fig. 2, Supplemental Digital Content 1, http://links.lww.com/CCM/E730; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/E731). Survival estimates with bundles adjusted for severity of illness or other characteristics were increased in the four studies providing data.
Figure 1.
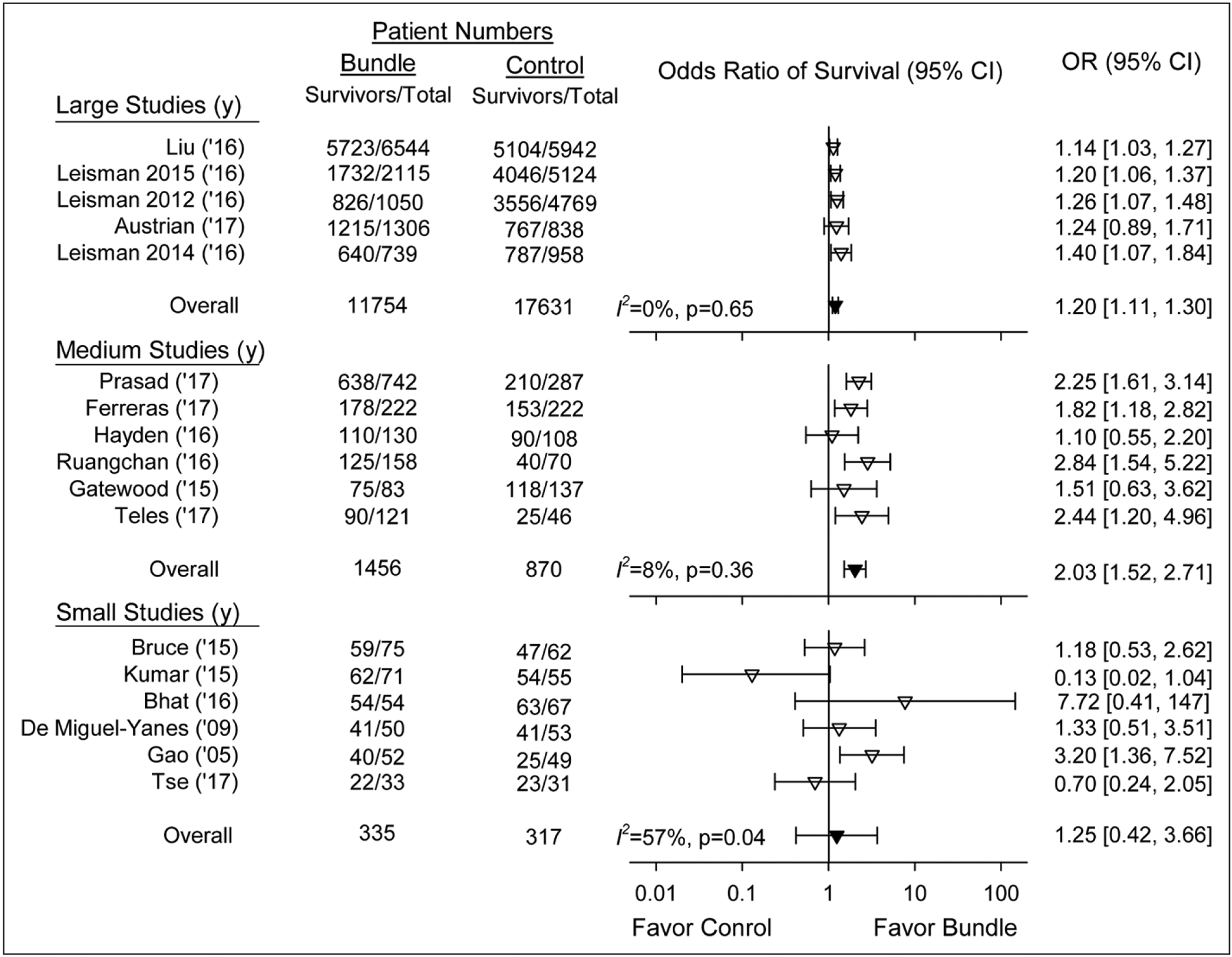
Effect of treatment with a sepsis bundle on the odds ratio (OR) of survival (95% CI) in the 17 individual studies analyzed (inverted open triangles). Studies are shown stratified by terciles into the five studies with the largest-sized number of patients enrolled (1,697–12,486 patients, 29,385 total), the six studies with medium-sized number of patients (167–1,029, 2,326 total), and the five studies with the smallest number of patients enrolled (64–137, 652 total). The overall ORs (95% CI) and I2 are shown for each of these three groups (inverted closed triangles).
Bundles were associated with similarly increased survival (p = 0.19) whether they specified antibiotic administration within 1 hour (n = 7 studies) (1.92 [0.92–4.00]; I2 = 57%; p = 0.03), 3 hours (n = 8) (1.34 [1.11–1.61]; I2 = 56%; p = 0.03), or without a specified time (n = 2) (1.21 [0.69–2.13]; I2 = 0; p = 0.77) (Fig. 2) Two of the 1-hour antibiotic studies had survival effects on the side of harm (I2 = 0), and for one study, this approached significance. Bundles were also associated with increased survival whether they specified 30 mL/kg fluid infusions (n = 7 studies) (1.23 [1.09–1.39]; I2 = 38%; p = 0.14), a volume other than 30 mL/kg (n = 6) (1.70 [0.94–3.05]; I2 = 41%; p = 0.13) or did not specify a volume (n = 4) (1.30 [0.17–10.13]; I2 = 77%; p < 0.01) (Fig. 3). Bundle survival effects were similar (p = 0.70) comparing studies specifying volumes other than 30 mL/kg (n = 6) versus those with individualized volumes (n = 4), and the overall survival effect of bundles in these 10 studies (1.62 [1.03–2.55]; I2 = 59%; p < 0.01) was not significantly different (p = 0.19) compared with studies requiring 30 mL/kg (1.23 [1.09–1.39]). This difference was also not significant (p = 0.27) when the one study requiring a fluid volume greater than or equal to 2 L was combined with those requiring 30 mL/kg. Finally, in the only bundle study requiring serial lactate measurements, survival (1.14 [1.03–1.27]) was no greater than all others (1.50 [1.20–1.87]; I2 = 58%; p < 0.01).
Figure 2.
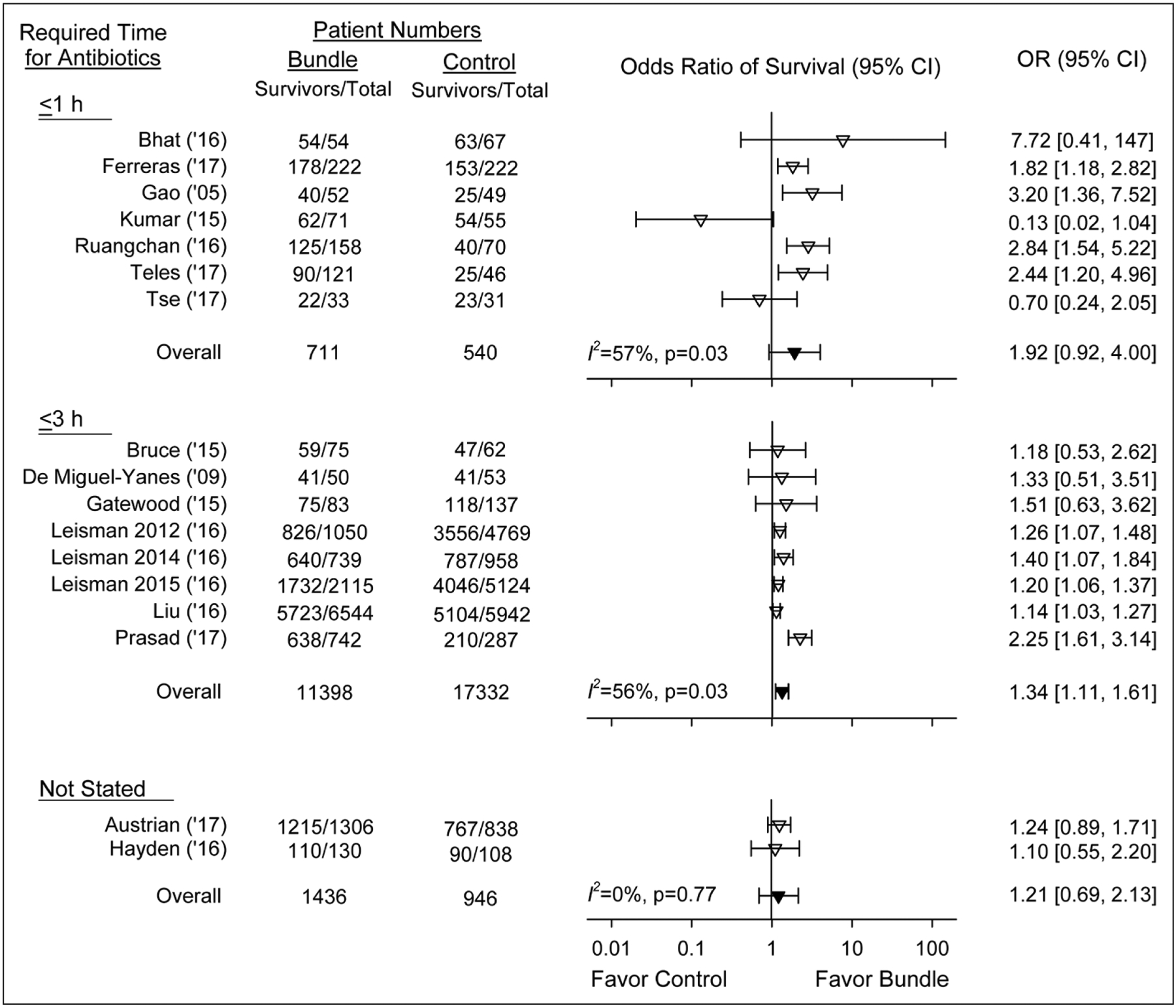
Effect of treatment with a sepsis bundle on the odds ratio (OR) of survival (95% CI) in studies grouped based on whether the bundles studied required antibiotics be administered (inverted open triangles). in less than or equal to 1 hr or less than or equal to 3 hr or the bundle did not state when antibiotics should be administered. The overall ORs (95% CI) and I2 are shown for each of these three groups. The overall effects of bundles did not differ significantly (p = 0.19) comparing the three groups (inverted closed triangles).
Figure 3.
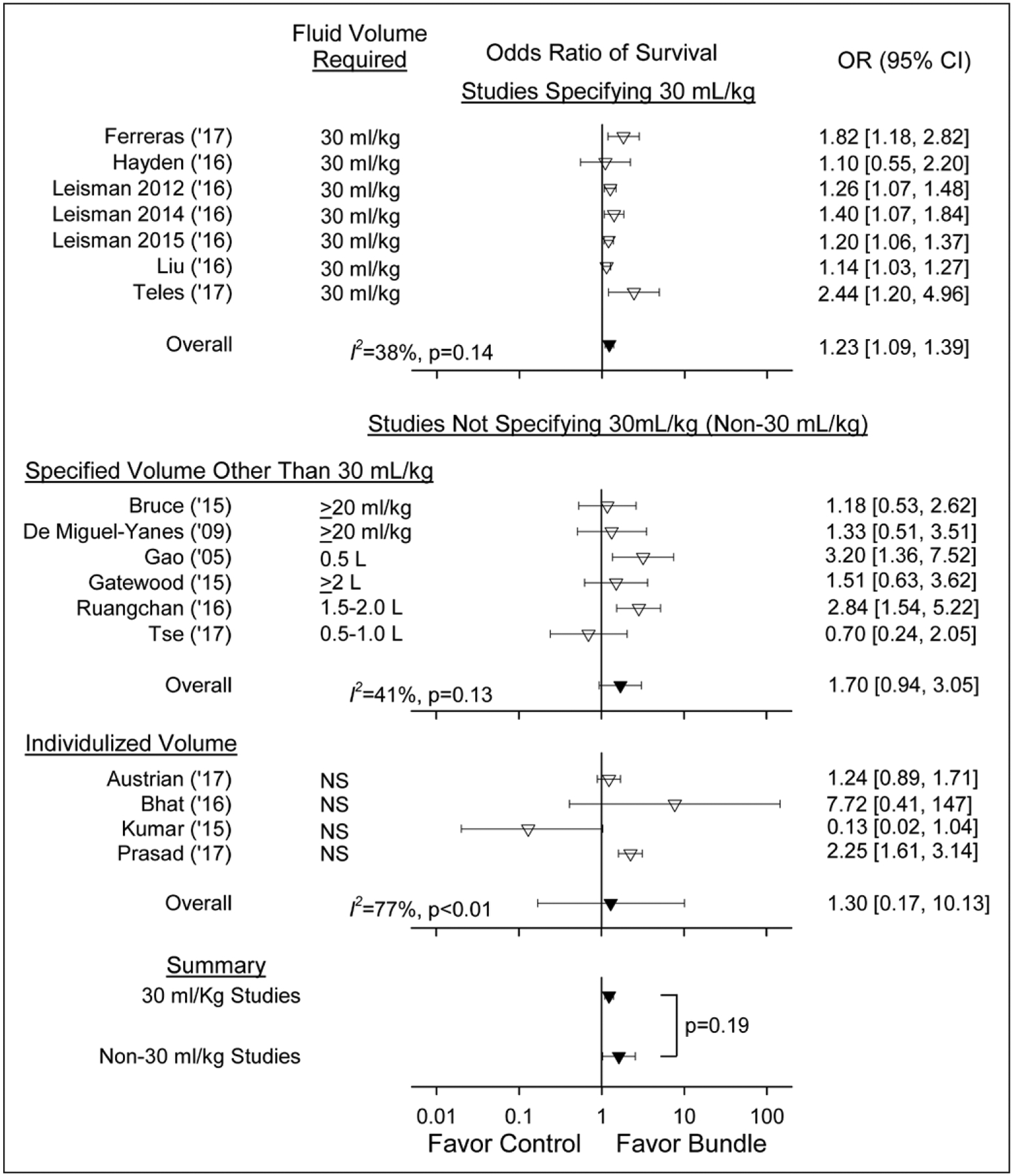
Effect of treatment with a sepsis bundle on the odds ratio (OR) of survival (95% CI) in studies grouped based on whether the bundles studied specified that 30 mL/kg fluid be administered, that a volume other than 30 mL/kg be given as shown in the figure, or that did not specify what volume be administered (i.e., an individualized volume) (inverted open triangles). The overall ORs (95% CI) and I2 are shown for each of these three groups (inverted closed triangles). The overall effects of bundles did not differ significantly (p = 0.19) comparing studies requiring 30 mL/kg to all other studies not requiring this volume. This difference was also not significant (p = 0.27) when the one study requiring a fluid volume greater than or equal to 2 L was combined with those requiring 30 mL/kg and compared with all other studies. NS = not significant.
Effect of Bundle Treatment on Antibiotic and Fluid Administration
In the 13 studies reporting antibiotic administration data, compared with controls, bundle treatment was associated with increased OR of receiving timely antibiotics (i.e., within the bundle’s targeted time) in 12 of 12 studies (10 significantly) (12.5 [2.4–65.6)]; I2 = 94%; p < 0.01) and reduced times to antibiotic administration (shown as a negative mean difference [95% CI]) in six of six studies (five significantly) (−42.0 min [−70.0 to −14.1]; I2 = 99%; p < 0.01) (Fig. 4; and Appendix Table 4, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). Only four studies reported subjects with positive cultures, and no study reported antibiotic microbial sensitivities. In 13 studies providing fluid data (Fig. 4; and Appendix Table 5, Supplemental Digital Content 1, http://links.lww.com/CCM/E730), bundles were associated with increased OR of receiving timely fluids in nine of nine studies (eight significantly) (29.9 [1.9–483.2]; I2 = 95%; p < 0.01) and with reduced times to fluid administration in five of five studies (−42.4 min [−65.8 to −18.9]; I2 = 98%; p < 0.01). Variability in antibiotic and fluid administration measures was not related to study size. In the only two studies reporting administered fluid volumes, one requiring 30 mL/kg fluid and one 1.5–2.0 L, bundle patients received more fluid than controls but administered volumes varied widely for both groups in each study (milliliters; mean [sd], 1,900 [1,200] vs 1,800 [1,200]; and median [IQR], 1,500 [1,000–2,000] vs 220 [160–628], respectively) (Appendix Table 5, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). Three studies reported proportions of patients receiving vasopressors, and one the time to vasopressors and were not analyzed further (Appendix Table 6, Supplemental Digital Content 1, http://links.lww.com/CCM/E730).
Figure 4.
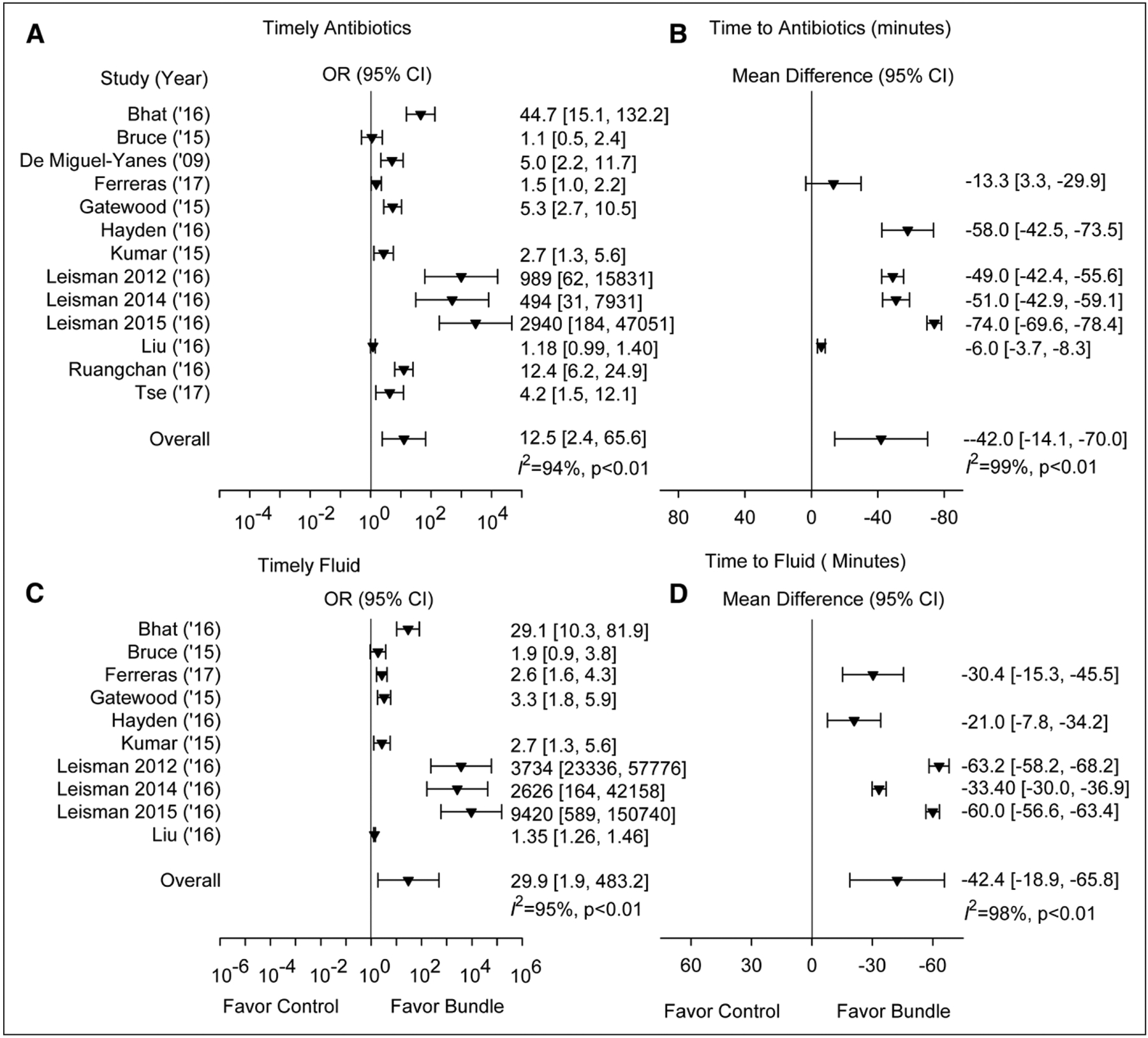
This figure shows whether bundle treatment increased the proportion of patients receiving timely antibiotics or fluids or decreased the mean time to antibiotics or fluids compared to no bundle treatment in studies providing data. A and C, The effect of treatment with a sepsis bundle on the odds ratio (OR [95% CI]) of patients receiving timely (i.e., within a prespecified time period) antibiotics or fluids is shown. B and D, The effect of treatment with a sepsis bundle on the time to (calculated as the mean difference in time [95% CI]) antibiotics or fluids is shown. Both individual and overall ORs are shown as inverted solid triangles.
No significant (p ≥ 0.50) relationships (slope ± se) were demonstrated between the log OR of survival and receipt of either timely antibiotics (−0.004 ± 0.028) or fluids (0.007 ± 0.011) with bundle treatment or with reduced time to antibiotic (0.001 ± 0.001) or fluid administration (−0.005 ± 0.004).
Other Findings
Bundle treatment was associated with increased ORs of obtaining initial lactate measurements and blood cultures (Appendix Fig. 3, Supplemental Digital Content 1, http://links.lww.com/CCM/E730 [legend, Supplemental Digital Content 2, http://links.lww.com/CCM/E731] and Appendix Table 6, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). Thirteen studies included educational and/or priority-care adjunctive aids (Appendix Table 7, Supplemental Digital Content 1, http://links.lww.com/CCM/E730). No study had a low risk of bias or assessed adverse effects of bundle treatment (Appendix Tables 7 and 8, Supplemental Digital Content 1, http://links.lww.com/CCM/E730).
DISCUSSION
We previously reported that some hemodynamic elements of the CMS SEP-1 performance improvement metric had only low-quality supporting evidence (8). We now report our study of other elements of this sepsis metric in the 17 observational studies analyzed here. In 15 of these studies, focused sepsis bundles combining antibiotic and fluid administration like SEP-1 had survival effects on the side of benefit and there were consistent significant beneficial effects across the five largest and six medium-sized studies. However, bundles had similar survival effects whether the required time to antibiotics was 1 or 3 hours or was not stated. Also, bundle effects on survival and antibiotic administration were not significantly related. There was substantial heterogeneity across studies of the 1- and 3-hour antibiotic treatment times (I2 = 57 and 56%), and two of the 1-hour antibiotic studies had survival effects on the side of harm with an I2 = 0, with one approaching significance. Bundles stipulating 30 mL/kg fluid did not have a significantly different or larger survival effect compared with bundles directing other volumes or allowing individualized volumes. Only one study requiring 30 mL/kg reported volumes delivered to patients and these varied greatly. Finally, survival was no better in the only study with a bundle requiring serial lactates than other studies. Antibiotics and cardiovascular resuscitation with fluids are essential sepsis therapies. Overall, the present studies are consistent with the notion that antibiotic- and fluid-focused bundles improve sepsis survival. But they do not provide concordant evidence that any specific time to antibiotic treatment or fluid volume was better or safer than any other tested or that serial lactate measurements are needed.
Consistent with these findings, other studies have suggested that decreasing the time from sepsis diagnosis to antibiotic administration improves survival (47–55). A retrospective analysis of SEP-1’s earlier version found that only antibiotic compliance was potentially associated with improved survival (56). However, at present, there is no high-quality evidence (i.e., randomized controlled trials [RCTs]) demonstrating that stipulating a specific time to antibiotics like 3 hours, either by itself or in a bundle, is more effective than a more flexible timeframe based on patients’ sepsis severity. Although most would agree that antibiotics should be administered rapidly in patients with septic shock, hastily administered antibiotics to patients less likely to have bacterial infection or not requiring emergent therapy can increase risks (e.g., renal toxicity or C. difficile or resistant nosocomial infection) (7, 9, 18–20). It is also accepted that septic shock patients require fluid and vasopressor support to prevent organ injury and death. But in the present studies, there was considerable variability in the fluid volumes targeted, ranging from 500 to 1,000 mL up to 30 mL/kg, and survival was increased whether bundles required lower or higher volumes or did not stipulate a volume. The actual volumes administered in the only two studies providing data varied markedly among patients suggesting fluids were titrated and no single volume was used for all patients. Experts debate the best volume and rate of fluid for sepsis, and a government-sponsored, multicenter trial enrolling septic shock patients (critically examined by C.N., P.Q.E.) is based on the premise that appropriate fluid management for early sepsis is unknown (23, 48, 57–59).
Importantly, none of the 17 analyzed studies reported or clearly investigated bundle-associated adverse events such as toxicities related to inappropriate antibiotic administration in subjects without documented infection, or cardiopulmonary complications related to inadequate or excessive fluid administration. Many subjects meeting systemic inflammatory response or organ failure assessment criteria for focused sepsis bundle administration are eventually found to not have sepsis. The potential harm of these interventions when administered in bundles to nonseptic patients or when comorbidities are unaccounted for has not been quantified.
We understand that important limitations undermine the conclusions regarding the efficacy of the bundles studied. All studies were observational non-RCTs and did not meet NQF standards for high-quality evidence which requires well-conducted RCTs (16). The NQF endorses PMs for CMS including SEP-1. These studies also do not meet NQF criteria for moderate-quality evidence, requiring that observational studies control for possible confounders. Thirteen studies (76%) enrolled patients retrospectively, and no study was at low risk of bias. Thirteen included educational or priority-care adjunctive aids potentially benefiting survival. Only four studies reported adjusted survival, yet the recent analysis of cases managed with the original SEP-1 found that although crude mortality was lower in compliant versus noncompliant patients, the groups did not differ after adjustment for clinical characteristics and illness severity (56). Ten studies (59%), including the largest, were before/after ones, and evidence indicates that increasing sepsis awareness will boost enrollment of less ill sepsis cases over time with this study design (60, 61). This problem may be more pronounced with studies using sepsis coding (e.g., ICD codes) and administrative datasets to identify septic patients, which four studies including the two largest did. In the seven concurrent-control studies, noncompliance in control patients may have been due to less evident sepsis symptomatology at patient presentation, a variable potentially increasing mortality independent of treatment (62). The funnel plot and Egger analysis cannot exclude publication bias. Finally, there was no measurable relationship between bundle effects on time to treatments and improved survival. Thus, multiple factors besides bundle interventions potentially influenced the observed survival improvements across the 17 studies. Observational studies designed similar to those analyzed here, some larger than the present ones, reported that sepsis bundles including early goal-directed therapy (EGDT) improved survival (49). However, subsequent well-conducted RCTs showed that EGDT was no more beneficial but more costly than usual care (63).
We previously showed that there was only low-quality evidence supporting the safety or benefit of serial lactate measurements to manage septic patients (8). The present study demonstrates that there is also no evidence that serial lactate measurements increase the effectiveness of focused sepsis bundles.
Two large retrospective studies examining focused bundles did not meet our inclusion criteria requiring a direct comparison between a bundle and nonbundle group. One examined whether there was a “correlation” between the time the components of a sepsis bundle were administered and survival (48). The other examined whether over 2 years of study, there were “temporal trends” in the initiation of a sepsis protocol, compliance with a sepsis bundle, or survival and then whether there was an association between the two former two measures and survival (64).
Sepsis is one of the most common causes of hospital mortality and may warrant care that is optimized by a PM. The best available data are consistent with the notion that bundles focusing on the two essential initial interventions for septic shock patients, antibiotics for infection and fluids for cardiovascular resuscitation, benefit care. But multiple factors limit how specific these bundle recommendations should be. Notably, there has been considerable variability in the volume of fluids and timing of antibiotics studied in focused bundles so far. Furthermore, studies have not examined bundle-associated adverse effects and lack rigorous methodology. Therefore, these studies are not fully informative or definitive. In sepsis, clinical experience and the belief that delays in treatment for most clinical conditions are detrimental support starting antibiotics for infection and resuscitating with fluids and vasopressors to normalize physiology as soon as possible. Patients with septic shock require prompt antibiotic therapy and hemodynamic support. A framework for the administration of these therapies, like a 3-hour window for antibiotic administration, will benefit some patients and could be part of a PM. However, consistent with the observations of others in the field of sepsis, as CMS further revises SEP-1, it should take into account that without high- or even moderate-quality evidence to support the benefit and safety of one specific antibiotic treatment time or volume of fluid, there is no justification for not allowing clinicians to adjust these treatments’ delivery as risk-benefit calculations require in individual cases (10–12). Although emphasizing the importance of early care for septic patients, flexibility is also necessary because it is unlikely that there will ever be definitive data showing a specific antibiotic time or fluid volume that fits all sepsis cases. Without high- or moderate-quality evidence, serial lactate measurements should also be guided by providers’ assessment. When revising SEP-1, broad input from all medical disciplines employing the measure may help ensure the measure is followed because there would be a consensus that it represents optimal medical care. A sepsis PM should focus care on the septic patient. However, without high-quality evidence for its safety and benefit, it should not preempt a qualified clinician’s judgment or hinder the clinician’s medical practice.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. Kelly Byrne for her editorial assistance.
Supported, in part, by National Institutes of Health intramural funding.
Drs. Pepper, Sun, Cui, Natanson, and Eichacker received support for article research from the National Institutes of Health. Drs. Pepper, Sun, and Cui, Ms. Welsh, and Dr. Eichacker disclosed government work.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Clinical Trial Registration numbers: International Prospective Register of Systematic Reviews (PROSPERO) 2017: CRD42017080258.
REFERENCES
- 1.Quality Reporting Center: SEP-1 Early Management Bundle, Severe Sepsis/Septic Shock: v5.4 Measure Updates. Available at: https://www.qualityreportingcenter.com/event/sep-1-early-management-bundle-severe-sepsis-septic-shock-v5-4-measure-updates/. Accessed December 4, 2018
- 2.Version 5.2 The Joint Commission: Specifications Manual for National Hospital Inpatient Quality Measures. Available at: www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed December 4, 2018
- 3.Version 5.3 The Joint Commission: Specifications Manual for National Hospital Inpatient Quality Measures. Available at: www.jointcom-mission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed December 4, 2018
- 4.Version 5.4 The Joint Commission: Specifications Manual for National Hospital Inpatient Quality Measures. Available at: www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed December 4, 2018
- 5.Klompas M, Rhee C: The CMS sepsis mandate: Right disease, wrong measure. Ann Intern Med 2016; 165:517–518 [DOI] [PubMed] [Google Scholar]
- 6.Aaronson EL, Filbin MR, Brown DF, et al. : New mandated centers for Medicare and Medicaid Services requirements for sepsis reporting: Caution from the Field. J Emerg Med 2017; 52:109–116 [DOI] [PubMed] [Google Scholar]
- 7.Faust JS, Weingart SD: The past, present, and future of the centers for Medicare and Medicaid services quality measure SEP-1: The early management bundle for severe sepsis/septic shock. Emerg Med Clin North Am 2017; 35:219–231 [DOI] [PubMed] [Google Scholar]
- 8.Pepper DJ, Jaswal D, Sun J, et al. : Evidence underpinning the centers for Medicare & Medicaid services’ severe sepsis and septic shock management bundle (SEP-1): A systematic review. Ann Intern Med 2018; 168:558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IDSA Sepsis Task Force: Infectious Diseases Society of America (IDSA) position statement: Why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis. 2018;66:1631–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klompas M, Calandra T, Singer M: Antibiotics for sepsis-finding the equilibrium. JAMA 2018; 320:1433–1434 [DOI] [PubMed] [Google Scholar]
- 11.Klompas M, Rhee C: Current sepsis mandates are overly prescriptive, and some aspects may be harmful. Crit Care Med 2018. Dec 4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Farkas JD, Spiegel R, et al. ; collaborating authors: POINT: should the surviving sepsis campaign guidelines be retired? Yes. Chest 2019; 155:12–14 [DOI] [PubMed] [Google Scholar]
- 13.Medicare: Hospital Compare. Available at: https://www.medicare.gov/hospitalcompare/about/what-is-HOS.html. Accessed December 4, 2018
- 14.Centers for Medicare and Medicaid Services: Hospital Compare. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalCompare.html. Accessed December 4, 2018
- 15.Centers for Medicare and Medicaid Services: Hospital Value-Based Purchasing. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Hospital-Value-Based-Purchasing-.html. Accessed December 4, 2018
- 16.NQF: Review and Update of Guidance for Evaluating Evidence and Measure Testing. Available at: https://www.qualityforum.org/Publications/2013/10/Review_and_Update_of_Guidance_for_Evaluating_Evidence_and_Measure_Testing_-_Technical_Report.aspx. Accessed December 4, 2018
- 17.Jaswal DS, Natanson C, Eichacker PQ: Endorsing performance measures is a matter of trust. BMJ 2018; 360:k703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers JD, Akram AR, Singanayagam A, et al. : Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J Infect 2016; 73:45–53 [DOI] [PubMed] [Google Scholar]
- 19.Kanwar M, Brar N, Khatib R, et al. : Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: Side effects of the 4-h antibiotic administration rule. Chest 2007; 131:1865–1869 [DOI] [PubMed] [Google Scholar]
- 20.Shime N, Saito N, Bokui M, et al. : Clinical outcomes after initial treatment of methicillin-resistant Staphylococcus aureus infections. Infect Drug Resist 2018; 11:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maitland K, Kiguli S, Opoka RO, et al. ; FEAST Trial Group: Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364:2483–2495 [DOI] [PubMed] [Google Scholar]
- 22.Andrews B, Semler MW, Muchemwa L, et al. : Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: A randomized clinical trial. JAMA 2017; 318:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald SPJ, Keijzers G, Taylor DM, et al. ; REFRESH trial investigators: Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): A pilot randomised controlled trial. Intensive Care Med 2018; 44:2070–2078 [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Malbrain MLNG: The SEP-1 quality mandate may be harmful: How to drown a patient with 30 mL per kg fluid! Anaesthesiol Intensive Ther 2017; 49:323–328 [DOI] [PubMed] [Google Scholar]
- 25.Marik PE, Bellomo R: Lactate clearance as a target of therapy in sepsis: A flawed paradigm. OA Critical Care 2013;1:3 [Google Scholar]
- 26.Bakker J: Lost in translation: On lactate, hypotension, sepsis-induced tissue hypoperfusion, quantitative resuscitation and Surviving Sepsis Campaign bundles. Crit Care Med 2015; 43:705–706 [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group: The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605 [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Wang W, Liu J, et al. : Estimating the sample mean and standard deviation from the sample size, median, range and/or inter-quartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp G, Hartung J: Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22:2693–2710 [DOI] [PubMed] [Google Scholar]
- 32.Austrian JS, Jamin CT, Doty GR, et al. : Impact of an emergency department electronic sepsis surveillance system on patient mortality and length of stay. J Am Med Inform Assoc 2018; 25:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhat A, Asghar M, Raulia G, et al. : Improving multidisciplinary severe sepsis management using the Sepsis Six. Clin Med (Lond) 2016; 16:503–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruce HR, Maiden J, Fedullo PF, et al. : Impact of nurse-initiated ED sepsis protocol on compliance with sepsis bundles, time to initial antibiotic administration, and in-hospital mortality. J Emerg Nurs. 2015; 41:130–137 [DOI] [PubMed] [Google Scholar]
- 35.De Miguel-Yanes JM, Muñoz-González J, Andueza-Lillo JA, et al. : Implementation of a bundle of actions to improve adherence to the Surviving Sepsis Campaign guidelines at the ED. Am J Emerg Med 2009; 27:668–674 [DOI] [PubMed] [Google Scholar]
- 36.Ferreras Amez JM, Arribas Entrala B, Sarrat Torres MA, et al. ; En nombre del Grupo Sepsis Aragón: [Before-after study of the effect of implementing a sepsis code for emergency departments in the community of Aragon]. Emergencias 2017; 29:154–160 [PubMed] [Google Scholar]
- 37.Gao F, Melody T, Daniels DF, et al. : The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: A prospective observational study. Crit Care 2005; 9:R764–R770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatewood MO, Wemple M, Greco S, et al. : A quality improvement project to improve early sepsis care in the emergency department. BMJ Qual Saf 2015; 24:787–795 [DOI] [PubMed] [Google Scholar]
- 39.Hayden GE, Tuuri RE, Scott R, et al. : Triage sepsis alert and sepsis protocol lower times to fluids and antibiotics in the ED. Am J Emerg Med 2016; 34:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar P, Jordan M, Caesar J, et al. : Improving the management of sepsis in a district general hospital by implementing the ‘Sepsis Six’ recommendations. BMJ Qual Improv Rep 2015; 4:u207871.w4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leisman DE, Doerfler ME, Ward MF, et al. : Survival benefit and cost savings from compliance with a simplified 3-hour sepsis bundle in a series of prospective, multisite, observational cohorts. Crit Care Med 2017; 45:395–406 [DOI] [PubMed] [Google Scholar]
- 42.Liu VX, Morehouse JW, Marelich GP, et al. : Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med 2016; 193:1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad PA, Shea ER, Shiboski S, et al. : Relationship between a sepsis intervention bundle and in-hospital mortality among hospitalized patients: A retrospective analysis of real-world data. Anesth Analg 2017; 125:507–513 [DOI] [PubMed] [Google Scholar]
- 44.Ruangchan S, Chusri S, Saengsanga P, et al. : Clinical outcomes of community-acquired severe sepsis after implementation of a simple severe sepsis fast Track. J Med Assoc Thai 2016; 99:877–885 [PubMed] [Google Scholar]
- 45.Teles F, Rodrigues WG, Alves MGTC, et al: Impact of a sepsis bundle in wards of a tertiary hospital. J Intensive Care 2017; 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tse CL, Lui CT, Wong CY, et al. : Impact of a sepsis guideline in emergency department on outcome of patients with severe sepsis. Hong Kong J Emerg Med 2017;24:123–131 [Google Scholar]
- 47.Kumar A, Roberts D, Wood KE, et al. : Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 48.Seymour CW, Gesten F, Prescott HC, et al. : Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalil AC, Johnson DW, Lisco SJ, et al. : Early goal-directed therapy for sepsis: A novel solution for discordant survival outcomes in clinical trials. Crit Care Med 2017; 45:607–614 [DOI] [PubMed] [Google Scholar]
- 50.Gaieski DF, Mikkelsen ME, Band RA, et al. : Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–1053 [DOI] [PubMed] [Google Scholar]
- 51.Puskarich MA, Trzeciak S, Shapiro NI, et al. ; Emergency Medicine Shock Research Network (EMSHOCKNET): Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston ANB, Park J, Doi SA, et al. : Effect of immediate administration of antibiotics in patients with sepsis in tertiary care: A systematic review and meta-analysis. Clin Ther 2017; 39:190–202.e6 [DOI] [PubMed] [Google Scholar]
- 53.Sterling SA, Miller WR, Pryor J, et al. : The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: A systematic review and meta-analysis. Crit Care Med 2015; 43:1907–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barochia AV, Cui X, Vitberg D, et al. : Bundled care for septic shock: An analysis of clinical trials. Crit Care Med 2010; 38:668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy MM, Dellinger RP, Townsend SR, et al. ; Surviving Sepsis Campaign: The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374 [DOI] [PubMed] [Google Scholar]
- 56.Rhee C, Filbin MR, Massaro AF, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program: Compliance with the national SEP-1 quality measure and association with sepsis outcomes: A multicenter retrospective cohort study. Crit Care Med 2018; 46:1585–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macdonald SPJ, Taylor DM, Keijzers G, et al. : Restricted fluid resuscitation in sepsis-associated hypotension (REFRESH): Study protocol for a pilot randomised controlled trial. Trials 2017; 18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical Trials: Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS). Available at: https://clinicaltrials.gov/ct2/show/NCT03434028. Accessed December 4, 2018 [DOI] [PubMed]
- 59.Public Citizen: Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis Trial. Available at: https://www.citizen.org/sites/default/files/2446.pdf. Accessed December 4, 2018 [DOI] [PubMed]
- 60.Rhee C, Dantes R, Epstein L, et al. : CDC prevention epicenter program: Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kadri SS, Rhee C, Strich JR, et al. : Estimating ten-year trends in septic shock incidence and mortality in united states academic medical centers using clinical data. Chest 2017; 151:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filbin MR, Lynch J, Gillingham TD, et al. : Presenting symptoms independently predict mortality in septic shock: Importance of a previously unmeasured confounder. Crit Care Med 2018; 46:1592–1599 [DOI] [PubMed] [Google Scholar]
- 63.Rowan KM, Angus DC, Bailey M, et al. ; PRISM Investigators: Early, goal-directed therapy for septic shock - A patient-level meta-analysis. N Engl J Med 2017; 376:2223–2234 [DOI] [PubMed] [Google Scholar]
- 64.Levy MM, Gesten FC, Phillips GS, et al. : Mortality changes associated with mandated public reporting for sepsis. The results of the New York state initiative. Am J Respir Crit Care Med 2018; 198:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


