Abstract
Context
Hemoglobin-based blood substitutes (HBBSs) are infusible oxygen-carrying liquids that have long shelf lives, have no need for refrigeration or cross-matching, and are ideal for treating hemorrhagic shock in remote settings. Some trials of HBBSs during the last decade have reported increased risks without clinical benefit.
Objective
To assess the safety of HBBSs in surgical, stroke, and trauma patients.
Data Sources
PubMed, EMBASE, and Cochrane Library searches for articles using hemoglobin and blood substitutes from 1980 through March 25, 2008; reviews of Food and Drug Administration (FDA) advisory committee meeting materials; and Internet searches for company press releases.
Study Selection
Randomized controlled trials including patients aged 19 years and older receiving HBBSs therapeutically. The database searches yielded 70 trials of which 13 met these criteria; in addition, data from 2 other trials were reported in 2 press releases, and additional data were included in 1 relevant FDA review.
Data Extraction
Data on death and myocardial infarction (MI) as outcome variables.
Results
Sixteen trials involving 5 different products and 3711 patients in varied patient populations were identified. A test for heterogeneity of the results of these trials was not significant for either mortality or MI (for both, I2=0%, P≥.60), and data were combined using a fixed-effects model. Overall, there was a statistically significant increase in the risk of death (164 deaths in the HBBS-treated groups and 123 deaths in the control groups; relative risk [RR], 1.30; 95% confidence interval [CI], 1.05–1.61) and risk of MI (59 MIs in the HBBS-treated groups and 16 MIs in the control groups; RR, 2.71; 95% CI, 1.67–4.40) with these HBBSs. Subgroup analysis of these trials indicated the increased risk was not restricted to a particular HBBS or clinical indication.
Conclusion
Based on the available data, use of HBBSs is associated with a significantly increased risk of death and MI.
THE DEVELOPMENT OF A BLOOD substitute—an infusible liquid that eliminates the need for refrigeration and cross-matching, has a long shelf life, and reduces the risk of iatrogenic infection—would provide a potentially lifesaving option for surgical patients and trauma patients with hemorrhagic shock, especially in rural areas and military settings. To date, a large proportion of blood substitutes in development have been hemoglobin-based products. Yet randomized controlled trials completed as early as 19961 have raised questions about the safety of these products and have failed to demonstrate clinical benefit. Nonetheless, at least 1 of these products is approved for use outside the United States and new clinical trials are being conducted or planned worldwide.2–8
Although there are biochemical differences between the products tested to date,9–15 all share the same mechanism of action and apparent mechanism of toxicity.16 Hemoglobin molecules used to manufacture these products are not contained by a red cell membrane, and when released into the vasculature, these molecules rapidly scavenge nitric oxide. This can result in systemic vasoconstriction, decreased blood flow, increased release of proinflammatory mediators and potent vasoconstrictors, and a loss of platelet inactivation,17–20 creating conditions that may lead to vascular thrombosis of the heart or other organs. This mechanism has recently been shown in preclinical models to be responsible for injury during hemolytic states, in which hemoglobin is also released into the circulation.21
Unlike naturally occurring hemoglobin, manufactured cell-free hemoglobin-based blood substitutes (HBBSs) can be chemically altered to theoretically minimize such toxicities. It has been postulated that cross-linking, polymerization, or pegylation of hemoglobin will create larger, more stable HBBS molecules, preventing extravasation and thereby leading to a reduction in toxicities related to nitric oxide scavenging. At least 1 manufacturer has also chemically increased the affinity of its HBBS for oxygen (lower P50, the partial pressure of oxygen required for 50% hemoglobin saturation) to decrease arteriole oxygen transfer and thereby potentially eliminate untoward cardiovascular effects.16,22–24
The primary purpose of this study was to review the association between these HBBSs and the risk of myocardial infarction (MI) and death in trials in different clinical settings. We also examine the regulatory process that permitted repeated trials with these agents despite persistent safety concerns.
METHODS
We conducted searches, most recently on March 25, 2008, using PubMed, EMBASE, and Cochrane Library to find all human randomized controlled trials published in English involving HBBSs. The searches began in 1980 and used the search terms blood substitutes and hemoglobin. Trials were excluded if they did not involve an HBBS, if all of the patients were healthy volunteers or younger than 19 years, or if the results were included in subsequent reports. Eligible trials had to include either death or MI as an outcome variable.
The most complete data for one of the products (Hemopure; Biopure Corp, Cambridge, Massachusetts) were presented in a slide presentation by the US Food and Drug Administration (FDA) at an advisory committee meeting.25 Companies are required to submit trial results to the FDA as the studies are completed, regardless of whether or not the results of these studies are published. The published articles on Hemopure represented 23.2% of patients in the FDA analysis14,26–31 but were not separately identified by the FDA. Instead, the FDA described a “pooled” analysis to enhance sample size,25 but pooling methods and the number of individual studies comprising the analysis were not reported. To prevent data duplication while including in our analysis the maximum number of patients studied with Hemopure, we used the FDA compilation only and it was treated as a single trial. The sponsor did not respond to our e-mail request for the data from the unpublished trials.
We also searched the Internet for press releases from any companies known to be involved in developing HBBSs. We used as keywords the names of these companies and their respective products (TABLE 1). Company communications with quantitative data from randomized controlled trials meeting our inclusion criteria are presented. The data from 2 trials of PolyHeme (Northfield Laboratories Inc, Evanston, Illinois) were available only in company press releases.32,33 A request to the sponsor for more detailed unpublished data from these 2 trials was declined, and we were directed to these same press releases. Qualitative data for a discontinued HBBS, Optro (Baxter Healthcare Corp, Deerfield, Illinois),34 and an additional trial of Hemolink (Hemosol BioPharma Inc, Mississauga, Ontario, Canada)35 were also available only as press releases. Requests for quantitative data were declined. Lacking data, we could not include these latter 2 trials in our meta-analysis.
Table 1.
Products Included in Meta-analysis
| Product and Source for Characteristics | Company | Chemical Alteration | P50, mm Hg | Percent Tetramer |
|---|---|---|---|---|
| HemAssist13 | Baxter Healthcare Corporation, Deerfield, Illinois | Cross-linking | 32 | >99 |
| Hemopure12,14 | Biopure Corp, Cambridge, Massachusetts | Pyridoxylation | 32–38 | <5 |
| Hemolink9,10 | Hemosol BioPharma Inc, Mississauga, Ontario, Canada | Polymerization | 34 | 30–40 |
| PolyHeme11 | Northfield Laboratories Inc, Evanston, Illinois | Polymerization | 26–30 | ≤1 |
| Hemospan15 | Sangart Inc, San Diego, California | Pegylation | 10 | 100 |
Abbreviation: P50, the partial pressure of oxygen required for 50% hemoglobin saturation.
Two of us (C.N. and S.J.K.) independently reviewed the included studies using a standardized data collection form. A third author resolved any discrepancies. Mortality and MI were selected as outcomes because, based on an initial review, these data were commonly reported. We also abstracted other descriptive data from included trials,1,13,23,25,32,33,36–45 such as blinding, therapy used in controls, and enrollment dates. We requested enrollment dates from the authors but in several cases received no response.
The intention-to-treat analysis was used when provided. Patients (n=5) were reported missing in only 1 of these studies.39 We considered patients with missing data from both the treatment (n=1) and control groups (n=4) to be survivors but also analyzed them as nonsurvivors to see if this affected the overall results. In 2 trials, the patients were first randomized to 1 of 3 groups representing different doses of the product. Each dose group was then randomized independently to be treated at that dose or to its own control condition.37,42 These data were treated as 3 independent studies in each trial. Most trials reported neither an adjudication process to confirm MIs nor a process for attributing deaths or MIs to the product. For consistency, outcomes for death and for MI were therefore analyzed in their raw forms.
To avoid denominators of 0 in the calculation of standard error, a correction value of 0.5 was added to every cell of any trial in which there was a single empty cell in the 2 × 2 table. We assessed the homogeneity of the trials’ treatment effects for the association between HBBSs and mortality and MI using the Breslow-Day test46 and an associated I2 statistic.47 We then used the Cochran-Mantel-Haenszel test48 to estimate the pooled relative risks (RRs) of mortality and MI of these products with associated 95% confidence intervals (CIs), using a fixed-effects model in the R package metabin (http://www.r-project.org). For all analyses of the complete data set for each outcome, a fixed-effects model was required because of the null values for the estimates of between-study variance. Relative risk was chosen as the summary measure of effect size to produce the smallest evidence of heterogeneity, as well as to produce an easily interpretable result.
Conventional forest plots were prepared, with the sizes of point estimates proportional to the inverse variance of each estimate.Cumulative meta-analyses of mortality and MI, using a fixed-effects model, were performed for each year that studies were known to have been completedor, if completion dates were unavailable, the year the studies were published or otherwise made public. Subgroup analyses of mortality and MI end points were performed to construct estimates of treatment effect for each clinical indication and product, tetramer content (dichotomized at the median for the various products), P50 (also dichotomized at the median), and publication status (published/unpublished).Differences between selected subgroups were tested using a decomposed Breslow-Day test.49
All tests of significance were performed at the α=.05 level. Tests of heterogeneity and the decomposed Breslow-Day test comparing the treatment effects between subgroups were 1-sided tests.49 Tests of significance of a treatment effect were 2-sided.
RESULTS
Sixteen trials of 5 distinct HBBSs met the inclusion criteria1,13,23,25,32,33,36–45 (FIGURE 1, TABLE 2). The P50 values varied from 10 mm Hg of oxygen (highest affinity) to 38 mm Hg (lowest affinity) and the percentage of hemoglobin tetramer varied from less than 1% to 100% (Table 1). Four trials were described as double-blind, 7 as single-blind, 4 as open-label or unblinded, and 1 was uninformative. Five trials investigated HBBSs in trauma patients, 10 in various surgical patients, and 1 in stroke patients. Twelve of these 16 trials reported deaths and 10 reported MIs. The median time from the completion of each of the 8 trials with known enrollment dates until the data were published or made public in press releases was 4 years, with a range of 1 to 6 years.
Figure 1.
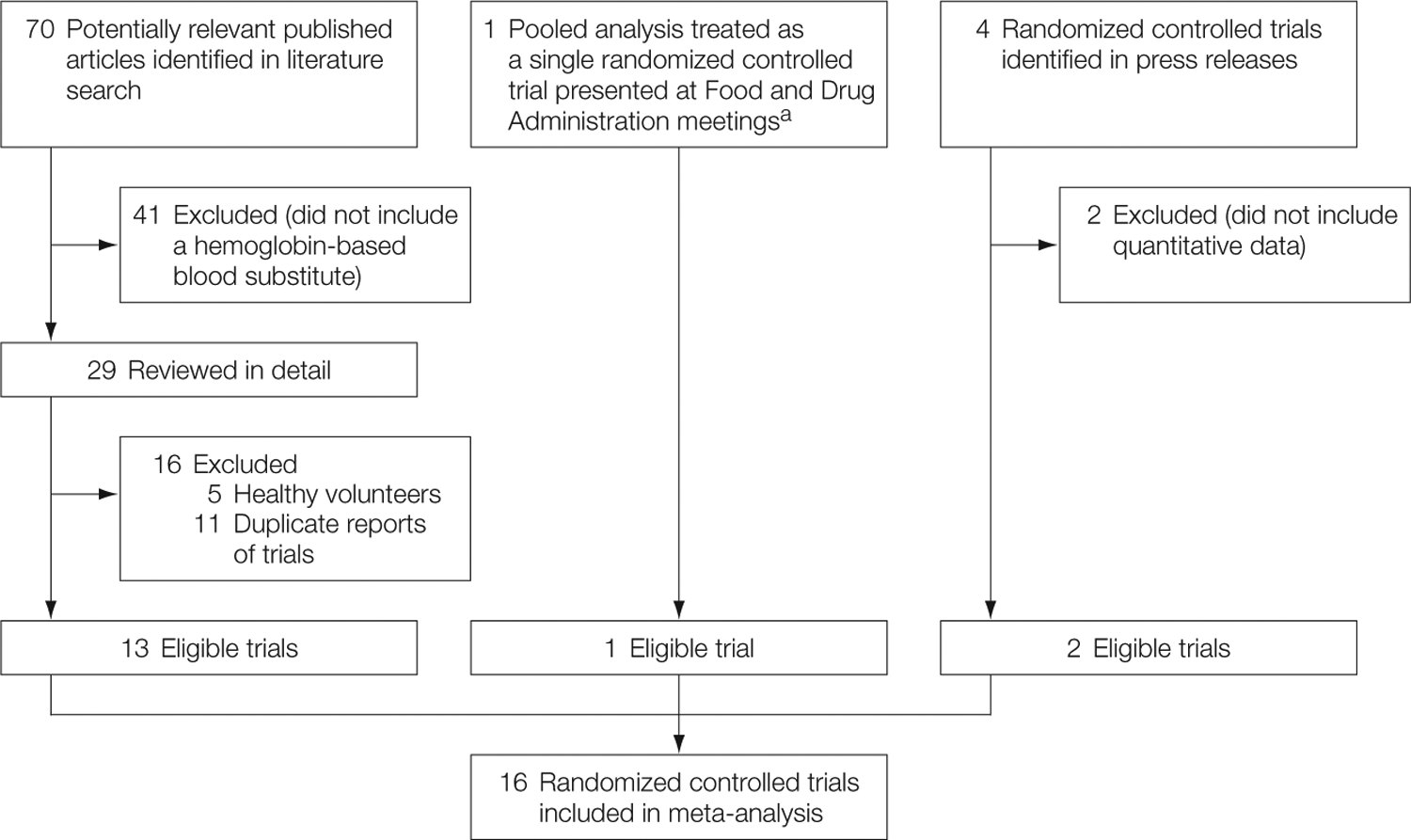
Study Selection
aPublished articles on Hemopure were not separately identified by the Food and Drug Administration (FDA). Instead, the FDA described a pooled analysis to enhance sample size but did not report the number of individual studies. We treated the FDA compilation as a single trial.
Table 2.
Characteristics of Studies Included in Meta-analysis
| Source | Product | Enrollment Dates | Patients | Blinding, Study Type | Dose of Product | Control | Patients, No. | 1 End Point | |
|---|---|---|---|---|---|---|---|---|---|
| 1 Treatment Control | |||||||||
| Gould et al,38 1998 | PolyHemea | NR | Trauma, emergency surgery | Unblinded, phase 2, multicenter | 1–6 Units | Allogeneic blood | 21 | 23 | Avoidance of allogeneic transfusion |
| Garrioch et al,37 1999 | HemAssistb | NR | Vascular surgery | Single-blind, phase 2, single-center | 50 mg/kg | LR solution | 5 | 5 | Vasoactive properties |
| 100 mg/kg | LR solution | 6 | 6 | ||||||
| 200 mg/kg | LR solution | 5 | 5 | ||||||
| Przybelski et al,42 1999 | HemAssist | NR | Hemorrhagic, hypovolemic shock | Double-blind during randomization only, phase 2, multicenter | 50 mL | Normal saline | 27 | 26 | Renal failure, myocardial ischemia or - injury, liver dysfunction |
| 100 mL | Normal saline | 22 | 20 | ||||||
| 200 mL | Normal saline | 22 | 20 | ||||||
| Saxena et al,1 1999 | HemAssist | August 1994-November 1996 | Acute ischemic stroke | Single-blind, phase 2, multicenter | 25, 50, or 100 mg/kg 10% every 6 hours for 72 hours (12 doses) | Normal saline | 40 | 45 | NIHSS, Barthel, and Rankin scales at 3 months |
| Sloan et al,45 1999 | HemAssist | February 1997-January 1998 | Severe traumatic hemorrhagic shock | Single-blind, phase 3, multicenter | 500–1000 mL | Normal saline | 58 | 53 | 28-Day mortality |
| Lamy et al,13 2000 | HemAssist | NR | Cardiac surgery | Single-blind, phase 2/3 multicenter | Up to three 250-mL infusions | PRBCs | 104 | 105 | Avoidance of transfusion |
| Schubert et al,43 2002 | HemAssist | NR | Orthopedic surgery | Unblinded, phase 2, single-center | Up to 750 mL | PRBCs | 12 | 12 | Avoidance of transfusion at 28 days |
| Hill et al,40 2002 | Hemolinkc | 1999–2000 | CABG | Single-blind, phase 2, multicenter | 3 Sequential dose blocks of 250 mL, 500 mL, or 750 mL | 6% Hetastarch | 28 | 32 | Avoidance of transfusion at 28 days |
| Schubert et al,44 2003 | HemAssist | 1996–1998 | Elective surgery | Double-blind, phase 2/3, multicenter | Up to three 10% 250-mL infusions | PRBCs | 92 | 89 | Avoidance of allogeneic transfusion |
| Kerner et al,41 2003 | HemAssist | July1997–June 1998 | Severe hemorrhagic shock | Single-blind, phase 3, multicenter | Maximum volume of 1000 mL | Standard hemorrhagic shock resuscitation | 58 | 63 | Reduction in organ failure scores and deaths at 5 days |
| Greenburg and Kim,39 2004 | Hemolink | NR | CABG | Double-blind, phase 3, multicenter | 750 mL | 10% Pentastarch | 148 | 151 | Need for allogeneic PRBC transfusion |
| Bloomfield et al,36 2004 | HemAssist | NR | Vascular surgery | Single-blind, phase 2, single-center | 50 mg/kg | Hetastarch | 5 | 5 | Safety and pharmacodynamics |
| FDA presentation,25 2006 | Hemopured | 1994–2000 | Elective surgery | NR | NR | LR solution, hetastarch, PRBCs | 797 | 661 | NR |
| Northfield Laboratories,32 2006 | PolyHeme | 1998–2000 | Vascular surgery | Unblinded, phase 3, multicenter | Up to 6 units | Standard solutions only | 81 | 71 | Avoidance of allogeneic infusion |
| Olofsson et al,23 2006 | Hemospane | August 2004–February 2005 | Orthopedic surgery | Double-blind, phase 2, multicenter | 250 mL+750 mL RA or 500 mL +500 mL RA | Ringer acetate | 46 | 28 | Serious adverse events |
| Northfield Laboratories,33 2007 | PolyHeme | NR | Trauma | Unblinded, phase 3, multicenter | NR | Standard fluid in ambulance, blood in hospital | 350 | 364 | Day 1 and day 30 mortality and durable serious adverse events |
Abbreviations: CABG, coronary artery bypass graft; FDA, Food and Drug Administration; LR, lactated Ringer; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; PRBCs, packed red blood cells.
Manufactured by Northfield Laboratories Inc, Evanston, Illinois.
Manufactured by Baxter Healthcare Corp, Deerfield, Illinois.
Manufactured by Hemosol BioPharma Inc, Mississauga, Ontario, Canada.
Manufactured by Biopure Corp, Cambridge, Massachusetts. Published articles on Hemopure were not separately identified by the FDA. Instead, the FDA described a pooled analysis to enhance sample size but did not report the number of individual studies. We treated the FDA compilation as a single trial.
Manufactured by Sangart Inc, San Diego, California.
Mortality and MI
There were a total of 164 deaths among the HBBS-treated patients and 123 deaths among the patients in the control groups. There was no evidence of heterogeneity between studies for the mortality end point (I2=0%, P=.60). Overall, this class of HBBS products was associated with a significantly increased risk of death (RR, 1.30; 95% CI, 1.05–1.61) (FIGURE 2).
Figure 2.
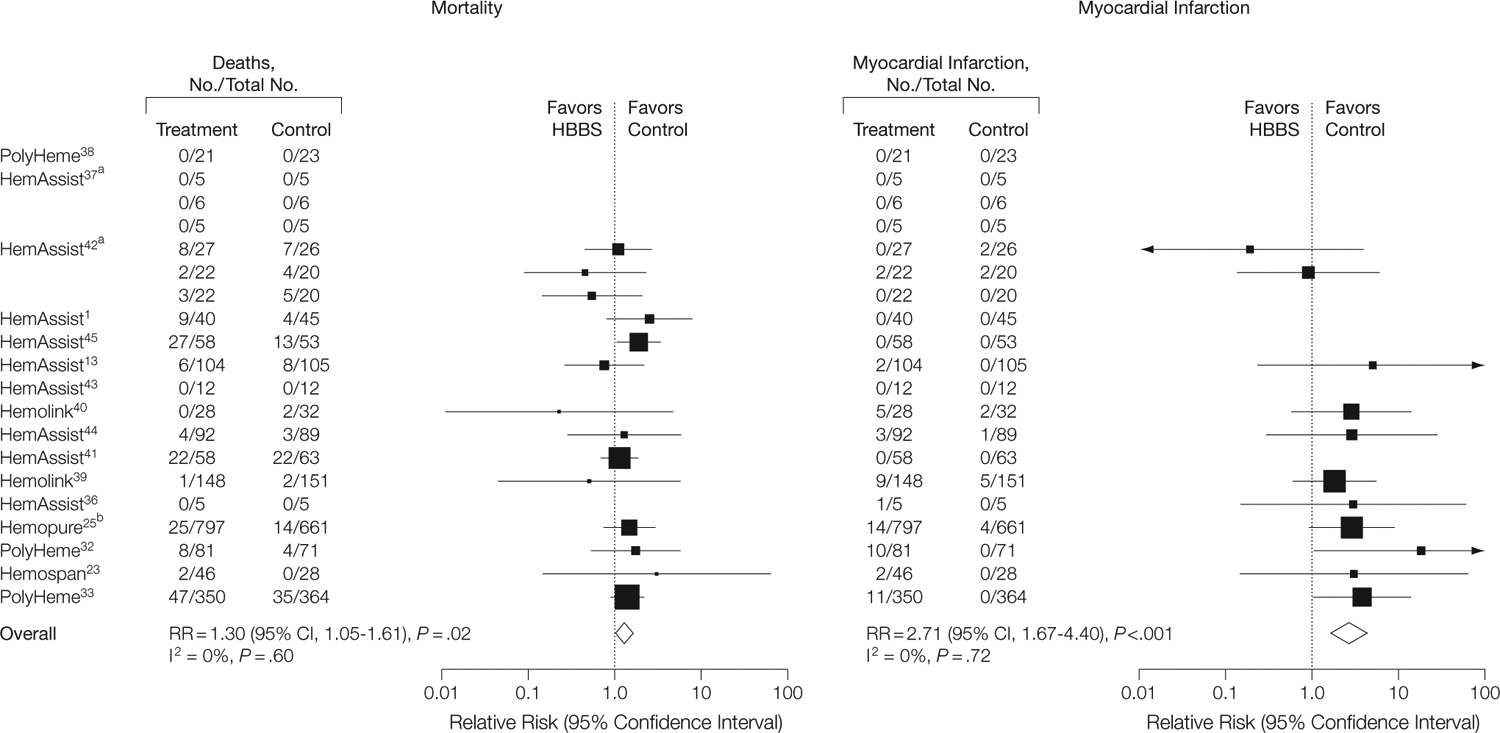
Mortality and Myocardial Infarction
The size of the data markers is proportional to the inverse variance of each point estimate. aTrials involved the randomization of patients to 1 of 3 doses, followed by independent randomizations to treatment and control groups. bPublished articles on Hemopure were not separately identified by the Food and Drug Administration (FDA). Instead, the FDA described a pooled analysis to enhance sample size but did not report the number of individual studies. We treated the FDA compilation as a single trial. RR indicates relative risk; CI, confidence interval.
There was a total of 59 MIs among the HBBS-treated patients and 16 MIs among the patients in the control groups. There was no evidence of heterogeneity across the individual studies for the MI end point (I2=0%, P=.72). For these studies combined, there was a significantly increased risk of MI among patients receiving HBBSs (RR, 2.71; 95% CI, 1.67–4.40) (Figure 2).
The only available data from which an estimate of the number needed to harm could be determined were from summary counts of total event rates across all studies (without adjustment for length of follow-up). A calculation from these summations yields an estimate for number needed to harm of 62 patients treated for each treatment-related death and 50 patients treated for each treatment-related MI.
Subgroup Analyses
FIGURE 3 shows the mortality and MI data according to subgroups. Except for cardiac surgery, the RRs for mortality in the patient subgroups were similarly elevated but were not statistically significant for the trauma and stroke subgroups. For cardiac surgery, the RR was less than 1, but this was not statistically significant compared with elective orthopedic or vascular surgery studies (P=.11, decomposed Breslow-Day test49). For MI, the RR was elevated in all patient groups for which there were relevant data but was not statistically significant for the cardiac surgery or trauma subgroups.
Figure 3.
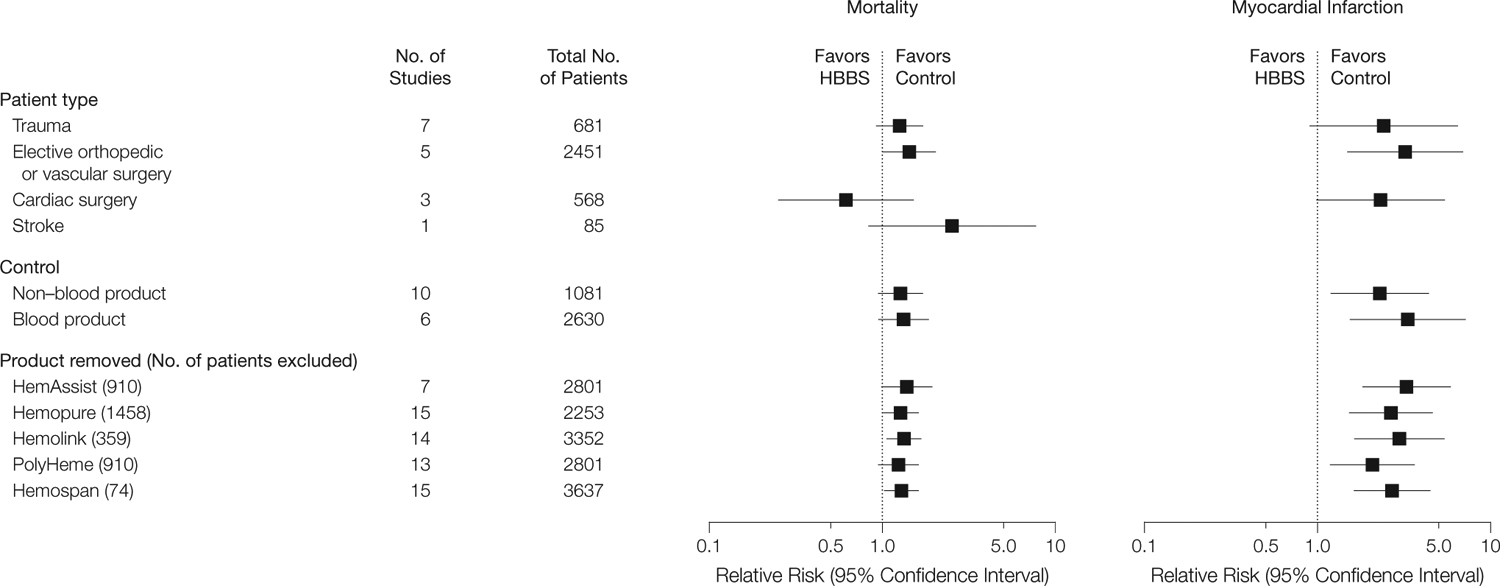
Subgroup Analysis
In the analysis comparing HBBS studies with non–blood product controls with those with blood product controls, the RRs for mortality and MI were elevated, but for mortality neither reached statistical significance. In analyses removing each HBBS product in turn, RRs for mortality and MI remained increased, except when PolyHeme was removed; in that case, the RR for mortality was increased but not statistically significantly.
Although the unpublished HBBS studies had a higher RR of death (RR, 1.45; 95% CI, 1.04–2.02; 3 trials) than the published studies (RR, 1.19; 95% CI, 0.90–1.56; 13 trials), a test of the equality of the 2 RRs did not reach statistical significance (P=.53). The findings for MI were similar. Dividing these 5 HBBS products into low vs high tetramer content and low vs high P50 values resulted in similar estimates of the increases in risk of MI and death as in the full data set. For mortality, the RR for low tetramer content was 1.37 (95% CI, 0.99–1.89) and for high tetramer content was 1.24 (95% CI, 0.94–1.64); for low P50, the RR was 1.46 (95% CI, 0.99–2.15) and for high P50 was 1.23 (95% CI, 0.95–1.58). For MI, the RR for low tetramer content was 3.28 (95% CI, 1.84–5.87) and for high tetramer content was 1.55 (95% CI, 0.62–3.88); for low P50, the RR was 5.61 (95% CI, 1.95–16.16) and for high P50 was 2.04 (95% CI, 1.17–3.57).
Cumulative Mortality and MI
FIGURE 4 displays the cumulative meta-analyses of mortality and MI by the year the studies completed enrollment or, if such data were not available, the year the study results were published or became public through press releases or an FDA presentation. By 1998, it was apparent that there was a significant increase in the RR of death associated with HemAssist (Baxter Healthcare Corp), one of the few products with available data at that point.
Figure 4.
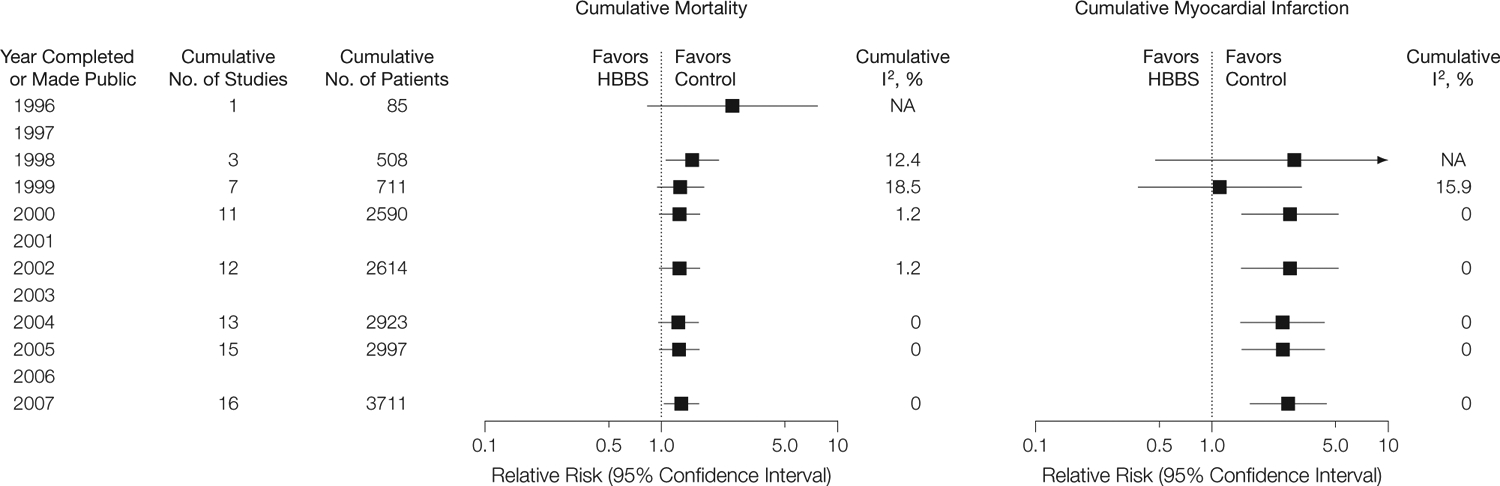
Cumulative Mortality and Myocardial Infarction
NA indicates not applicable.
By the end of 2000, at least 81,13,37,41–45 of the 9 HemAssist studies, all of the Hemopure studies,25 2 PolyHeme studies,32,38 and the first Hemolink40 study had been completed. At that time, the RRs for mortality and for MI were both increased (RR, 1.27; 95% CI, 0.99–1.63, and RR, 2.77; 95% CI, 1.49–5.15, respectively). Additional trial data that have become available since 2000 have had little effect on the cumulative RRs for either mortality or MI.
COMMENT
Based on data from randomized controlled trials of 5 different HBBSs conducted over the last decade in elective surgery, trauma, and stroke patients, there was an overall 30% statistically significant increase in mortality risk. There was also a statistically significant 2.7-fold increase in MI risk associated with these products. Subgroup analyses indicated that the increased risks generally were consistent, regardless of the patient population or type of product, although these analyses have reduced statistical power. The pattern of increased risk demonstrated by a variety of HBBSs across an array of clinical settings argues for a policy whereby any new or existing HBBSs should be subjected to preclinical studies in animal models that replicate the known toxicities of HBBSs demonstrated in humans before further clinical trials of this class of product are allowed to proceed.
Sponsors are required by law to report their results to the FDA in a timely fashion after studies are completed, even if they do not publish their findings. However, the data reported by sponsors to the FDA are not made public by the FDA unless the product is approved or an advisory committee is convened to discuss the product. The cumulative mortality analysis shown in Figure 4 indicates that prompt meta-analyses of the HBBS trials by the FDA most likely would have demonstrated significant risks by 2000. Had the agency placed a moratorium on trials at that point, product-related deaths and MIs in subsequent trials most likely would have been prevented.
However, such data were not available to scientists, the public, institutional review boards, or competing HBBS manufacturers. For instance, at least 7 of the 9 HemAssist trials were completed by 1998; however, because of times to publication of 3 to 5 years, these trials were published between 1999 and 2003.1,13,41–45 Data on a large proportion of patients (approximately 75%) in Hemopure trials, all of which were completed by 2000, have not been published, and these data only became publicly known after Public Citizen (Washington, DC) sued the FDA to make open to the public a December 2006 FDA advisory committee meeting50 at which the data were presented. The data from the first large trial of PolyHeme were only made public when the company, responding to a critical article in the lay press,51 issued a press release on December 19, 2006, 6 years after the trial was completed.32,33
The data from at least 2 additional trials still have not been published in the medical literature; only qualitative descriptions are available from press releases.Both studies were terminated early for safety reasons. In a study involving vascular surgery patients conducted in the late 1990s, Optro was associated with gastrointestinal adverse effects, hypertension, and increases in total peripheral resistance.34 In 2003, Hemolink was reported to produce an increase in MIs,35 as had been described in 2 previously published trials in cardiac surgery patients for this product.39,40 It is possible that there are still more clinical trials that have not been made public.
The most recent PolyHeme trial requires special mention for 2 reasons. First, the FDA gave approval for this trial in trauma patients even though the FDA presumably had unpublished data showing a significant increase in MIs in the prior PolyHeme trial in vascular surgery patients32; the FDA had the results from trials involving other HBBS products also showing harm; and the FDA had placed a clinical hold on a Hemopure trauma trial because of serious adverse events in previous, mostly unpublished, trials of this HBBS.25 The results of the PolyHeme trauma trial were made public in a company press release in 2007 and showed nonsignificant increased mortality risk and a significant increase in MI risk among patients who received PolyHeme.33 Second, the failure to publish the results of the earlier PolyHeme vascular surgery trial and previous trials of some other HBBSs meant that thorough review of previous trial results by institutional review boards reviewing the PolyHeme trauma trial at the many participating sites was not possible.
Today, 5 trials of HBBSs are ongoing and at least 1 is being planned. A Hemopure trial is presently enrolling trauma patients in South Africa,2 where the product is approved for human use for treatment of acute anemia in adult surgical patients. Additional ongoing Hemopure studies involve coronary artery bypass graft surgery patients in the United Kingdom, Greece, and South Africa4 and elective percutaneous coronary revascularization patients in the Netherlands.3 There are also 2 ongoing trials for Hemospan (Sangart Inc, San Diego, California), both for treatment of hypotension in patients undergoing hip arthroplasty, in the United Kingdom, Belgium, the Netherlands, Poland, Sweden, and the Czech Republic.5 The US Navy and the manufacturer of Hemopure had submitted to the FDA another proposed trial in trauma patients. In December 2006, the FDA’s Blood Products Advisory Committee voted 11 to 8 that the benefits of this proposed phase 3 trial did not out-weigh the risks for individual patients.50 The Navy Medical Research Center and the manufacturers of Hemopure have since submitted a new protocol for a phase 2 out-of-hospital trauma study, but the FDA has placed this trial on clinical hold.7
The risks of these HBBS products should be weighed against any benefits the products may have demonstrated. The primary efficacy outcomes studied across the HBBS trials varied. Seven trials examined the ability of HBBSs to limit blood transfusions. Two of these trials reported that HBBSs acutely prevented the need for blood transfusions, but this was completely offset by increased blood requirements later.13,43 Two trials were stopped early for safety concerns,32,44 and the other 3 trials reported a decrease in transfusion requirements, 1 statistically significant39 and the other 2 not significant.38,40 Other trials investigated whether HBBSs could improve neurological outcome from stroke1 or prevent organ failure and death in different clinical settings.33,41,45 Some of these trials found a significant increase in morbidity, mortality, or both; however, no HBBS study reported a statistically significant, meaningful, long-term beneficial outcome.
We acknowledge that our meta-analysis has several limitations. Details on some study protocols were unavailable due in part to the failure of companies to publish data and our resultant reliance on press releases and advisory committee presentations for some data.25,32,33 Data for Hemopure were based on a pooled FDA analysis using unclear methods encompassing an unknown number of trials; we treated the pooled analysis as if it were a single trial.25 The number of patients in the pooled Hemopure analysis represented 39% of the total number of patients in the entire meta-analysis for all products. The effect on the meta-analysis of treating all the Hemopure studies as a single trial is uncertain, but similarity of the RR estimates for the pooled Hemopure analysis and the RR for all other studies combined suggests that this approach most likely had a limited effect on the overall risk estimates.
Although the control group varied from trial to trial (eg, from saline to red blood cells to various plasma expanders), in each case the control intervention represented usual care for that patient population. The levels of blinding in the trials also varied, ranging from various forms of single-blinding to comprehensive double-blinding,23 evidence that such blinding is feasible. One study23 adjudicated whether serious adverse events were attributable to the treatment. For consistency, we analyzed nonadjudicated data throughout. The overall results of our meta-analysis are un-changed if the nonattributable serious adverse events from this 1 study are considered to be nonevents. Similarly, in the singlestudy39 with missing outcome data, the overall results of the meta-analysis are unaffected by whether the patients (n=5) with missing data are treated assurvivors or nonsurvivors.
The results of all trials of experimental agents conducted in human beings—from phase 1 to phase 4—should be fully and expeditiously disclosed to the scientific and medical communities. The case study detailed here underscores both the scientific inefficiency and the real risks to patients of the current failure to report data promptly. When “secret science” is allowed, scientists are unable to build on the successes or failures of other researchers testing similar products, and patients can be repeatedly exposed to risks unnecessarily.
One straightforward solution to these problems would be for Congress to reverse the FDA’s policy of treating as confidential all corporate materials submitted during the product development process, including the investigational new drug application. The agency will not even confirm the existence of an investigational new drug, new drug, or biologics license application until (and only if) the product is approved, unless it is one of the minority of products that receive an advisory committee hearing52 or the application is formally abandoned—a rare occurrence.
As far back as 3 decades ago, the Review Panel on New Drug Regulation questioned the longstanding practice of keeping investigational new drug and new drug applications confidential and concluded that “the need to make scientific data concerning the safety and effectiveness of drugs available to the public is urgent [and] can be achieved without eliminating the existing incentive to invest in drug research and development.” The panel went on to recommend that “Congress immediately amend the Federal Food, Drug, and Cosmetic Act to provide that safety and effectiveness data are not trade secrets for purposes of federal prohibitions against release on confidential and privileged information.”53
A second, complementary approach would involve congressional modification of the Freedom of Information Act. Exemption 4 to the act permits the with-holding by any federal agency of material deemed trade secret or confidential commercial information; it is the exemption most frequently cited by the FDA and is the one most relevant in this setting.52 Courts have held that the exemption does not provide for a balancing of the commercial interest against the public interest.54 That is, if the material sought is confidential commercial information, the exemption is triggered, regardless of the strength of the public interest in the disclosure of that information. A relatively minor alteration in the statutory language of exemption 4 to allow for consideration of the public interest would markedly increase data disclosure.
Finally, much attention of late has been directed toward clinical trial registries.55 This approach is independent of changes at the FDA level. Trials should be registered at their inception so that the failure to complete or publish a trial can be detected. The recently passed FDA reauthorization bill56 provides for a database that includes ongoing trials as well as the eventual posting of study results and adverse effects. However, for now there is no requirement that results for unapproved products be posted. If the public interest in expanded dissemination of scientific information is to be fulfilled and patients are to be protected, all 3 of these remedies will need to be enacted.
In conclusion, in this analysis of available data from clinical trials, the use of HBBSs was associated with a significantly increased risk of death and MI.
Acknowledgment:
Dr Banks, senior mathematical statistician, Critical Care Medicine Department, National Institutes of Health, died unexpectedly during the final stages of writing the manuscript. Dr Banks dedicated his life’s work to advancing science; his colleagues mourn his loss.
Funding/Support:
The Warren G. Magnuson Clinical Center of the National Institutes of Health, Bethesda, Maryland, and Public Citizen, Washington, DC, provided support for this study.
Role of the Sponsor:
Public Citizen and the National Institutes of Health had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures:
In 2004, Dr Natanson reports being paid $10 000 by Hemosol Inc on a 1-time basis to review a cardiac surgery trial of its HBBS product, Hemolink. There are no other financial conflicts reported for Dr Natanson with manufacturers of these types of products or other blood products. At present, Dr Natanson reports being an unpaid special government consultant to the FDA on the HBBS product Hemopure (Biopure Corp, Cambridge, Massachusetts). None of the other authors reported financial disclosures.
Disclaimer: The opinions expressed are those of the authors only and do not represent the official position or policies of the National Institutes of Health.
Contributor Information
Charles Natanson, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland.
Steven J. Kern, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland.
Peter Lurie, Health Research Group, Public Citizen, Washington, DC.
Steven M. Banks, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland.
Sidney M. Wolfe, Health Research Group, Public Citizen, Washington, DC.
REFERENCES
- 1.Saxena R, Wijnhoud AD, Carton H, et al. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke. 1999; 30(5):993–996. [DOI] [PubMed] [Google Scholar]
- 2.A single-center study to evaluate the safety and tolerability of hemoglobin-based oxygen carrier-201 (HBOC 201) in trauma subjects (phase II–safety and tolerability). http://clinicaltrials.gov/ct2/show/NCT00301483. Accessed December 10, 2007.
- 3.Phase II, open-label study in the catheterization laboratory setting to challenge the concept that HBOC-201 administration might improve myocardial “oxygenation” and myocardial function at the moment of (brief) coronary occlusion. http://clinicaltrials.gov/ct2/show/NCT00479895. Accessed December 10, 2007.
- 4.Enhancement of tissue preservation during cardio-pulmonary bypass with HBOC-201 (registry study). http://clinicaltrials.gov/ct2/show/NCT00301535. Accessed December 10, 2007.
- 5.A randomized, double-blind, phase III study of the efficacy and safety of an oxygen-carrying plasma expander, Hemospan, compared with Voluven to treat hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia. http://clinicaltrials.gov/ct2/show/NCT00420277. Accessed January 8, 2007.
- 6.Biopure announces 2007 first quarter financial results [news release]. Cambridge, MA: Biopure Corporation; 2007. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/02-15-2007/0004528855&EDATE=. Accessed April 9, 2008. [Google Scholar]
- 7.Biopure announces 2007 third quarter financial results [news release]. Cambridge, MA: Biopure Corporation; 2007. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=109&STORY=/www/story/08-23-2007/0004650729&EDATE=. Accessed April 9, 2008. [Google Scholar]
- 8.Dawson James Securities reiterates speculative buy recommendation on Biopure Corp (Nasdaq: BPUR) with $3.00 price target [news release]. Boca Raton, FL: Dawson James Securities; 2007. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/05-22-2007/0004593850&EDATE=. Accessed April 9, 2008. [Google Scholar]
- 9.Caron A, Menu P, Faivre-Fiorina B, Labrude P, Alayash AI, Vigneron C. Cardiovascular and hemorheo-logical effects of three modified human hemoglobin solutions in hemodiluted rabbits. J Appl Physiol. 1999; 86(2):541–548. [DOI] [PubMed] [Google Scholar]
- 10.Workshop on criteria for safety and efficacy evaluation of oxygen therapeutics as red cell substitutes. Food and Drug Administration Center for Biologics Evaluation and Research. September 27, 1999; Bethesda, MD. http://www.fda.gov/cber/minutes/oxygen092799.pdf. Accessed April 9, 2008. [Google Scholar]
- 11.Gould SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–452. [DOI] [PubMed] [Google Scholar]
- 12.Jahr JS, Osgood S, Rothenberg SJ, et al. Lactate measurement interference by hemoglobin-based oxygen carriers (Oxyglobin, Hemopure, and Hemolink). Anesth Analg. 2005;100(2):431–436. [DOI] [PubMed] [Google Scholar]
- 13.Lamy ML, Daily EK, Brichant JF, et al. Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery: the DCLHb Cardiac Surgery Trial Collaborative Group. Anesthesiology. 2000;92 (3):646–656. [DOI] [PubMed] [Google Scholar]
- 14.Levy JH, Goodnough LT, Greilich PE, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124(1):35–42. [DOI] [PubMed] [Google Scholar]
- 15.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43(4):509–516. [DOI] [PubMed] [Google Scholar]
- 16.Rohlfs RJ, Bruner E, Chiu A, et al. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998; 273(20):12128–12134. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin G, Macdonald RL, Marton LS, Kowalczuk A, Solenski NJ, Weir BK. Hemoglobin increases endothelin-1 in endothelial cells by decreasing nitric oxide. Biochem Biophys Res Commun. 2001;280(3): 824–830. [DOI] [PubMed] [Google Scholar]
- 19.Phelan M, Perrine SP, Brauer M, Faller DV. Sickle erythrocytes, after sickling, regulate the expression of the endothelin-1 gene and protein in human endothelial cells in culture. J Clin Invest. 1995;96(2): 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extra-cellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. [DOI] [PubMed] [Google Scholar]
- 21.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextrava-sating hemoglobin polymer. J Appl Physiol. 2002; 93(4):1479–1486. [DOI] [PubMed] [Google Scholar]
- 23.Olofsson C, Ahl T, Johansson T, et al. A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol-modified hemoglobin (Hemospan) in patients undergoing major orthopedic surgery. Anesthesiology. 2006;105(6):1153–1163. [DOI] [PubMed] [Google Scholar]
- 24.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108(10):3603–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration presentation to Blood Products Advisory Committee: NMRC RESUS protocol using HBOC-21. December 14, 2006. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4270S_9.ppt. Accessed April 9, 2008.
- 26.Brauer P, Standl T, Wilhelm S, Burmeister MA, Schulte am Esch J. Transcranial Doppler sonography mean flow velocity during infusion of ultrapurified bovine hemoglobin. J Neurosurg Anesthesiol. 1998; 10(3):146–152. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez P, Hackney AC, Jones S, et al. A phase I/II study of polymerized bovine hemoglobin in adult patients with sickle cell disease not in crisis at the time of study. J Investig Med. 1997;45(5):258–264. [PubMed] [Google Scholar]
- 28.Kasper SM, Grune F, Walter M, Amr N, Erasmi H, Buzello W. The effects of increased doses of bovine hemoglobin on hemodynamics and oxygen transport in patients undergoing preoperative hemodilution for elective abdominal aortic surgery. Anesth Analg. 1998;87(2):284–291. [DOI] [PubMed] [Google Scholar]
- 29.Kasper SM, Walter M, Grune F, Bischoff A, Erasmi H, Buzello W. Effects of a hemoglobin-based oxygen carrier (HBOC-201) on hemodynamics and oxygen transport in patients undergoing preoperative hemodilution for elective abdominal aortic surgery. Anesth Analg. 1996;83(5):921–927. [PubMed] [Google Scholar]
- 30.LaMuraglia GM, O’Hara PJ, Baker WH, et al. The reduction of the allogenic transfusion requirement in aortic surgery with a hemoglobin-based solution. J Vasc Surg. 2000;31(2):299–308. [DOI] [PubMed] [Google Scholar]
- 31.Sprung J, Kindscher JD, Wahr JA, et al. The use of bovine hemoglobin glutamer-250 (Hemopure) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg. 2002;94(4):799–808. [DOI] [PubMed] [Google Scholar]
- 32.Northfield Laboratories releases summary observations from its elective surgery trial [news release]. Evanston, IL: Northfield Laboratories Inc; 2006. http://phx.corporate-ir.net/phoenix.zhtml?c=91374&p=irol-newsArticle_print&ID=833808&highlight=. Accessed April 9, 2008. [Google Scholar]
- 33.Northfield Laboratories reports results of pivotal phase III trauma study [news release]. Evanston, IL: Northfield Laboratories Inc; 2007. http://phx.corporate-ir.net/phoenix.zhtml?c=91374&p=irol-newsArticle&ID=1005951&highlight=. Accessed April 9, 2008. [Google Scholar]
- 34.Lowe KC. Blood substitutes: from chemistry to clinic. J Mater Chem. 2006;16(43):4189–4196. [Google Scholar]
- 35.Hemosol to review safety data prior to continuing enrolment in cardiac trial [news release]. Toronto, ON: Hemosol Inc; 2003. http://www.secinfo.com/dRx61.23h.htm. Accessed April 9, 2008. [Google Scholar]
- 36.Bloomfield EL, Rady MY, Esfandiari S. A prospective trial of diaspirin cross-linked hemoglobin solution in patients after elective repair of abdominal aortic aneurysm. Mil Med. 2004;169(7):546–550. [DOI] [PubMed] [Google Scholar]
- 37.Garrioch MA, McClure JH, Wildsmith JA. Haemodynamic effects of diaspirin crosslinked haemoglobin (DCLHb) given before abdominal aortic aneurysm surgery. Br J Anaesth. 1999;83(5):702–707. [DOI] [PubMed] [Google Scholar]
- 38.Gould SA, Moore EE, Hoyt DB, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187(2):113–120. [DOI] [PubMed] [Google Scholar]
- 39.Greenburg AG, Kim HW. Use of an oxygen therapeutic as an adjunct to intraoperative autologous donation to reduce transfusion requirements in patients undergoing coronary artery bypass graft surgery. J Am Coll Surg. 2004;198(3):373–383. [DOI] [PubMed] [Google Scholar]
- 40.Hill SE, Gottschalk LI, Grichnik K. Safety and preliminary efficacy of hemoglobin raffimer for patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2002;16(6):695–702. [DOI] [PubMed] [Google Scholar]
- 41.Kerner T, Ahlers O, Veit S, Riou B, Saunders M, Pison U. DCL-Hb for trauma patients with severe hemorrhagic shock: the European “On-Scene” multicenter study. Intensive Care Med. 2003;29(3): 378–385. [DOI] [PubMed] [Google Scholar]
- 42.Przybelski RJ, Daily EK, Micheels J, et al. A safety assessment of diaspirin cross-linked hemoglobin (DCLHb) in the treatment of hemorrhagic, hypovolemic shock. Prehosp Disaster Med. 1999;14(4):251–264. [PubMed] [Google Scholar]
- 43.Schubert A, O’Hara JF Jr, Przybelski RJ, et al. Effect of diaspirin crosslinked hemoglobin (DCLHb HemAssist) during high blood loss surgery on selected indices of organ function. Artif Cells Blood Substit Immobil Biotechnol. 2002;30(4):259–283. [DOI] [PubMed] [Google Scholar]
- 44.Schubert A, Przybelski RJ, Eidt JF, et al. Diaspirin-crosslinked hemoglobin reduces blood transfusion in noncardiac surgery: a multicenter, randomized, controlled, double-blinded trial. Anesth Analg. 2003; 97(2):323–332. [DOI] [PubMed] [Google Scholar]
- 45.Sloan EP, Koenigsberg M, Gens D, et al. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999; 282(19):1857–1864. [DOI] [PubMed] [Google Scholar]
- 46.Breslow NE, Day NE. Statistical methods in cancer research: volume I, the analysis of case-control studies. IARC Sci Publ. 1980;(32):5–338. [PubMed] [Google Scholar]
- 47.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 49.Cochran WG. Some methods for strengthening the common chi-square tests. Biometrics. 1954; 10(4):417–451. [Google Scholar]
- 50.Food and Drug Administration Blood Products Advisory Committee 88th Meeting: FDA presentations. December 13, 2006. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4270B1-index.htm. Accessed August 28, 2007.
- 51.Burton TM. Blood-substitute study is criticized by US agency. Wall Street Journal (Eastern Edition). March 10, 2006:A3. [PubMed] [Google Scholar]
- 52.Lurie P, Zieve A. Sometimes the silence can be like the thunder: access to pharmaceutical data at the FDA. Law Contemp Probl. 2006;69(3):85–97. [Google Scholar]
- 53.Dorsen N, Weiner N, Astin A. Review Panel on New Drug Regulation: Final Report. Washington, DC: Dept of Health, Education, and Welfare; 1977. [Google Scholar]
- 54.Public Citizen Health Research Group v Food and Drug Administration (Schering), 185 F3d 898 (DC Cir 1999).
- 55.Damle A, Lurie P, Wolfe SM. A Policy Study of Clinical Trial Registries and Results Databases. Washington, DC: Public Citizen’s Health Research Group; (http://www.citizen.org/publications/release.cfm?ID=7534); 2007. [Google Scholar]
- 56.Food and Drug Administration Amendments Act of 2007, Pub L No. 110–85, 110th Cong, 1st Sess (2007).


