Abstract
Objective
Evaluate the effects of methylprednisolone (MP) on markers of inflammation, coagulation and angiogenesis during early ARDS
Design
Retrospective analysis
Setting
Four intensive care units
Subjects
79 of 91 patients with available samples enrolled in randomized, blinded controlled trial
Interventions
Early methylprednisolone infusion (n=55) compared to placebo (n = 24)
Measurements
Interleukin-6, tumor necrosis factor α, vascular endothelial growth factor, protein C, procalcitonin and proadrenomedullin were measured in archived plasma. Changes from baseline to day 3 and day 7 were compared between groups and in subgroups based on the precipitating cause of ARDS.
Main Results
MP therapy was associated with greater improvement in Lung Injury Score (p=0.003), shorter duration of mechanical ventilation (p=0.005) and lower ICU mortality (p=0.05) than controls. On days 3 and 7, MP decreased IL-6 and increased protein C levels (all p<0.0001) compared to controls. Proadrenomedullin levels were lower by day 3 with MP treatment (p=0.004). MP decreased IL-6 by days 3 and 7 in patients with pulmonary causes of ARDS, but only at day 3 in those with extra-pulmonary causes of ARDS. Protein C levels were increased with MP on days 3 and 7 in patients with infectious and/or pulmonary causes of ARDS (all p<0.0001) but not in patients with non-infectious or extra-pulmonary cause of ARDS. Proadrenomedullin levels were decreased with MP on day 3 in patients with infectious or extra-pulmonary causes of ARDS (both p≤0.008) but not in non-infectious or pulmonary ARDS.
TNF, VEGF and procalcitonin were elevated but not differentially affected by MP therapy.
Conclusions
In early ARDS, administration of MP was associated with improvement in important biomarkers of inflammation and coagulation and clinical outcomes. Biomarker changes varied with the precipitating cause of ARDS, suggesting that the underlying mechanisms and response to anti-inflammatory therapy may vary with the cause of ARDS.
Keywords: ARDS, coagulation, corticosteroids, inflammation, protein C, proadrenomedullin
INTRODUCTION
The severity of lung and systemic inflammation is strongly associated with survival from the acute respiratory distress syndrome (ARDS). Nonsurvivors of ARDS have significantly higher levels of bronchoalveolar lavage (BAL) and blood levels of inflammatory cytokines (1–6), chemokines (7), and markers of fibrogenesis and alveolar-capillary membrane permeability at ARDS onset that persist for at least seven days (3, 8, 9). At the tissue level, the continued production of inflammatory mediators sustains recurrent tissue injury, intra- and extra-vascular coagulation, and proliferation of mesenchymal cells that result in maladaptive lung repair (10). Persistent endothelial and epithelial injury leads to a protracted increase in vascular permeability in the lung and systemic circulation. The former may result in intravascular coagulation that decreases the patency of the pulmonary vascular bed and leads to intra-alveolar fibrin deposition that promotes fibroproliferation (11).
Contemporary approaches to the management of ARDS include treatment of the primary insult and supportive measures that limit further lung injury by avoiding extreme mechanical forces during ventilatory support. Although the mechanisms underlying acute lung injury may be different with a direct insult to the lungs compared to indirect lung injury (12), the current treatment approach is uniform. The use of corticosteroids to attenuate persistent inflammatory injury remains controversial. In ARDS, the failure to down-regulate excessive inflammation is associated with worsening lung function (4). Two meta-analyses evaluating the efficacy of prolonged corticosteroid use in patients with acute lung injury (ALI) or ARDS describe improved PaO2/FiO2 (P/F ratio), decreased duration of mechanical ventilation and time in intensive care unit (ICU), decrease in multiple organ dysfunction scores and improvement in markers of systemic inflammation (13, 14). Overall effects on mortality in the meta-analyses were limited because of heterogeneity among the studies. (15).
Alterations in hemostasis and fibrinolysis are inextricably associated with the inflammatory injury that results in diffuse alveolar damage in ARDS. Enhanced coagulation and depressed fibrinolysis have been described in the alveolar lining fluid of patients with ARDS (16, 17). Furthermore, decreased blood protein C, an endogenous anti-coagulant that degrades factors Va and VIIa, is associated with an increased risk of death in ALI (6). Limited data are available describing the effect of corticosteroid treatment on hemostatic biomarkers in ARDS. We hypothesized that methylprednisolone treatment would attenuate inflammation and thus improve components of coagulopathy in patients with ARDS. We studied inflammatory and hemostatic biomarkers during the first seven days after the onset of ARDS in patients receiving standard therapy compared to a group of patients receiving low-dose continuous methylprednisolone infusion as adjunctive therapy for early ARDS. Further, we analyzed the effect of methylprednisolone therapy on these biomarkers with regard to the precipitating cause (pulmonary or extra-pulmonary as well as infectious or non-infectious) of ARDS.
METHODS
Patient Characteristics
Plasma samples were analyzed from patients with ARDS who were enrolled in a trial conducted by one of the authors (GUM), comparing standard supportive therapy to standard therapy and early continuous low-dose methylprednisolone infusion (1 mg/kg/day for 14 days and then tapered over two weeks). The study employed a 2:1 randomization schedule, enrolling 63 patients in the methylprednisolone arm and 28 in the standard therapy arm. Patients in both arms of the clinical study received mechanical ventilation for ARDS based on the standard practice at the time of enrollment. Ventilator settings were initially set to limit plateau pressure to < 35 cm H2O and when new data became available were changed to conform with the ARDSnet protocol of 6 mL/kg predicted body weight while limiting plateau pressure to < 30 cm H2O (1, 18). Therapy with methylprednisolone was associated with significant improvement in the primary endpoints: extubation or a one-point reduction in Lung Injury Score (LIS) by day seven of the study (1).
Since the clinical trial design allowed crossover to open-label higher dose methylprednisolone for non-improvers after day seven, samples were analyzed from time of study entry (day 0), day 3 and day 7 prior to any crossover. Of the 91 patients enrolled in the clinical study, 79 had plasma samples available for analysis during the first 7 days.
Patients were enrolled from medical and surgical ICUs of Baptist Memorial Medical Center and East Hospitals, the Regional Medical Center, St. Francis Hospital, University of Tennessee Bowld Medical Center and the Veterans Affairs Medical Center, all in Memphis, TN. All collected samples were heparinized plasma and all patients or a legally authorized representative consented to sample collection. Each protocol for sample collection was approved by the institutional review boards of the participating centers.
Analyte Measurements
Based on a pilot analysis, six biomarkers were chosen for analysis in the full 79 patient cohort. Tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and vascular endothelial growth factor (VEGF) concentrations were measured by using the Fluorokine Multiplex Human Cytokine Panel A (R&D Systems, Minneapolis, MN). Protein C levels were measured by immunoassay (Helena Laboratories, Beaumont, TX). All samples were plated onto 96 well plates and all reference standards, controls and patient samples were run in duplicate. ELISA plates were read with a SpectraMax Plus plate reader (Molecular Devices, Sunnyvale, CA) with Softmax Pro 5 software. Procalcitonin (proCT) and proadrenomedullin (proADM) were both measured using KRYPTOR autoimmunofluorescent assay system (Brahms, Henningsdorf, Germany). Minimum detectable concentrations as well as inter- and intra-assay variation for all assays are summarized in the appendix.
Statistical Analysis
To account for baseline clinical differences affecting biomarker concentration between the group of patients receiving methylprednisolone and the group receiving usual care for ARDS, we compared biomarker concentration between the two groups based on the difference in biomarker concentrations at days 3 and 7 compared to baseline (day 0).
Continuous patient characteristics and baseline variables were first assessed using probability plots. Summary statistics are reported as the mean and standard error (SE) or associated 95% confidence interval or median and range. T tests or Wilcoxon tests were used to compare the two groups (methylprednisolone vs. usual care); Chi-squared test was used to compare categorical variables between groups when appropriate(less than 25% of cells in the contingency table had expected counts less than 5); otherwise, Fisher's exact test was used. For the comparison of change-from-baseline values between treatment groups, linear mixed models were used to account for repeated measures within each patient. Missing values were accounted for in the linear mixed model under the missing at random assumption (19). Data were log-transformed first when not normally distributed, and standard diagnostics were used to check model assumptions. Subgroup analyses were done similarly. Two-tailed p-values less than 0.05 were considered statistically significant for the primary analysis of all 79 patients. For patient subgroup analyses, two-tailed p-values less than 0.01 were considered significant. We pre-identified four subgroups to analyze based on the precipitating cause of ARDS, pulmonary versus extra-pulmonary and infectious versus non-infectious. All analyses were done using SAS version 9.2 (Cary, NC).
RESULTS
The baseline characteristics of the 79 patients studied are summarized in Table 1. Age, sex, P/F ratio, APACHE III and LIS were similar between the two groups at time of study entry. In addition, these clinical characteristics were statistically similar between the 79 patients who had samples available and the 12 patients enrolled in the clinical trial who did not have sample available for analysis. The causes of ARDS by site of injury (pulmonary or extra-pulmonary) or by infection status (infectious or non-infectious) are summarized in Table 2A and 2B respectively.
Table 1.
Baseline Characteristics of Included Patients
| Methylprednisolone (n=55) |
Usual Care (n=24) |
p-value | |
|---|---|---|---|
| Age (years): mean (SE) | 49.7 (2.1) | 53.9 (3.4) | 0.30 |
| Sex (male): number (%) | 29 (53%) | 12 (50%) | 0.82 |
| APACHE III score at ICU Admission: mean (SE) | 57.4 (2.2) | 66.3 (4.1) | 0.07 |
| PaO2/FIO2 at Study Entry: mean (SE) | 119.5 (6.9) | 126.2 (8.2) | 0.53 |
| Lung Injury Score at Study Entry: Median (range) | 3.25 (2.50, 4.00) | 3.13 (2.50, 4.00) | 0.44 |
95 % confidence interval in parentheses
Table 2.
| A-Causes of ARDS Classified By Site of Injury | ||
|---|---|---|
| Methylprednisolone (n=55) |
Usual Care (n=24) |
|
| PULMONARY | (n=38) | (n=14) |
| Community-acquired pneumonia | 21 | 8 |
| Aspiration | 10 | 4 |
| Hospital-acquired pneumonia | 5 | 1 |
| Near-Drowning | 2 | 0 |
| Other | 0 | 1 |
| EXTRA-PULMONARY | (n=17) | (n=10) |
| Post-operative | 6 | 2 |
| Pancreatitis | 3 | 0 |
| Other infectionsa | 5 | 7 |
| Other (non-infectious) | 3 | 1 |
| B-Cause of ARDS Classified by Infection Status | ||
|---|---|---|
| Methylprednisolone (n=55) |
Usual Care (n=24) |
|
| INFECTIOUS | (n = 31) | (n = 16) |
| Community-acquired pneumonia | 21 | 8 |
| Hospital-acquired pneumonia | 5 | 1 |
| Intra-Abdominal | 2 | 2 |
| Urinary Tract Infection | 1 | 1 |
| Endocarditis | 1 | 1 |
| Necrotizing Fasciitis | 0 | 1 |
| Bacteremia Unknown Source | 1 | 1 |
| Ehrlichiosis | 0 | 1 |
| NON-INFECTIOUS | (n=24) | (n=8) |
| Aspiration | 10 | 4 |
| Post-operative | 6 | 2 |
| Pancreatitis | 3 | 0 |
| Other (non-infectious)a | 5 | 2 |
Other infections include urinary tract infection, endocarditis, peritonitis and ehrlichiosis
Other non-infectious causes include TRALI, near-drowning, vasculitis and placental abruption
Table 3.
Clinical Outcomes in Studied Patients*
| Methylprednisolone (n=55) |
Usual Care (n=24) |
p-value | |
|---|---|---|---|
| Lung Injury Score Improved by Day 8: number (%) | 40 (73%) | 9 (38%) | 0.003 |
| ICU Survival number (%) | 44 (80%) | 14 (58%) | 0.05 |
| Duration of MV Median (range) | 5.0 (0.0, 64.0) | 9.5 (0.0, 63.0) | 0.005 |
Subgroup of 79 patients from original cohort of 91 patients previously described(1)
Clinical Outcomes
Of the 79 patients who had plasma sample available for analysis, the patients who received methylprednisolone had greater improvement in Lung Injury Score (p=0.003), shorter duration of mechanical ventilation (p=0.005) and lower ICU mortality (p=0.05) than the patients who received usual care (Table 3). These data are consistent with the previously reported clinical outcomes from the entire 91 patient cohort (1).
Inflammatory Biomarkers
At study entry, plasma levels of TNF-α, VEGF, protein C, proADM and proCT were similar between the ARDS patients receiving usual care compared to those treated with methylprednisolone at time of study entry. Study entry levels of TNF-α and proCT were elevated in both groups compared to normal values: methylprednisolone median TNF-α 6.52 (range 0.60–93.69) pg/mL compared to usual care 7.36 (range 1.37–112.26) pg/mL and methylprednsiolone proCT 1.81 (range 0.08–34.79) ng/mL compared to usual care 3.95 (range 0.24–49.46) ng/mL. The fall in plasma TNF-α and proCT levels from study entry to day 7 was similar in both groups. The temporal changes in IL-6, protein C, proADM and VEGF levels are described below.
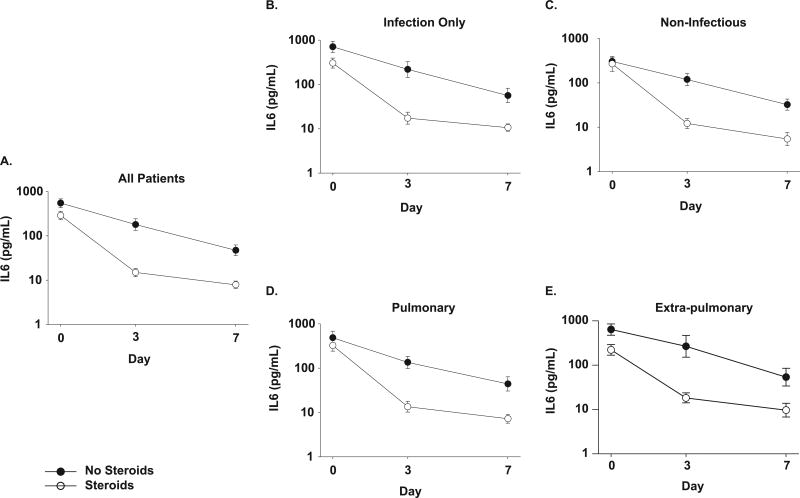
At study entry, plasma IL-6 was higher in the usual care group (median 558; range 64 – 4,461 pg/mL) than the methylprednisolone group (median 264; 16 −34,595 pg/ml, p=0.03). Methylprednisolone treatment was associated with a greater decline in IL-6 compared to levels in patients receiving usual therapy (Figure 1a) on days 3 (p<0.0001) and 7 (p=0.002).
Figure 1. Effects of methylprednisolone on plasma concentrations of interleukin-6 in patients with ARDS.
Panel A). IL-6 concentrations at time of study entry (day 0), day 3 and day 7 for all patients (usual care for ARDS ● and those treated with methylprednisolone ○). Data are presented as mean ± SEM of log transformed values (labeled in anti-log). Methylprednisolone treatment was associated with a decrease in IL-6 from baseline compared to patients receiving usual care on both day 3 and day 7 (p<0.0001 for both days).
In the subgroup analyses (panels B-E), a p=0.01 was used as a threshold for significance.
Panels B and C show plasma IL-6 concentration over time in the subgroups of patients with B). infectious causes of ARDS and C). non-infectious causes of ARDS. Both subgroups had a decline in plasma IL-6 with methylprednisolone at day 3 compared to baseline (p=0.0007 for infectious subgroup, p=0.002 for non-infectious subgroup) but not at day 7 (p=0.03 for infectious subgroup, p=0.02 for non-infectious subgroup).
Panels D and E show log plasma IL-6 concentration over time in the subgroups of patients with D). pulmonary causes of ARDS and E). extra-pulmonary causes of ARDS. Both subgroups had a decline in plasma IL-6 with methylprednisolone treatment at day 3 compared to baseline (p=0.0003 for pulmonary subgroup, p=0.001 for extra-pulmonary subgroup) but at day 7, only the pulmonary subgroup had a significant decrease in interleukin-6 with corticosteroid therapy compared to baseline (p=0.002).
To determine if the anti-inflammatory effect of methylprednisolone related to the cause of ARDS, we compared responses in four subgroups (infectious versus non-infectious and pulmonary versus extra-pulmonary etiologies). A greater decline in plasma IL-6 occurred by day 3 with methylprednisolone therapy in both patients with infectious (p=0.0007) and non-infectious causes of ARDS (p=0.002). However, this effect was only marginal in these subgroups on day 7 (p=0.03 for infectious (n = 47), p=0.02 for non-infectious (n =32). (Figures 1b and 1c). In patients with pulmonary causes (n=52), methylprednisolone treatment was associated with a greater decline in plasma IL-6 from baseline than usual care by day 3 (p=0.0003) and day 7 (p=0.002). In contrast, in patients with extra-pulmonary causes (n=27), the decline in IL-6 with methylprednisolone compared to usual care was significant by day 3 (p=0.001) but not day 7 (Figures 1d and 1e).
Coagulation
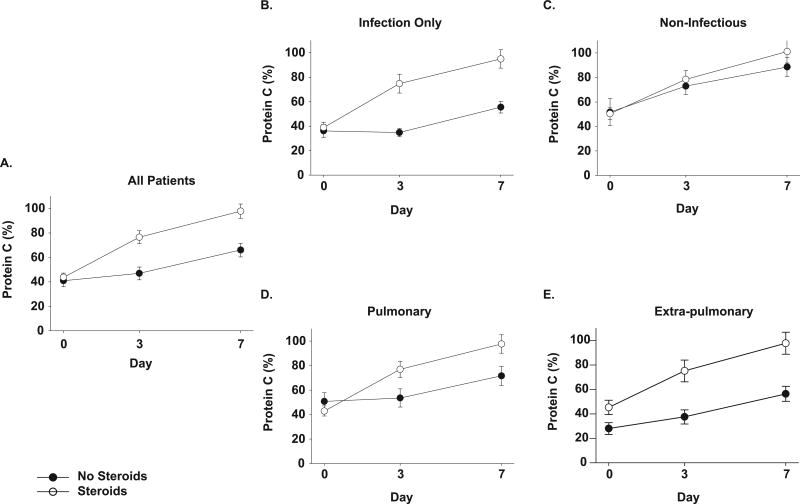
At study entry, both treatment groups had protein C levels less than 50% of the normal pooled plasma reference range (methylprednisolone median 42.39, range 5.00 – 108.10 and usual care 43.74, 5.00 −118.21 %, p= NS) (Figure 2a). Methylprednisolone administration resulted in a rapid rise in protein C by day 3, with levels significantly higher than patients receiving usual care (p<0.0001) that persisted on day 7 (p<0.0001).
Figure 2. Effects of methylprednisolone on plasma protein C levels in patients with ARDS.
Panel A) Protein C levels at time of study entry (day 0), day 3 and day 7 for all patients (usual care for ARDS ● and those treated with methylprednisolone ○). Data are presented as mean ± SEM. Methylprednisolone treatment was associated with a significant increase in plasma protein C activity from baseline compared to patients receiving usual care, at both days 3 and day 7 (p<0.0001 for both days 3 and 7).
In the subgroup analyses (panels B-E), a p=0.01 was used as a threshold for significance.
Panels B and C show plasma protein C levels over time in the subgroups of patients with B). infectious causes of ARDS and C). non-infectious causes of ARDS respectively. Patients receiving methylprednisolone in the infectious subgroups had a significant increase in plasma protein C at both day 3 and day 7 compared to usual care (p<0.0001 for both days). No significant difference in protein C levels over time in the non-infectious subgroup with methylprednisolone therapy at day 3 or day 7.
Panels D and E show plasma protein C over time in the subgroups of patients with D). pulmonary and E). extra-pulmonary causes of ARDS. The subgroup with pulmonary causes of ARDS had significantly higher protein C levels at both day 3 and day 7 (p<0.0001 for both days) with methylprednisolone therapy. There was no difference in protein C levels over time with methylprednisolone in the subgroup with extra-pulmonary etiology of ARDS.
In patients with an infectious cause of ARDS, methylprednisolone therapy was associated with significant rise in plasma protein C levels when compared to usual care on both day 3 (p<0.0001) and day 7 (p<0.0001). In contrast, no difference in protein C levels was found with methylprednisolone compared to usual care in the subgroup with non-infectious causes of ARDS (Figures 2b and 2c).
Methylprednisolone treatment was associated with a rise in protein C levels over time compared to usual care on both day 3 (p<0.0001) and day 7 (p<0.0001) in patients with direct pulmonary injury but there was no difference in protein C levels over time in the subgroup with an extra-pulmonary cause of ARDS (Figures 2d and 2e).
Markers of Vascular Tone and Angiogenesis
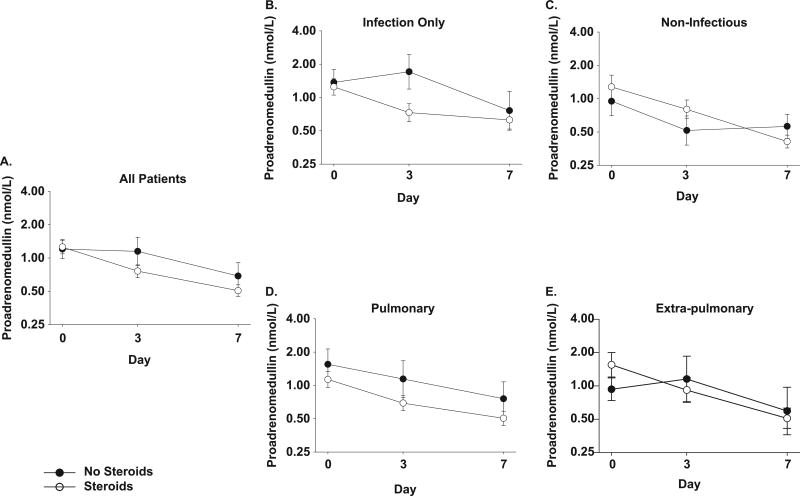
ProADM, an ADM precursor fragment that is measured more reliably than the rapidly cleared ADM, directly reflects ADM levels(20). Baseline values of proADM were: methylprednisolone median 1.01 (range 0.25 – 8.30) nmol/L and usual care median 1.03 (0.25 – 8.83) nmol/L. Methylprednisolone therapy resulted in a decrease in proADM (figure 3a) by day 3 compared to patients receiving usual care (p=0.004) but these differences were not sustained on day 7 (p=0.07).
Figure 3. Effects of methylprednisolone on proadrenomedullin concentration in patients with ARDS.
Panel A) Proadrenomedullin concentration is shown at time of study entry (day 0), day 3 and day 7 for all patients (usual care for ARDS ● and those treated with methylprednisolone ○). Data are presented as mean ± SEM of log transformed values (labeled in anti-log). Methylprednisolone treatment was associated with a significant decrease in plasma proadrenomedullin from baseline compared to patients receiving usual care at day 3 (p=0.004) but not day 7 (p=0.07).
In the subgroup analyses (panels B-E), a p=0.01 was used as a threshold for significance.
Panels B and C show log plasma proadrenomedullin concentration over time in the subgroups of patients with B). infectious causes of ARDS and C). non-infectious causes of ARDS. In patients with infectious cause of ARDS, there was a significant decline in plasma proadrenomedullin with methylprednisolone at day 3 compared to baseline (p=0.0007) but not at day 7. There was no difference with methylprednisolone therapy at day 3 or 7 in the non-infectious subgroup.
Panels D and E show log plasma proadrenomedullin over time in the subgroups of patients with D). pulmonary and E). extra-pulmonary causes of ARDS. Patients with extra-pulmonary causes of ARDS had a significant decline in plasma proadrenomedullin with methylprednisolone at day 3 compared to baseline (p=0.008) but not at day 7. There was no difference in proADM concentration with methylprednisolone therapy at day 3 or 7 in the subgroup with direct pulmonary injury causing ARDS.
In the patients with infectious causes of ARDS, methylprednisolone was associated with decreased proADM compared to usual care on day 3 (p=0.0007) but not by day 7. In patients with non-infectious causes of ARDS, no differences in proADM levels were found by day 3 and day 7 with methylprednisolone therapy (Figures 3b and 3c).
In the patients with direct pulmonary causes of ARDS, patients given methylprednisolone had similar levels of proADM on day 3 and day 7 compared to usual care. In patients with extra-pulmonary causes of ARDS, proADM was lower with methylprednisolone therapy compared to usual care on day 3 (p=0.008) but not on day 7 (Figures 3d and 3e).
Plasma VEGF concentration did not differ between the two study groups at study entry (methylprednisolone median 112.34 (range 0.81 – 497.14) and usual care 59.80 (0.81 – 483.89) pg/ml) or on days 3 or 7 (all, p = NS).
DISCUSSION
We studied the effects of a continuous low-dose methylprednisolone infusion on six plasma biomarkers of inflammation and hemostasis over a seven-day period in patients with early ARDS. Methylprednisolone significantly decreased plasma levels of IL-6 and proADM, and restored plasma protein C to normal levels. These biomarker changes occurred in the context of methylprednisolone treatment improving ICU mortality, decreasing mechanical ventilation duration and improving LIS in this cohort.
Protein C is activated by cell surface thrombin-thrombomodulin complexes and acts as an anti-coagulant by inactivating factors Va and VIIIa. Several recent studies suggest a critical role of protein C in the link between coagulation and inflammation (21–23). The protein C system is suppressed in patients with pulmonary inflammation and pre-clinical models of lung injury suggest that activated protein C can reduce lung injury and the associated pulmonary coagulopathy (24) (25). Plasma protein C levels are lower in acute lung injury compared to normal controls, both in the presence and absence of sepsis, and low protein C levels correlate with poor clinical outcome (16, 26). However, a trial of activated protein C administration for the treatment of acute lung injury failed to improve outcomes (27).
Our study demonstrates that methylprednisolone is associated with restoration of plasma protein C levels to near normal after seven days compared to usual care. Corticosteroid therapy increases protein C levels in patients with autoimmune thrombocytopenic purpura, minimal change nephrotic syndrome and systemic lupus erythematosus (28–30) but this effect has not been described in ARDS. These findings suggest that beneficial effects of corticosteroids in ARDS may be mediated in part by earlier restoration of protein C levels. Whether methylprednisolone-induced reversal of protein C suppression is a mediator or a marker of an improved rate of resolution of lung injury is unknown. Restoration of protein C levels with methylprednisolone occurred in subgroups of patients with either infectious or direct pulmonary injuries causing ARDS.
Corticosteroid therapy was also associated with significant temporal decline in plasma IL-6, an important cytokine, in the first week of ARDS. This may have important clinical consequences. A prior study found that plasma IL-6 levels decrease less over time in nonsurvivors of severe sepsis, than in survivors (31). Our results are consistent with previous studies showing that corticosteroids decrease plasma IL-6 levels when compared to placebo during the first week of septic shock (32). The decrease in IL-6 parallels the decrease in C-reactive protein levels, another global marker of systemic inflammation, described in the original cohort (1).
Methylprednisolone treatment was also associated with a significant decrease in plasma proADM, the prohormone of adrenomedullin, at study day 3. Adrenomedullin is a potent vasodilator with immune-modulating and bactericidal activity. The reduction in plasma proADM with methylprednisolone found at day 3 in the entire cohort was found at day 3 in patients with infectious and extra-pulmonary causes of ARDS. Prior studies describe the association of elevated proADM with poor short-term outcome in sepsis (33) and long-term outcome in community-acquired pneumonia (34).
Plasma levels of proCT, a global marker of inflammation, and the inflammatory cytokine TNF were elevated at study entry but were not altered by methylprednisolone treatment. A previous study of human experimental inflammation after intravenous endotoxin showed that pre-treatment with prednisolone reduced the rise in blood proADM concentration but did not alter the rise in proCT (35). These data are consistent with our findings and suggest that corticosteroids have differential effects on inflammatory biomarkers based in part on dose and timing around an inflammatory insult.
There are several limitations to our study. Ideally, the measurement of serial markers from bronchoalveolar lavage fluid or lung tissue would best describe changes in inflammation and coagulation in the lung but these samples were not available from the entire cohort. While the ventilator management of ARDS was appropriate for the study time period, it is conceivable that using a low tidal volume protocol throughout the study may have influenced biomarker concentrations. Though desirable, a more comprehensive evaluation of multiple biomarkers was not feasible due to limited sample volume. The number of subjects studied in the usual care group was relatively small due the 2:1 randomization strategy of the clinical trial (1). Some patients did not have plasma samples through day 7 because they improved and were discharged from the ICU. We did not study plasma after day 7 as the clinical trial allowed open label corticosteroid treatment for patients not improving by day 7, with patients in both the methylprednisolone (8%) and usual care (34%) groups receiving open-label methylprednisolone. Since biomarkers were not measured at later time points, it is possible that survivors that did not receive steroids may have had normalization of IL-6, protein C and proADM levels after the one-week study period.
Conclusions
This study shows that infusion of methylprednisolone early in the course of ARDS decreases plasma IL-6 concentrations, consistent with a global decrease in systemic inflammation, decreases plasma proADM concentration, a biomarker prognostic of mortality in community-acquired pneumonia and severe sepsis, and restores one part of the altered hemostatic profile by increasing plasma protein C levels. The finding of increased protein C levels with corticosteroid treatment suggests that attenuation of inflammation may improve aspects of altered coagulation in critically ill patients. In addition, methylprednisolone treatment improved important clinical outcomes in this group of patients, decreasing ICU length of stay, decreasing duration of mechanical ventilation and increasing the percentage of patients with improvement in LIS by day 8. These data suggest important clinical benefits of corticosteroids that may be related to its effects on key components of inflammation and hemostasis in patients with ARDS.
The consensus definition of ARDS is based on a constellation of common clinical and pathologic findings that result in hypoxemic respiratory failure (36). However, this definition does not account for the diverse mechanisms that lead to the common final pathway of acute lung injury. Our subgroup analyses suggest that these underlying mechanisms differ with the precipitating cause of ARDS, which may be an important determinant of responsiveness to anti-inflammatory therapy.
Acknowledgments
This research was supported by the Intramural Research Program of the Critical Care Medicine Department of the National Institutes of Health. We thank Reba Umberger PhD for data gathering, Debra Reda RN for technical support in figure production and help with sample preparation and Kyle Quinn for assay completion.
Funding support: This research was supported by the Intramural Research Program of the Critical Care Medicine Department of the National Institutes of Health.
Appendix - Assay Characteristics
| Assay | Mean MDC | Mean Inter- Assay CV |
Mean Intra- Assay CV |
Manufacturer |
|---|---|---|---|---|
| Protein C (%) | 5% in respect to normal | 7.50% | 7.00% | Helena Laboratories |
| VEGF | 0.81 pg/mL | 7.80–9.50% | 3.70–6.70% | R and D Systems |
| IL-6 | 0.36 pg/mL | 5.90–7.90% | 4.30–4.70% | R and D Systems |
| TNF-alpha | 0.60 pg/mL | 6.20–7.30% | 3.70–4.80% | R and D Systems |
| proCT | 0.019 ng/mL | < 15% | < 10% | Brahms |
| proADM | 0.25 nmol/L | < 20% | < 10% | Brahms |
MDC: Minimum detectable concentration
CV: Coefficient of Variation
Data in table from data in manufacturer’s manual for respective assays
Ranges provided for assay variation when %CV varied for testing of samples at low and high concentrations
For protein C, standard curve range was 12.5–150% activity and assay detection range was 5 % −200% activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nitin Seam, Honghui Wang, Junfeng Sun, and Anthony Suffredini received funding from NIH. The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 2.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 3.Meduri GU, Kohler G, Headley S, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 4.Meduri GU, Muthiah MP, Carratu P, et al. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005;12(6):321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 5.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 6.McClintock D, Zhuo H, Wickersham N, et al. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair S, Bijoy J, Golden E, et al. Interleukin-8 amd soluble intercellular adhesion molecule-1 during acute respiratory distress syndrome and in response to prolonged methylprednisolone treatment. Minerva Pneumologica. 2006;45:93–104. [Google Scholar]
- 8.Steinberg KP, Milberg JA, Martin TR, et al. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150(1):113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 9.Meduri GU, Tolley EA, Chinn A, et al. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 10.Meduri GU. The role of the host defence response in the progression and outcome of ARDS: pathophysiological correlations and response to glucocorticoid treatment. Eur Respir J. 1996;9(12):2650–2670. doi: 10.1183/09031936.96.09122650. [DOI] [PubMed] [Google Scholar]
- 11.Cordier J-F, Peyrol S, Loire R. Bronchiolitis obliterans organizing pneumonia as a model of inflammatory lung disease. In: Epler G, editor. Diseases of the bronchioles. New York: Raven Press, Ltd; 1994. pp. 313–345. [Google Scholar]
- 12.Pelosi P, Caironi P, Gattinoni L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001;22(3):259–268. doi: 10.1055/s-2001-15783. [DOI] [PubMed] [Google Scholar]
- 13.Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34(1):61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 14.Tang BM, Craig JC, Eslick GD, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37(5):1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 15.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 16.Bastarache JA, Ware LB, Bernard GR. The role of the coagulation cascade in the continuum of sepsis and acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):365–376. doi: 10.1055/s-2006-948290. [DOI] [PubMed] [Google Scholar]
- 17.Idell S, James KK, Levin EG, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84(2):695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John W. Wiley and Sons; 2002. [Google Scholar]
- 20.Koo DJ, Yoo P, Cioffi WG, et al. Mechanism of the beneficial effects of pentoxifylline during sepsis: maintenance of adrenomedullin responsiveness and downregulation of proinflammatory cytokines. J Surg Res. 2000;91(1):70–76. doi: 10.1006/jsre.2000.5916. [DOI] [PubMed] [Google Scholar]
- 21.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 22.Toltl LJ, Beaudin S, Liaw PC. Activated protein C up-regulates IL-10 and inhibits tissue factor in blood monocytes. J Immunol. 2008;181(3):2165–2173. doi: 10.4049/jimmunol.181.3.2165. [DOI] [PubMed] [Google Scholar]
- 23.Weiler H. Regulation of inflammation by the protein C system. Crit Care Med. 38(2 Suppl):S18–25. doi: 10.1097/CCM.0b013e3181c9cbb5. [DOI] [PubMed] [Google Scholar]
- 24.Hataji O, Taguchi O, Gabazza EC, et al. Activation of protein C pathway in the airways. Lung. 2002;180(1):47–59. doi: 10.1007/s004080000080. [DOI] [PubMed] [Google Scholar]
- 25.Hofstra JJ, Juffermans NP, Schultz MJ, et al. Pulmonary coagulopathy as a new target in lung injury--a review of available pre-clinical models. Curr Med Chem. 2008;15(6):588–595. doi: 10.2174/092986708783769696. [DOI] [PubMed] [Google Scholar]
- 26.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35(8):1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costallat LT, Ribeiro CC, Annichino-Bizzacchi JM. Antithrombin, protein S and protein C and antiphospholipid antibodies in systemic lupus erythematosus. Sangre (Barc) 1998;43(5):345–348. [PubMed] [Google Scholar]
- 29.Oner AF, Bay A, Kuru M, et al. Effects of high-dose methylprednisolone therapy on coagulation factors in patients with acute immune thrombocytopenic purpura. Clin Appl Thromb Hemost. 2005;11(4):489–492. doi: 10.1177/107602960501100418. [DOI] [PubMed] [Google Scholar]
- 30.Ueda N. Effect of corticosteroids on some hemostatic parameters in children with minimal change nephrotic syndrome. Nephron. 1990;56(4):374–378. doi: 10.1159/000186178. [DOI] [PubMed] [Google Scholar]
- 31.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33(11):2457–2464. doi: 10.1097/01.ccm.0000186370.78639.23. [DOI] [PubMed] [Google Scholar]
- 33.Guignant C, Voirin N, Venet F, et al. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med. 2009;35(11):1859–1867. doi: 10.1007/s00134-009-1610-5. [DOI] [PubMed] [Google Scholar]
- 34.Kruger S, Ewig S, Giersdorf S, et al. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 182(11):1426–1434. doi: 10.1164/rccm.201003-0415OC. [DOI] [PubMed] [Google Scholar]
- 35.de Kruif MD, Lemaire LC, Giebelen IA, et al. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34(3):518–522. doi: 10.1007/s00134-007-0955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]