Abstract
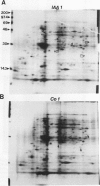
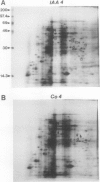
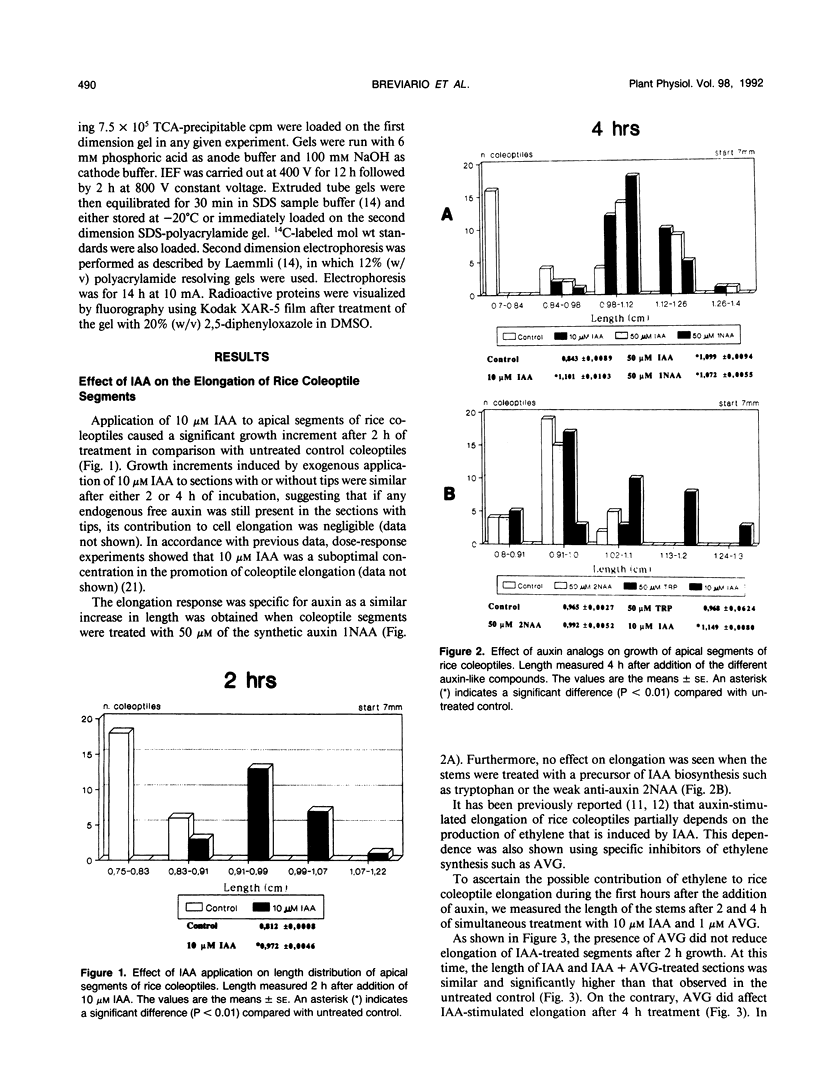
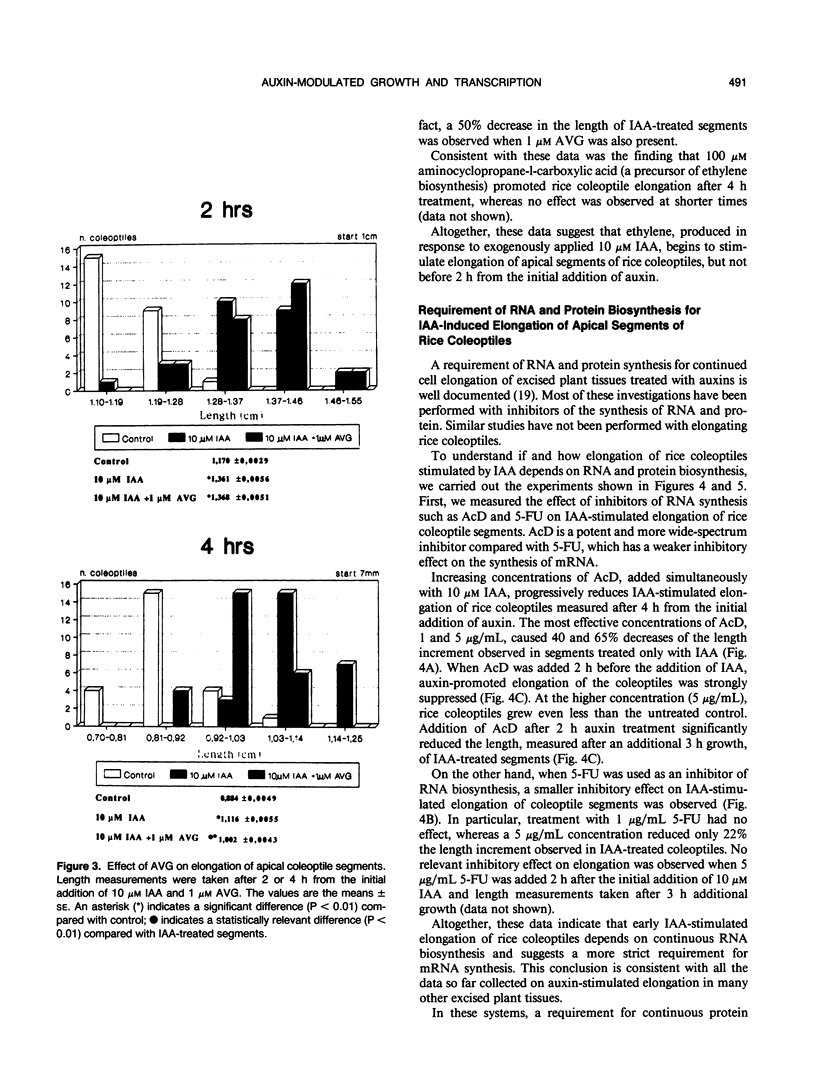
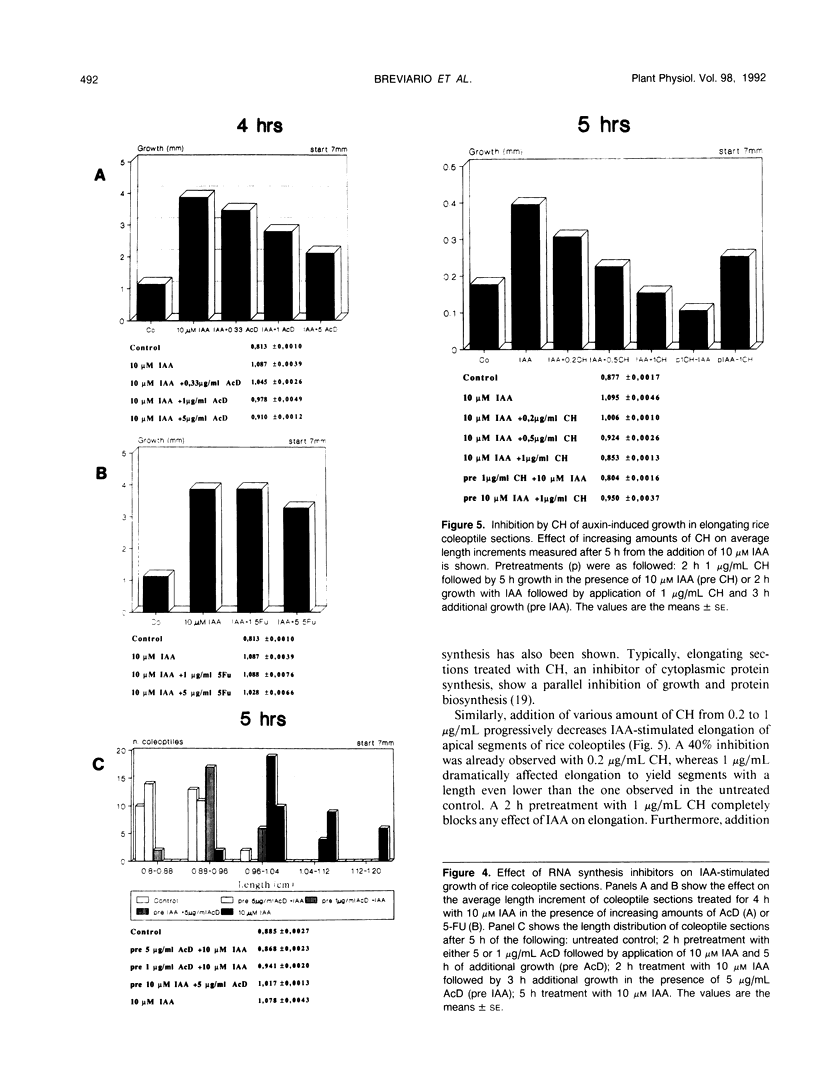
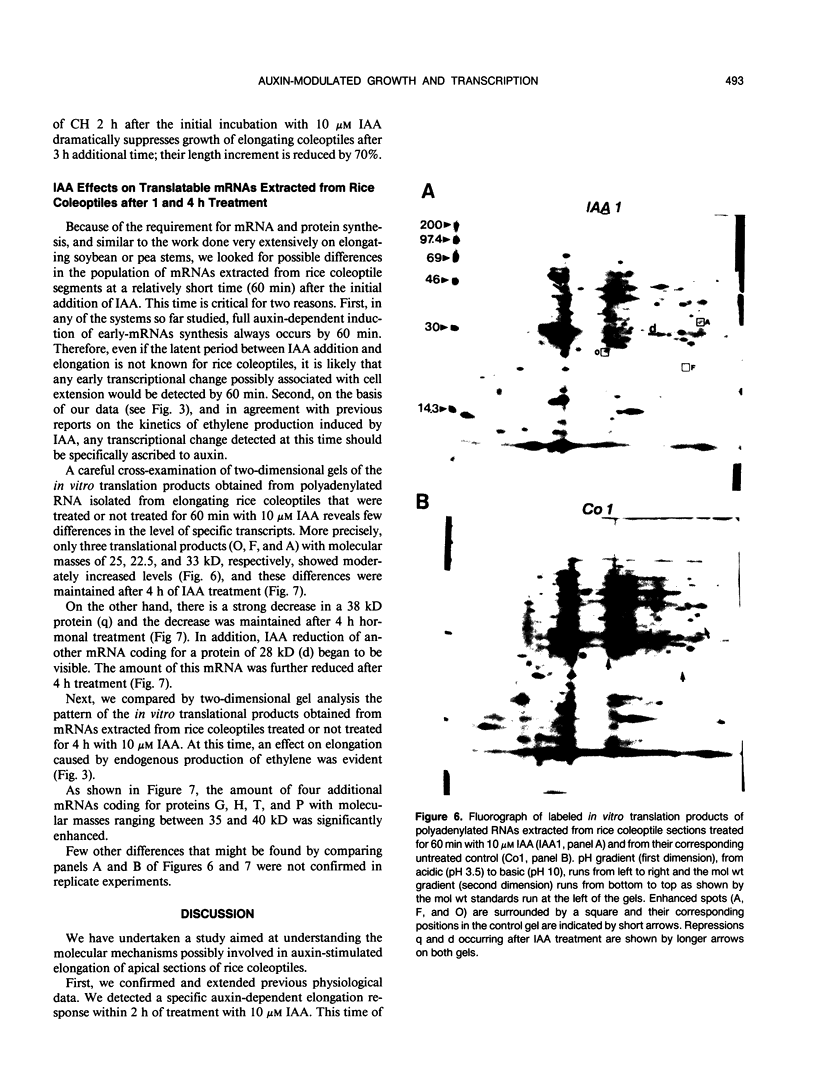
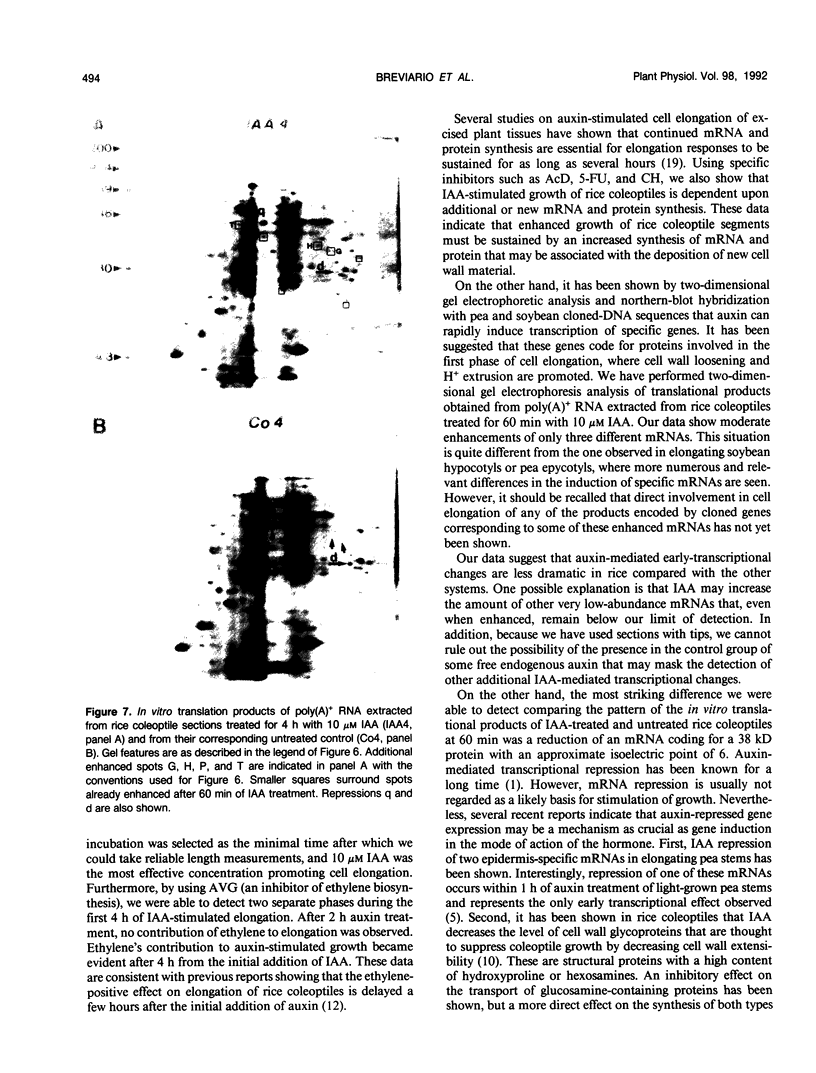
Auxin-stimulated elongation of apical segments of rice (Oryza sativa L. cv Arborio) coleoptiles occurring in the first 4 hours of treatment has been studied. Cell extension promoted in the first 2 hours by 10 micromolar indole-3-acetic acid (IAA) is specifically auxin-dependent, whereas after 4 hours, elongation also depends on endogenous production of ethylene. Similar to other systems, rice coleoptile cell elongation stimulated by auxin requires continuous synthesis of RNA and protein. Two-dimensional gel analysis of the in vitro translation products obtained from polyadenylated RNAs extracted from treated and untreated segments after 1 or 4 hours from the initial addition of IAA shows few transcriptional differences. At 60 minutes of treatment, the level of three mRNAs coding for proteins of 22.5, 25, and 33 kilodaltons was moderately enhanced while the disappearance of a 38 kilodalton translation product was observed. Additional repression of another mRNA coding for a 28 kilodalton product begins to show by this time, but becomes more evident after 4 hours treatment. At 4 hours, four IAA-specific mRNA enhancements coding for proteins with molecular masses ranging between 35 to 40 kilodaltons were also observed. We discuss these data in relation to the possible involvement of IAA-mediated transcriptional regulation in growth promotion of rice coleoptiles and, more widely, in control of cell elongation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conner T. W., Goekjian V. H., LaFayette P. R., Key J. L. Structure and expression of two auxin-inducible genes from Arabidopsis. Plant Mol Biol. 1990 Oct;15(4):623–632. doi: 10.1007/BF00017836. [DOI] [PubMed] [Google Scholar]
- Dietz A., Kutschera U., Ray P. M. Auxin Enhancement of mRNAs in Epidermis and Internal Tissues of the Pea Stem and Its Significance for Control of Elongation. Plant Physiol. 1990 Jun;93(2):432–438. doi: 10.1104/pp.93.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Barnett N. M., Lin C. Y. RNA and protein biosynthesis and the regulation of cell elongation by auxin. Ann N Y Acad Sci. 1967 Aug 9;144(1):49–62. doi: 10.1111/j.1749-6632.1967.tb34000.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClure B. A., Hagen G., Brown C. S., Gee M. A., Guilfoyle T. J. Transcription, organization, and sequence of an auxin-regulated gene cluster in soybean. Plant Cell. 1989 Feb;1(2):229–239. doi: 10.1105/tpc.1.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Reddy A. S., Poovaiah B. W. Molecular cloning and sequencing of a cDNA for an auxin-repressed mRNA: correlation between fruit growth and repression of the auxin-regulated gene. Plant Mol Biol. 1990 Feb;14(2):127–136. doi: 10.1007/BF00018554. [DOI] [PubMed] [Google Scholar]
- Yamada N. Auxin Relationships of the Rice Coleoptile. Plant Physiol. 1954 Jan;29(1):92–96. doi: 10.1104/pp.29.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]