Abstract
PD98059, a specific inhibitor of MEK-1 mitogen-activated protein (MAP) kinase kinase, blocked Listeria monocytogenes invasion into HeLa epithelial cells. The effects of PD98059 were reversible, as adherent extracellular bacteria were internalized upon removal of the drug. Previously, we reported that L. monocytogenes could activate ERK-1 and ERK-2 MAP kinases through the action of listeriolysin O (LLO) on the host cell (P. Tang, I. Rosenshine, P. Cossart, and B. B. Finlay, Infect. Immun. 64:2359–2361, 1996). We have now found that two other MAP kinase pathways, those of p38 MAP kinase and c-Jun N-terminal kinase, are also activated by wild-type L. monocytogenes. Mutants lacking functional LLO (hly mutants) were still invasive but only activated ERK-2 and only activated it at later (90-min) postinfection times. Two inhibitors of L. monocytogenes invasion, cytochalasin D, which disrupts actin polymerization, and wortmannin, which blocks phosphatidylinositol (PI) 3-kinase activity, did not block ERK-2 activation by wild-type L. monocytogenes and hly mutants. However, genistein, an inhibitor of tyrosine kinases, and PD98059 both blocked invasion and decreased ERK-2 activation. These results suggest that MEK-1 and ERK-2 activities are essential for L. monocytogenes invasion into host epithelial cells. This is the first report to show that a MAP kinase pathway is required for bacterial invasion.
Listeria monocytogenes is a gram-positive facultative intracellular bacterium that can cause severe infections in newborns, elderly people, and immunocompromised individuals (14, 15). Invasion into host cells is a primary step required for the pathogenesis of the disease listeriosis (12). The model for the invasion process requires the bacteria to bind host cell receptors. This leads to the activation of host cell signal transduction molecules which eventually trigger the host cell cytoskeletal rearrangements necessary for uptake of the surface-bound bacteria.
Recently, the bacterial surface protein internalin and the host epithelial cell adhesion molecule E-cadherin were found to be involved in the L. monocytogenes internalization process (10, 23). Strains with mutations in inlA, which codes for internalin, have a reduced ability to enter Caco-2 epithelial cells. Nonpathogenic Listeria innocua expressing inlA can invade epithelial cells, though not as efficiently as wild-type L. monocytogenes. Fibroblasts transfected with E-cadherin show an increased ability to uptake L. monocytogenes and L. innocua expressing inlA. Antibodies against E-cadherin and internalin also reduce the invasion of L. monocytogenes into host cells.
Phosphatidylinositol (PI) 3-kinase plays a role in the host cell signal transduction required for efficient uptake of L. monocytogenes (18). It has been implicated in the regulation of actin cytoskeletal rearrangements and certain endocytic processes (5). PI 3-kinase is activated when L. monocytogenes binds to E-cadherin on the host epithelial cell surface. Inhibition of PI 3-kinase with wortmannin or LY294002 or expression of the dominant-negative form of the p85 subunit of PI 3-kinase leads to a decrease in L. monocytogenes invasion. Internalin-related protein InlB is required for activating PI 3-kinase and for invasion into certain cell lines including Vero, CHO, and HeLa cells and hepatocytes (18). Given the complexity of signal transduction pathways, the other molecules involved have yet to be identified.
Mitogen-activated protein (MAP) kinases ERK-1 and ERK-2 (extracellular signal-regulated protein kinases) were originally found to be activated during L. monocytogenes invasion (34). However, subsequent studies found that purified listeriolysin O (LLO) alone could induce the activation of these two isoforms of MAP kinase (33, 38). LLO is a sulfhydryl-activated hemolysin specific to L. monocytogenes that plays a crucial role in freeing the bacteria from the phagolysosome after uptake into the host cell (26). Mutants lacking LLO (hly mutants) can still invade host cells as efficiently as wild-type L. monocytogenes (20). The MAP kinase activation was believed to be caused by the action of LLO on the host cell membrane rather than by the invasion of the bacteria (33). This suggested that MAP kinases ERK-1 and ERK-2 were not involved in the signal transduction required for L. monocytogenes uptake.
MAP kinases are central in many host responses including the mitogenic response to the growth factors for which they are named. MAP kinases have also been implicated in the regulation of cytokine responses, stress responses, and cytoskeletal reorganization (7). Other isoforms of MAP kinase are also known, including p38 MAP kinase, also known as hyperosmolarity glycerol (HOG) kinase (16), and c-Jun N-terminal kinase (JNK), also known as stress-activated protein kinase (8, 21). More recently, ERK-3 (6), ERK-5 (40), and ERK-6 (22) have been discovered. MAP kinases, with the exception of ERK-3, are activated upon phosphorylation of both tyrosine and threonine residues by MAP kinase kinases or MEKs (MAPK/ERK kinases). Many different MEKs have been described, and in vitro assays indicate that each has only one or at most two specific targets in the MAP kinase pathways: MEK-1 and MEK-2 act on ERK-1 and ERK-2; MEK-3 and MEK-6 act on p38; MEK-4 acts on JNKs; and MEK-5 possibly acts on ERK-5 (4, 17, 37). Most eucaryotic surface receptors use at least one of these highly conserved MAP kinase cascades for signalling inside the cell (28).
Using a more sensitive assay, we reexamined the roles of ERK-1 and ERK-2 and looked at the involvement of two other MAP kinase pathways, those of p38 (HOG) and JNK, in the L. monocytogenes invasion process. The availability of PD98059, a highly specific inhibitor of MEK-1 activation (3, 9), also allowed us to adequately address the requirement for this signalling pathway during L. monocytogenes internalization.
MATERIALS AND METHODS
Bacteria.
L. monocytogenes was grown in brain heart infusion broth or on Trypticase soy agar plates with the appropriate antibiotics. For all infections, Listeria was grown to logarithmic phase at 37°C with shaking. L. monocytogenes 1/2a3 is a streptomycin-resistant derivative of wild-type strain SLCC 5764. M3 is a nonhemolytic strain with a mutation in hly induced by the Tn916 transposon; it was derived from 1/2a3 (19). L028 is a wild-type strain of L. monocytogenes and the parent of BUG337. BUG337 has a single amino acid mutation (Trp-492 to Ala) in LLO (24). BUG337 secretes normal levels of LLO, but the mutation decreases the hemolytic activity of its LLO by 1,000-fold. L. monocytogenes EGD SmR (bof297) is the parent of BUG11, a noninvasive mutant with a Tn1545 insertion upstream of inlAB (10). Salmonella typhimurium SL1344 was grown in Luria-Bertani (LB) broth or on LB agar plates. For infections, Salmonella was grown overnight to stationary phase at 37°C without shaking. All bacterial media were purchased from Difco Laboratories (Detroit, Mich.).
Tissue culture cells.
HeLa cells were maintained between passages 5 and 25 in Eagle’s minimum essential medium (MEM; GIBCO, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (GIBCO) and no antibiotics. Cells were cultured at 37°C and 5% CO2.
Inhibitors.
Inhibitors of invasion were added at various times before or during the infection process. Cytochalasin D was obtained from Sigma Chemicals (St. Louis, Mo.). Wortmannin and genistein were purchased from Calbiochem (San Diego, Calif.). PD98059 was obtained from New England Biolabs (Beverly, Mass.). All drugs were stored in dimethyl sulfoxide (DMSO) at −20°C.
Invasion assay.
For the invasion assay, HeLa cells were seeded at 5 × 104 cells per well in 24-well plates (Costar, Cambridge, Mass.). Cells were allowed to grow for 2 days to reach confluency, and then the medium was replaced with MEM with no supplements for 2 h. Bacteria were grown as described above, washed twice in phosphate-buffered saline (PBS), pH 7.4, and resuspended in plain MEM before being added to the HeLa cells at a multiplicity of infection of approximately 50:1. Listeria and Salmonella infections were carried out for 90 min and 30 min, respectively, at 37°C in 5% CO2. The infected monolayers were then washed twice with PBS, pH 7.4. MEM containing 100 μg of gentamicin per ml was then added to kill extracellular bacteria. The cells were then washed three times with PBS, pH 7.4, and intracellular bacteria were released with 1% Triton X-100. Bacteria were diluted and quantitated by being plated on appropriate agar plates.
Preparation of cell lysates.
HeLa cells were seeded at 106 cells in 10-cm-diameter tissue culture dishes (Costar) and allowed to grow for 2 days. The medium was then replaced with MEM with no supplements for 2 h. Infection with bacteria was carried out in the same manner as described for the invasion assays. After the infection period, the monolayer was washed twice with ice-cold 1 mM Na3VO4 in PBS, pH 7.4. Cells were scraped into 1.5 ml microcentrifuge tubes with disposable cell scrapers (Costar). The supernatant was removed after centrifugation at 8,000 × g for 1 min at 4°C. The appropriate lysis buffer was added to the pellets, and the samples were spun down at 16,000 × g for 2 min at 4°C. The soluble fraction was transferred to a new microcentrifuge tube.
Lysis buffers.
The following buffers were used to solubilize the cells: buffer A (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 1% Nonidet P-40, 1 mM Na2MoO4, 40 μg of phenylmethylsulfonyl fluoride [PMSF] per ml, 0.2 mM Na3VO4, 10 μg of aprotinin per ml, 1 mM dithiothreitol [DTT], 2 μg of leupeptin per ml, 0.7 μg of pepstatin per ml, 10 μg of soybean trypsin inhibitor per ml), buffer B (137 mM NaCl, 20 mM Tris-HCl [pH 8.0], 5 mM EDTA, 1 mM Na6P2O7, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 10 mM NaF, 10 mM β-glycerophosphate, 20 μg of aprotinin per ml, and 20 μg of leupeptin per ml), and buffer C (137 mM NaCl, 20 mM Tris-HCl [pH 7.4], 10% Triton X-100, 10% glycerol, 2 mM Na6P2O7, 2 mM EDTA, 25 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, and 10 μg of leupeptin per ml).
Kinase assay buffers.
The kinase assay buffers used were as follows: buffer D (20 mM HEPES [pH 7.2], 5 mM MgCl2, 1 mM EGTA, 5 mM β-mercaptoethanol, 2 mM Na3VO4, 10 μg of aprotinin per ml, 1 mM PMSF), buffer E (25 mM HEPES [pH 7.6], 20 mM MgCl2, 20 mM β-glycerophosphate, 1 mM Na3VO4, 2 mM DTT), and buffer F (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM DTT, 100 μM Na3VO4).
Immunoprecipitation.
The immunoprecipitation steps were all carried out at 4°C. The samples were precleared with Sepharose-A or Sepharose-G beads (Pharmacia, Uppsala, Sweden) for 45 min. Immunoprecipitation was done with the appropriate antibodies and beads for 90 min. The beads were washed with lysis buffer followed by the appropriate kinase assay buffer. Beads were transferred to 1.5-ml screw-cap tubes for the kinase assays. Rabbit anti-ERK-1, anti-ERK-2, and anti-JNK-1 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.), Sheep anti-MAPKAP kinase-2 antibodies were obtained from Upstate Biotechnology, Inc. (Lake Placid, N.Y.).
ERK in vitro kinase assay.
Cells were solubilized in buffer A as described above. ERK-1 and ERK-2 were immunoprecipitated with the appropriate antibodies and Sepharose-A beads in buffer A. The beads were then washed twice with buffer A and once with buffer D. The in vitro kinase reaction was initiated by adding 1 mg of myelin basic protein (MBP; Sigma) per ml and 167 μCi of [γ-32P]ATP (Amersham, Arlington Heights, Ill.) per ml in 30 μl of buffer D. After 15 min at 30°C, the reaction was terminated by adding 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (500 mM Tris-HCl [pH 6.8], 10% SDS, 0.5 M DTT, 50% glycerol) and boiling for 5 min. Samples were loaded onto SDS–15% PAGE gels. The gels were dried and exposed to film. 32P incorporation into MBP was also quantitated with a phosphorimager (Molecular Dynamics PhosphorImager SI; Sunnyvale, Calif.).
JNK in vitro kinase assay.
Cells were lysed in buffer B as described above. JNK was immunoprecipitated with anti-JNK-1 antibodies and Sepharose-A beads in buffer B. The anti-JNK-1 antibodies recognize JNK-1 but also bind JNK-2 to a lesser degree. The beads were washed twice with buffer B and once with buffer E. Kinase reactions were initiated with a mixture containing 67 μg of glutathione S-transferase (GST)–c-Jun(1-169) (Upstate Biotechnology, Inc.) per ml, 20 μM ATP, and 333 μCi of [γ-32P]ATP per ml in 30 μl of buffer E. The reactions were stopped after 15 min at 30°C by adding 5× SDS-PAGE sample buffer and boiling for 5 min. Samples were loaded onto SDS–12% PAGE gels. Gels were dried and subjected to autoradiography, and 32P incorporation into GST–c-Jun was determined with a phosphorimager.
MAPKAP kinase-2 in vitro kinase assay.
Cells were solubilized in buffer C as described above. MAPKAP kinase-2 was immunoprecipitated with anti-MAPKAP kinase-2 antibodies and Sepharose-G beads in buffer C. The beads were then washed twice with buffer C and once with buffer F. The in vitro kinase reaction was initiated by adding a mixture containing 33 μg of recombinant murine Hsp25 (StressGen Biotechnologies Corp., Victoria, Canada) per ml, 50 μM ATP, and 333 μCi of [γ-32P]ATP per ml in 30 μl of buffer F. After 15 min at 30°C, the reaction was terminated by adding 5× SDS-PAGE sample buffer and boiling for 5 min. Samples were loaded onto SDS–12% PAGE gels. The gels were dried and exposed to film. 32P incorporation into Hsp25 was measured using a phosphorimager.
RESULTS
PD98509 reversibly blocks L. monocytogenes invasion into HeLa cells.
PD98059 is a highly selective inhibitor of MEK-1 activation with a 50% inhibitory concentration (IC50) of 5 to 10 μM (9). It binds inactive MEK-1 to prevent upstream activation by kinases such as Raf-1 and MEK kinase-1 (3). PD98059 also blocks MEK-2 activation, but this inhibition is much weaker, with an IC50 of 50 μM. Thus, PD98059 can suppress the activation of ERK-1 and ERK-2 by different stimuli. PD98059 has been tested on over 20 different kinases, including various MAP kinases, protein kinase C, v-Src, epidermal growth factor receptor kinase, MAPKAP kinase, and PI 3-kinase, and has been found to have no in vitro inhibitory effects on the activities of these kinases (3, 9).
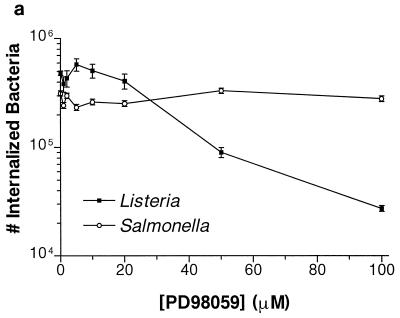
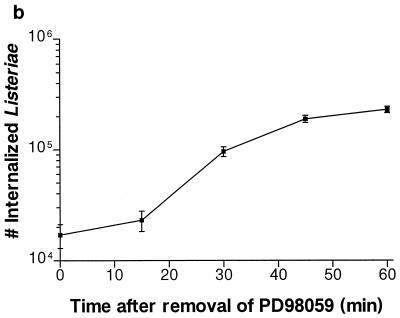
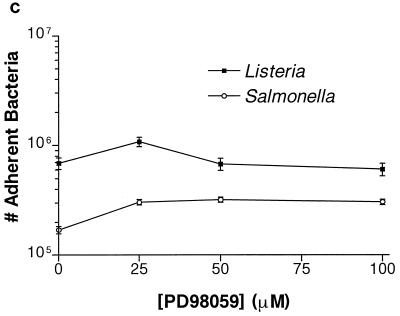
HeLa cells were preincubated with PD98059 for 60 min prior to bacterial infection. L. monocytogenes invasion was reduced by 10-fold at 100 μM PD98059 (Fig. 1a). S. typhimurium invasion was unaffected at the same concentrations. To investigate the reversibility of the PD98059 inhibition, we preincubated HeLa cells for 60 min. These cells were then infected with L. monocytogenes for an additional 60 min. The infected monolayers were washed twice with PBS to remove any PD98059 before the medium was replaced with plain MEM with no inhibitors. At various time points, the plain MEM was then replaced with 100 μg of gentamicin per ml in MEM to kill extracellular bacteria. After the PD98059 was washed away for 60 min, its effects were reversed and the ability of the cells to internalize L. monocytogenes was restored (Fig. 1b). Adherence of S. typhimurium and L. monocytogenes to the HeLa cells was not affected by PD98059 at the concentrations used to block L. monocytogenes invasion (Fig. 1c). Adherence was assayed at 15 min postinfection for S. typhimurium and at 45 min postinfection for L. monocytogenes, at which times the numbers of internalized bacteria are relatively insignificant (data not shown).
FIG. 1.
Effect of PD98059 on Listeria and Salmonella invasion into HeLa cells. HeLa cells were pretreated for 60 min with various concentrations of PD98059 prior to bacterial infection. Appropriate amounts of the solvent DMSO were added so that all wells contained equivalent concentrations of DMSO. (a) PD98059 blocks Listeria but not Salmonella invasion into HeLa cells. L. monocytogenes 1/2a3 and S. typhimurium SL1344 were allowed to invade for 60 min and 30 min, respectively, before gentamicin was added to kill extracellular bacteria. (b) Inhibition of L. monocytogenes invasion by PD98059 is reversible. HeLa cells were incubated with 100 μM PD98059 for 60 min. The cells were then infected with L. monocytogenes 1/2a3 for 60 min before the PD98059 was washed away. At various time points after the removal of PD98059, the medium was replaced with 100 μg of gentamicin per ml in MEM to kill extracellular bacteria. (c) PD98059 does not affect adherence of Listeria or Salmonella. L. monocytogenes 1/2a3 was allowed to infect the cells for 45 min, and S. typhimurium SL1344 was allowed to infect for 15 min. Monolayers were washed three times with PBS. Adherent (and some internalized bacteria) were released by the lysis of the cells and were quantitated by plating. For each panel, experiments were repeated at least three times with similar results, and the graph depicts one representative experiment; each data point is the mean of data from three wells, and the standard deviation is shown by the error bar.
PD98059 must be present during the actual infection to inhibit invasion.
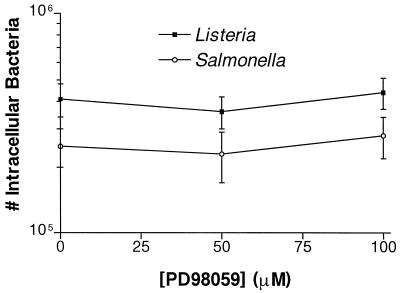
HeLa cells were also pretreated with PD98059 in the following manner. PD98059 was added 60 min prior to infection. The HeLa cells were then washed twice with PBS to remove the drug and then resuspended in plain MEM. These pretreated cells were infected with L. monocytogenes for 90 min or with S. typhimurium for 30 min. Pretreatment with PD98059 did not affect the invasion of either bacterium (data not shown). The survival of intracellular Listeria or Salmonella was also not affected by PD98059. HeLa cells were infected with L. monocytogenes for 60 min or S. typhimurium for 30 min. PD98059 was added along with 100 μg of gentamicin per ml in MEM for 2 h. The numbers of intracellular Listeria and Salmonella cells recovered from treated and untreated cells were the same (Fig. 2). In addition, PD98059 did not affect the viability of Listeria, Salmonella, or HeLa cells (data not shown) during up to a 4-h incubation with 100 μM PD98059. Bacteria pretreated for up to 2 h with 100 μM PD98059 were also unaffected in their ability to invade HeLa cells (data not shown).
FIG. 2.
PD98059 does not kill intracellular Listeria or Salmonella. HeLa cells were infected with L. monocytogenes 1/2a3 for 60 min or S. typhimurium SL1344 for 30 min. Various concentrations of PD98059 were then added along with 100 μg of gentamicin per ml in MEM for 120 min. Appropriate amounts of the solvent DMSO were also added so that all wells contained equivalent concentrations of DMSO. The monolayers were lysed, and the numbers of intracellular bacteria were determined by plating. Experiments were repeated at least three times with similar results, and the graph depicts one representative experiment. Each data point is the mean of data from three wells, and the standard deviation is shown by the error bar.
Wild-type L. monocytogenes activates ERK-1, JNK, and MAPKAP kinase-2.
We previously reported that L. monocytogenes can activate ERK-1 and ERK-2 because of the action of LLO on the host cell. Using a more sensitive in vitro kinase assay, we reexamined ERK-1 and ERK-2 activities as well as those of JNK and MAPKAP kinase-2. MAPKAP kinase-2 is activated by p38 MAP kinase (30). The various kinases were immunoprecipitated from cell lysates and then incubated with their appropriate substrates and [γ-32P]ATP: ERK-1 and -2 with MBP, JNK with c-Jun, and MAPKAP kinase-2 with Hsp25. The degree of phosphorylation of the substrates was measured to determine the relative activities of the kinases in cells that were subjected to bacterial infection. The activities of these various kinases were assayed in uninfected cells at time points from 0 to 2 h, and no changes were detected during this time period (data not shown).
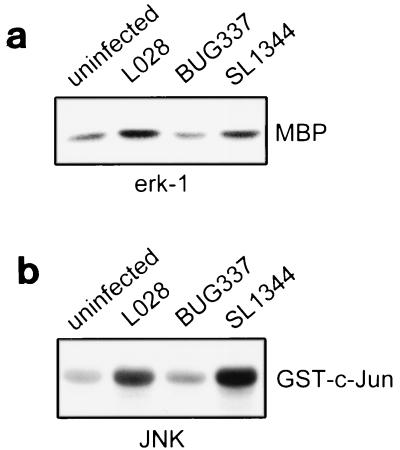
In agreement with our previous results, wild-type L. monocytogenes L028 activated ERK-1, while BUG337, a mutant with 1,000-fold-reduced hemolytic activity, did not (Fig. 3a) (33). HeLa cells were infected with Listeria for 90 min. We also confirmed previous findings that S. typhimurium activates ERK-1 (13, 29). Using the same infection times, we found that wild-type L028 also induced JNK activity, while the mutant BUG337 did not, at 90 min postinfection (Fig. 3b). S. typhimurium SL1344 also activated JNK at 45 min postinfection.
FIG. 3.
ERK-1 and JNK are activated by wild-type L. monocytogenes. HeLa cells were infected with wild-type L. monocytogenes L028 or BUG337, which carries a mutation in hly, for 90 min or were infected with S. typhimurium SL1344 for 45 min. (a) ERK-1 activity is induced by wild-type L. monocytogenes. ERK-1 activity was measured as described in Materials and Methods. The autoradiograph shows the level of phosphorylation of MBP by ERK-1. (b) JNK activity is induced by wild-type L. monocytogenes. JNK activity was measured as described in Materials and Methods. The autoradiograph shows the level of phosphorylation of GST–c-Jun by JNK. Both autoradiographs are representative of results from three experiments.
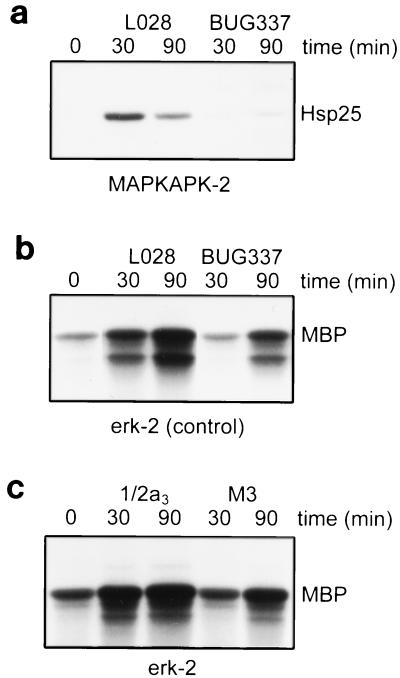
Wild-type L. monocytogenes L028 also induced MAPKAP kinase-2 activity at 30 min and at 90 min postinfection (Fig. 4a). BUG337 did not activate MAPKAP kinase-2 at 30 min postinfection but weakly activated MAPKAP kinase-2 at 90 min postinfection. The BUG337-induced activity was about 10-fold less than that induced by its parental strain, L028.
FIG. 4.
MAPKAP kinase-2 and ERK-2 are activated by wild-type and mutant L. monocytogenes. HeLa cells were infected with wild-type L. monocytogenes L028 or BUG337, which carries a mutation in hly, for 30 or 90 min. (a) MAPKAP kinase-2 activity is induced by wild-type L. monocytogenes and is only weakly induced by mutant L. monocytogenes. MAPKAP kinase-2 activity was measured as described in Materials and Methods. The autoradiograph shows the level of phosphorylation of Hsp25 by MAPKAP kinase-2. (b) ERK-2 activity is induced by wild-type and mutant L. monocytogenes. (c) ERK-2 activity is induced by wild-type and nonhemolytic L. monocytogenes. HeLa cells were infected with wild-type L. monocytogenes 1/2a3 or nonhemolytic mutant M3 for 30 or 90 min. For panels b and c, ERK-2 activity was measured as described in Materials and Methods and the autoradiographs show the level of phosphorylation of MBP by ERK-2. For all panels, the autoradiographs are representative of results from at least three experiments.
Both wild-type L. monocytogenes and the hly mutant can activate ERK-2.
The activation pattern of ERK-2 was different from that of the other kinases. As we observed before, ERK-2 was activated by wild-type L. monocytogenes L028 at 30 min and 90 min postinfection (Fig. 4b) (34). Also in agreement with our previous results, BUG337 did not activate ERK-2 at 30 min postinfection (33). However, at 90 min postinfection, ERK-2 was activated by BUG337. While BUG337 still had a slight amount of hemolytic activity (1,000-fold reduced), the same pattern of results was seen with nonhemolytic mutant M3 and its wild-type parent, 1/2a3 (Fig. 4c). M3 no longer secretes LLO because of a Tn916 transposon insertion in the hly gene.
inlAB mutants also activate ERK-2.
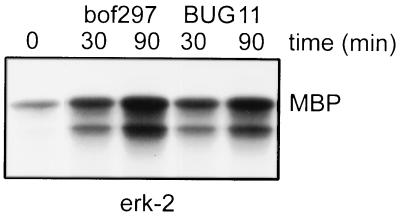
inlAB mutant BUG11 also activated ERK-2 at levels similar to that of its wild-type parent, bof297 (Fig. 5). This was expected, as BUG11 is still capable of producing LLO at the same levels as those of its parental strain. It is likely that the LLO activation of the MAP kinases, including ERK-2, masked any small differences in ERK-2 activation that might be attributed to the lack of invasion by BUG11.
FIG. 5.
ERK-2 is activated by inlAB mutants. HeLa cells were infected with wild-type L. monocytogenes bof297 or BUG11, which carries a mutation in inlAB, for 30 or 90 min. ERK-2 activity was measured as described in Materials and Methods. The autoradiograph shows the level of phosphorylation of MBP by ERK-2. This is representative of results from three experiments.
Cytochalasin D and wortmannin do not affect ERK-2 activation.
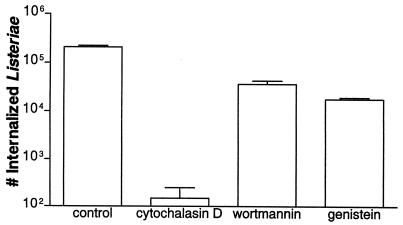
We looked at whether various inhibitors of Listeria invasion also affected ERK-2 activation in the host cells. Cytochalasin D, an inhibitor of actin polymerization, dramatically reduced L. monocytogenes invasion (11) (Fig. 6) but did not affect ERK-2 activation patterns (34) (Fig. 7a). Recently wortmannin, an inhibitor of PI 3-kinase (35), was demonstrated to inhibit L. monocytogenes invasion (18). In agreement with those results it was found that wortmannin did inhibit the uptake of Listeria by HeLa cells (Fig. 6) but that wortmannin had no effect on ERK-2 activation (Fig. 7b).
FIG. 6.
Various inhibitors reduce L. monocytogenes invasion. HeLa cells were pretreated with 1 μg of cytochalasin D per ml, 100 nM wortmannin, or 250 μM genistein for 30 min prior to bacterial infection. Control cells were treated with 0.2% DMSO in MEM. L. monocytogenes 1/2a3 was allowed to invade for 60 min before the addition of 100 μg of gentamicin per ml. The monolayers were lysed, and the numbers of intracellular bacteria were determined by plating. The assay was repeated at least three times with similar results, and the graph depicts one representative experiment. Each data point is the mean of data from three wells, and the standard deviation is shown by the error bar.
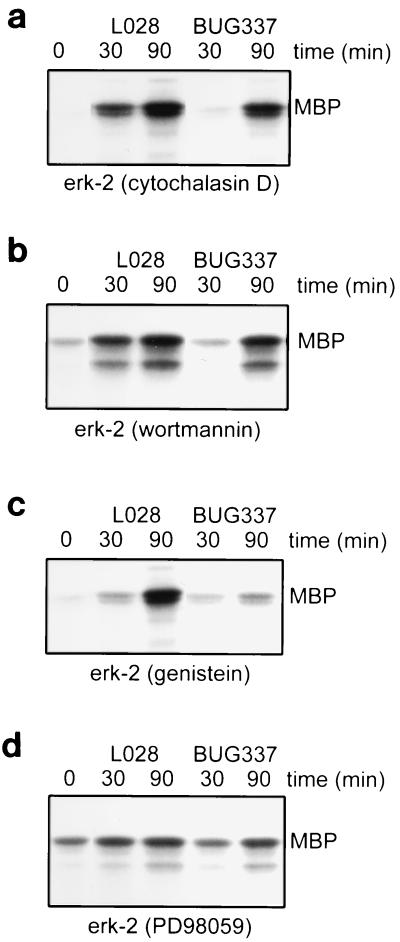
FIG. 7.
The effect of various inhibitors on ERK-2 activation. HeLa cells were pretreated with 1 μg of cytochalasin D per ml, 100 nM wortmannin, or 250 μM genistein for 30 min or with 100 μM PD98059 for 60 min prior to bacterial infection. Control cells were treated with 0.2% DMSO in MEM. ERK-2 activity was measured as described in Materials and Methods. The autoradiographs show the level of phosphorylation of MBP by ERK-2. (a) Cytochalasin D does not affect ERK-2 activation by Listeria. (b) Wortmannin does not affect ERK-2 activation by Listeria. (c) Genistein reduces ERK-2 activation by Listeria. (d) PD98059 blocks ERK-2 activation by Listeria. These autoradiographs are each representative of results from three experiments.
Genistein and PD98059 reduce ERK-2 activation.
We and others had previously found that genistein, an inhibitor of tyrosine kinases (2), blocked L. monocytogenes invasion and ERK-1 and -2 activation by L. monocytogenes (34, 36). Here, we report that genistein also reduced the ERK-2 activity induced by BUG337 (Fig. 7c). Genistein decreases ERK-2 activity due to wild-type L028 at 30 min postinfection but was not capable of blocking ERK-2 activation at 90 min. PD98059, a specific inhibitor of MEK-1 activation, was much more effective at blocking ERK-2 activation by both the wild-type L028 and the mutant BUG337, which has 1,000-fold-reduced hemolytic activity (Fig. 7d).
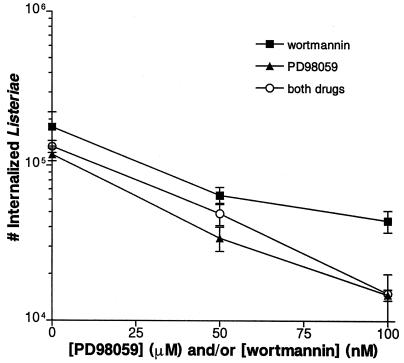
Inhibitory effects of wortmannin and PD98059 are not additive.
Since both the PI 3-kinase and the MEK-1/ERK-2 pathways were involved in Listeria invasion, we looked at the combined effects of wortmannin and PD98059. HeLa cells were pretreated with one or both drugs for 60 min prior to infection. At the concentrations used, the effect of PD98059 on Listeria invasion was stronger than that of wortmannin (Fig. 8). When the two drugs were used in combination, there was no additive effect on L. monocytogenes uptake by HeLa cells. Also, there was no effect on Salmonella invasion (data not shown).
FIG. 8.
Inhibition of L. monocytogenes invasion by PD98059 and wortmannin is not additive. HeLa cells were pretreated with various concentrations of PD98059, wortmannin, or both drugs for 60 min prior to bacterial infection. Appropriate amounts of the solvent DMSO were also added so that all wells contained equivalent concentrations of DMSO. Cells were infected with L. monocytogenes 1/2a3 for 60 min. This experiment was repeated at least three times with similar results, and the graph depicts one representative experiment. Each data point is the mean of data from three wells, and the standard deviation is shown by the error bar.
DISCUSSION
Using PD98059, a highly specific inhibitor of MEK-1 activation, we were able to demonstrate for the first time the requirement of the MEK-1 signalling pathway for the uptake of a bacterium by host epithelial cells. Cells treated with PD98059 were still capable of internalizing S. typhimurium, indicating that PD98059 shuts down a cellular process specific for Listeria invasion but not for Salmonella invasion. PD98059 had no effect on the adherence of these two bacteria to host cells. If the drug was removed, the adherent Listeria then invaded the cells. To exert its effect on Listeria invasion, PD98059 must be present during the infection process. If the PD98059 was washed away prior to infection with L. monocytogenes, there was no effect on invasion. These results show the reversibility of the effect of PD98059. In addition, PD98059 did not affect Listeria or Salmonella cells that had already been internalized and did not alter the viability of Listeria, Salmonella, or HeLa cells at the concentrations and treatment times used in this study.
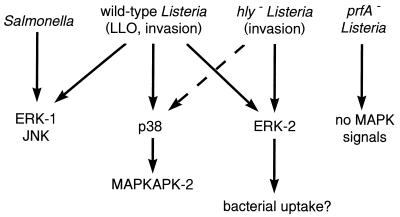
At the concentrations used, PD98059 has a weaker inhibitory effect on MEK-2; thus, it is possible that a low level of MEK-2 inhibition may also play a role in blocking invasion. However, MEK-1 and MEK-2 have the same downstream targets, ERK-1 and ERK-2 (39). With these inhibition results, we investigated which MAP kinase cascades were activated during L. monocytogenes invasion. Wild-type L. monocytogenes activated all four of the MAP kinase pathways we tested: those of ERK-1, ERK-2, JNK, and p38 MAP kinase (p38 MAP kinase activity was determined by assaying MAPKAP kinase-2, which is the downstream target of p38) (Fig. 9). More importantly, we wanted to identify pathways which were independent of LLO since LLO is not required for host cell invasion but can induce signal transduction in the host cell. A mutant with 1,000-fold-reduced hemolytic activity was still able to induce ERK-2 activity and weakly trigger MAPKAP kinase-2 at 90 min postinfection but did not activate ERK-1 or JNK. In our previous study, we failed to detect ERK-2 activation by nonhemolytic L. monocytogenes since we only looked for activation at 30 min postinfection (33). At 30 min postinfection, only a few of the bacteria are invading so any changes in host cell signal transduction are likely due to secreted factors such as LLO. However, at 90 min postinfection, the bacteria invade in greater numbers, thus allowing the detection of invasion-related signals in the host cell. Our finding that ERK-2 is activated by the invasion of wild-type and nonhemolytic L. monocytogenes suggests that the activation of ERK-2 is not due to the action of LLO but instead is part of the signalling required for uptake of the bacteria. This is consistent with the requirement for MEK-1, which is the upstream activator of ERK-2.
FIG. 9.
Summary of in vitro kinase assay results.
Whether these signals are induced by internalin, InlB, or another factor remains to be determined. Using inlAB mutants, we found ERK-2 activation by the noninvasive mutants to be similar to that by wild-type L. monocytogenes. These results suggest that the LLO produced by both the mutant and parent is activating ERK-2 as previously reported (33, 38). This activation may be masking any differences in ERK-2 activation that may be attributed to inlAB. Alternatively, other bacterial factors may be involved in the LLO-independent activation of ERK-2. In addition, other host cell factors may also be involved. The minor activation of MAPKAP kinase-2 may also be specific to invasion, but the selective inhibition of the p38 MAP kinase pathway will be required to address this issue. Activation of ERK-1 and JNK by S. typhimurium may represent a host cell stress response during Salmonella infection. The invasion mechanisms of Salmonella and Listeria are quite different, most notably in that Salmonella causes marked membrane ruffling. More work is required to fully characterize MAP kinase involvement in S. typhimurium invasion and the bacterial factors involved.
Various inhibitors were also tested for their effects on the ERK-2 activation. Cytochalasin D, which disrupts actin polymerization and inhibits invasion, did not block ERK-2 activation by L. monocytogenes. This indicates that the bacteria induced this signal while still extracellular. Wortmannin is an inhibitor of PI 3-kinase, which is required for L. monocytogenes invasion. Treatment of cells with wortmannin did not affect ERK-2 activation by L. monocytogenes. Thus, the ERK-2 activation is not downstream of PI 3-kinase activation. One possibility is that ERK-2 and PI 3-kinase are associated with two separate pathways required for triggering L. monocytogenes uptake by host cells. Also, inhibition of both pathways with wortmannin and PD98059 did not lead to an additive effect on L. monocytogenes internalization. These two pathways may eventually converge to initiate the cytoskeletal rearrangements necessary for L. monocytogenes uptake.
Genistein, an inhibitor of tyrosine kinases, blocks L. monocytogenes invasion and ERK-1 and -2 activation by L. monocytogenes (34, 36). We confirmed our previous findings and also found that genistein blocked ERK-2 activation by mutant L. monocytogenes with reduced hemolytic activity. Genistein decreased ERK-2 activity due to wild-type L. monocytogenes at 30 min postinfection but was not capable of blocking ERK-2 activation at 90 min. It is possible that the dual stimulatory effects of invasion and LLO production by wild-type L. monocytogenes on ERK-2 were too strong for inhibition by genistein. PD98059, which is a specific inhibitor of MEK-1 activation, was more effective at blocking ERK-2 activation by both wild-type L. monocytogenes and the mutant with reduced hemolytic activity. These results again suggest that ERK-2 activation is required for signalling L. monocytogenes uptake in the host epithelial cell.
The ERK MAP kinases have been implicated in regulation of the cytoskeleton in various systems. The high proportion of ERKs that colocalize in the cytoskeleton in activated cells suggests that they play a role in cytoskeletal reorganization (27). Some of the targets for MAP kinases include proteins associated with microfilament organization such as tau and caldesmon (1, 25). Endothelial cell motility requires phospholipase A2, which is activated by the ERK-2 pathway (31). The MEK-1/ERK pathway is required for immunoglobulin G-mediated phagocytosis by polymorphonuclear leucocytes (32).
The overall mechanism for Listeria invasion is likely to involve multiple converging signal transduction pathways in the host cell. As we are beginning to discover with many other cellular processes, the summation and integration of various stimulatory and inhibitory signals are required to induce the final event in the cell. This is especially true of the MAP kinases, where different combinations of ERK, JNK, and p38 MAP kinase activity lead to different actions by the cell (28). The same may apply in the case of L. monocytogenes internalization by the host cell: the PI 3-kinase and the MEK-1/ERK-2 pathways may form only two components of the signal transduction system required for uptake of the bacteria.
ACKNOWLEDGMENTS
We thank Steven Pelech (Kinetek Biotechnology Corp.) for his many invaluable suggestions.
P.T. was supported by a studentship from the Medical Research Council of Canada. This work was supported by a Howard Hughes International Scholar Award to B.B.F.
REFERENCES
- 1.Adam L P, Hathaway D R. Identification of mitogen-activated protein kinase phosphorylation sequences in mammalian h-Caldesmon. FEBS Lett. 1993;322:56–60. doi: 10.1016/0014-5793(93)81110-l. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 3.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Pouyssegur J. Identification of MAP kinase domains by redirecting stress signals into growth factor responses. Science. 1996;272:1652–1655. doi: 10.1126/science.272.5268.1652. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter C L, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 6.Cheng M, Boulton T G, Cobb M H. ERK3 is a constitutively nuclear protein kinase. J Biol Chem. 1996;271:8951–8958. doi: 10.1074/jbc.271.15.8951. [DOI] [PubMed] [Google Scholar]
- 7.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 8.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 9.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard J L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galan J E, Pace J, Hayman M J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992;357:588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- 14.Gellin B G, Broome C V. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 15.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 18.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 19.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn M, Kathariou S, Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988;56:79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 22.Lechner C, Zahalka M A, Giot J F, Moller N P, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 24.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 25.Pelech S L. Networking with proline-directed protein kinases implicated in tau phosphorylation. Neurobiol Aging. 1995;16:247–256. doi: 10.1016/0197-4580(94)00187-6. . (Discussion, 16:257–261.) [DOI] [PubMed] [Google Scholar]
- 26.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reszka A A, Seger R, Diltz C D, Krebs E G, Fischer E H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 29.Rosenshine I, Ruschkowski S, Foubister V, Finlay B B. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect Immun. 1994;62:4969–4974. doi: 10.1128/iai.62.11.4969-4974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 31.Sa G, Murugesan G, Jaye M, Ivashchenko Y, Fox P L. Activation of cytosolic phospholipase A2 by basic fibroblast growth factor via a p42 mitogen-activated protein kinase-dependent phosphorylation pathway in endothelial cells. J Biol Chem. 1995;270:2360–2366. doi: 10.1074/jbc.270.5.2360. [DOI] [PubMed] [Google Scholar]
- 32.Suchard S J, Mansfield P J, Boxer L A, Shayman J A. Mitogen-activated protein kinase activation during IgG-dependent phagocytosis in human neutrophils: inhibition by ceramide. J Immunol. 1997;158:4961–4967. [PubMed] [Google Scholar]
- 33.Tang P, Rosenshine I, Cossart P, Finlay B B. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 36.Velge P, Bottreau E, Kaeffer B, Yurdusev N, Pardon P, Van Langendonck N. Protein tyrosine kinase inhibitors block the entries of Listeria monocytogenes and Listeria ivanovii into epithelial cells. Microb Pathog. 1994;17:37–50. doi: 10.1006/mpat.1994.1050. [DOI] [PubMed] [Google Scholar]
- 37.Waskiewicz A J, Cooper J A. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 38.Weiglein I, Goebel W, Troppmair J, Rapp U R, Demuth A, Kuhn M. Listeria monocytogenes infection of HeLa cells results in listeriolysin O-mediated transient activation of the Raf-MEK-MAP kinase pathway. FEMS Microbiol Lett. 1997;148:189–195. doi: 10.1111/j.1574-6968.1997.tb10287.x. [DOI] [PubMed] [Google Scholar]
- 39.Zheng C F, Guan K L. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993;268:23933–23939. [PubMed] [Google Scholar]
- 40.Zhou G, Bao Z Q, Dixon J E. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]