Abstract
We present BATH, a tool for highly sensitive annotation of protein-coding DNA based on direct alignment of that DNA to a database of protein sequences or profile hidden Markov models (pHMMs). BATH is built on top of the HMMER3 code base, and simplifies the annotation workflow for pHMM-based annotation by providing a straightforward input interface and easy-to-interpret output. BATH also introduces novel frameshift-aware algorithms to detect frameshift-inducing nucleotide insertions and deletions (indels). BATH matches the accuracy of HMMER3 for annotation of sequences containing no errors, and produces superior accuracy to all tested tools for annotation of sequences containing nucleotide indels. These results suggest that BATH should be used when high annotation sensitivity is required, particularly when frameshift errors are expected to interrupt protein-coding regions, as is true with long read sequencing data and in the context of pseudogenes.
Keywords: Sequence Alignment, Genome Annotation, Profile Hidden Markov Models, Frameshift Mutations
Introduction
The process of annotating the protein-coding DNA within sequenced genomes by comparison to a database of known protein sequences is called translated search (1, 2). Labeling of genomic sequences by comparison to proteins is generally more sensitive than comparison to the DNA that encodes those proteins (DNA-to-DNA search), due to the additional information captured in the larger amino acid alphabet and the ability to distinguish between synonymous and non-synonymous substitutions (3, 4).
A common strategy in translated search is to translate genomic open reading frames (ORFs) into putative peptides across all 6 frames, then to compare each sufficiently-long peptide to a database of previously annotated protein sequences (5, 6). We will call this approach “standard” transition. The task of translating an ORF to the encoded protein can be performed within the annotation tool (as in tblastn (7)), or by a separate preprocessing tool that may extend the ORF selection process beyond simple length filtering (such as a do novo gene prediction (8) or special handling of short ORFs (9)).
Profile hidden Markov models.
Recent years have seen tremendous gains in the speed of sequence annotation, particularly with MMseqs2 (10) and DIAMOND (11), but state of the art for maximum sensitivity continues to be observed by using profile hidden Markov models (pHMMs (12, 13)) with full Forward scores (see (10, 14) and Fig 3). These probabilistic models of sequence homology yield substantially higher sensitivity in sequence annotation (15), and serve as the basis of a wide range of annotation pipelines (10, 16–18) and sequence-family databases (19–21). Profile HMM sensitivity gains are of paramount importance, particularly in the context of microbial community datasets, where annotation efforts often fail to identify large fractions (and in many cases, the majority) of putative proteins (22–24).
Fig. 3.
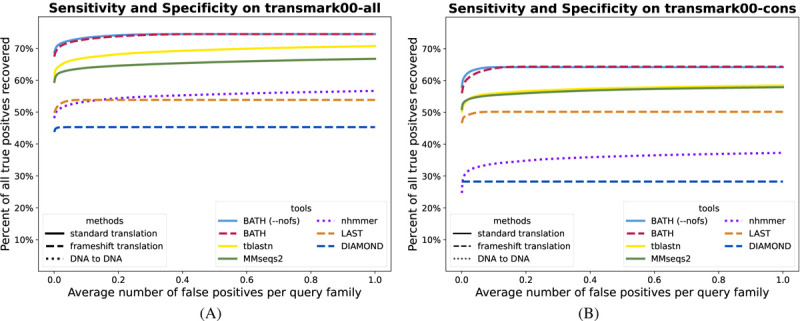
ROC plots showing sensitivity (true positives) vs specificity (false positives) for all tested tools on a translated search benchmark using either (A) 10 – 30 query sequences per family (B) a single query sequence per family. These benchmarks contain no simulated frameshifts.
The classic tool for pHMM-based sequence annotation, HMMER3 (14), does not provide direct translated search functionality. To perform a translated search using HMMER, the user must first produce a collection of ORFs, and translate them into their encoded peptides. These candidate peptides can then be compared to the user-provided protein database using HMMER’s protein-protein search tools. Positional bookkeeping and E-value adjustment requires additional post-processing by the user.
Here, we introduce a new tool for translated pHMM search, BATH, which fills the gap left by available tools. BATH is built on top of the HMMER3 code base, and its core functionality is to provide full HMMER3 sensitivity with automatic management of 6-frame codon translation, positional bookkeeping, and E-value computation. Importantly, BATH also explicitly models nucleotide insertions or deletions (indels) that can introduce shifts in the reading frame of a protein-coding region.
Frameshifts.
A key challenge in translated search is the presence in the genomic sequence of nucleotide indels that lead to shifts in reading frame. These may represent errors during sequencing (most commonly in homopolymer regions), true mutations as in pseudogenes, or instances of programmed ribosomal frameshifting (25).
Frameshifts in protein-coding DNA lead peptide translation software using standard translation to predict fragmented peptides, which in turn may reduce the ability to identify a full-length protein, or even to annotate the protein at all. Frameshifts due to sequencing error are of concern in the context of modern long read sequencing, where indels remain a challenge (26, 27). This is particularly true for metagenomic datasets, in which low read depth for low abundance taxa (28, 29), or high population diversity as in viral genomes (30), leaves little opportunity for error correction. For example, Sheetlin et al (31) showed that the majority of metagenomic reads from a polluted soil sample contain frameshifts.
The negative effect of frameshift errors on translated alignment has been a topic of interest for over 30 years (32, 33). One way to overcome frameshift concerns is to employ a de novo gene finding tool that models and corrects frameshift mismatches (34, 35). An example of this approach is FragGeneScan (34), which corrects for frameshift errors while predicting ORFs in DNA sequence and is currently utilized in the MGnify pipeline (36). In principle, by correcting sequencing errors prior to ORF calls, FragGeneScan should enable annotation of some reads that are not annotated using naive ORF calls. But if error correction is overly aggressive, it can introduce new errors, causing other sequences to escape annotation. Zhang and Sun (37) showed that this concern is legitimate, and that FragGeneScan induces more errors than it fixes, leading to reduced downstream sequence homology sensitivity.
An alternative strategy is to perform homology search directly on the DNA, avoiding the calling of ORFs and explicitly modeling frameshifts. In effect, this uses homology to guide ORF prediction, which in turn leads to better homology detection. We refer to this strategy as “frameshiftaware” (FA) translated alignment. Instead of translating the target DNA into proteins and aligning amino acids to amino acids, this approach computes an alignment by comparing the amino acids of the query directly to the target DNA, assigning scores for alignment between an amino acid and a codon based on the scoring model’s value for the amino acid encoded by that codon. Under this framework, frameshifts can be addressed with the addition of what Pearson et al (38) called “quasicodons”. Rather than limiting aligned codons to the standard length of three nucleotides, Pearson’s quasicodons could be 2 or 4 nucleotides long; other implementations (39) of this concept also include quasi-codons with lengths 1 and 5 nucleotides.
Variations on the FA approach have been implemented within both scoring-matrix based tools LAST (40) and DIAMOND (11), as well as by HMM-based tools (37, 39, 41). Of these, only DIAMOND and LAST produce E-value computations to enable evaluation of the statistical significance of their annotations, and the HMM-based approaches are all orders of magnitude slower than HMMER3. None of these methods provides full profile HMM sensitivity.
To fill this hole in the landscape of annotation software, we developed BATH. The BATH pipeline utilizes the fast filtering and accurate E-value estimates of HMMER3 to perform a combination of standard and frameshift-aware translation that maximizes the advantages of each approach while minimizing the disadvantages. BATH’s software package includes the alignment search tool and several helper tools for creating and manipulating pHMM files and is available on GitHub at https://github.com/TravisWheelerLab/BATH.
Methods
BATH is implemented on top of the HMMER3 code base, merging aspects of hmmsearch and nhmmer pipelines with modifications specific to translated (and frameshift-aware) search. The primary tool is bathsearch, which requires two inputs – a protein query and a DNA target. The DNA target is simply a sequence file. The query may be provided as a BATH-formatted pHMM file containing one or more pHMMs, either created from a file containing one or more multiple sequence alignments (MSAs) using the tool bathbuild, or converted from a HMMER-formatted pHMM file using the tool bathconvert. Alternatively, the user can simply supply a file containing MSAs or independent sequences as the query input to bathsearch, which will then build the requisite model on the fly.
BATH then implements a HMMER3-like accelerated analysis pipeline (14):
Identify ORFs within the target DNA, and convert them to peptides using standard translation. By default, all 6 frames are considered, and peptides of length ≥ 20 are retained.
Apply HMMER3’s MSV filter to comparisons between target peptides and the set of query proteins. This computes an un-gapped alignment between each query-target pair, and retains only those pairs with a score corresponding to a P-value of 0.02 (theoretically removing 98% of unrelated sequence pairings from further consideration).
Apply HMMER3’s Viterbi filter to remaining peptide-to-protein pairs. This computes a maximum-scoring gapped alignment for each query-target pair, then retains only those pairs with a score corresponding to a P-value of 0.001 (theoretically removing 99.9% of unrelated sequence pairings from further consideration).
For each unfiltered match from the Viterbi stage, select a window of genomic DNA around the surviving peptide. The length L of the window is such that only 1e-7 of sequences emitted by the query pHMM are expected to exceed length L (as in nhmmer (17)).
Compute a protein-to-protein Forward score, following the implementation in HMMER3’s hmmsearch Forward filter implementation. The score of the resulting alignment is converted to a P-value (see below), and this P-value is captured as F0.
If F0 > 1e−5 and F1 > 1e−5, then filter the pair.
For any unfiltered pair, if F0 ≤ F1, then follow a non-frameshifted (HMMER3-style) post-processing pipeline to compute protein-protein alignment boundaries, alignments, E-values, etc. Otherwise, follow an FA variant of each post-processing step, in which amino acid positions of the query are aligned to (quasi-)codons in the target.
Compute E-values, produce translated search output that provides necessary bookkeeping information.
Frameshift-aware model.
The protein pHMM shown in Figure 1A is based on the model used in HMMER3 (14). A protein pHMM consists of transition probabilities between states (shown as arrows), emission probabilities for each amino acid within the Match (M) states (single green circles), and emission probabilities for each amino acid in the Insert states (a single grey circle). To understand how bathsearch converts a protein pHMM to an FA codon pHMM it is helpful to consider the construction of an intermediate non-FA codon model 1B. The emission probability for a codon in a match state is set to that of its encoded amino acid, under a given codon translation table. This effectively collapses all synonymous codons down to a single class, yielding probabilities that are equivalent to the protein pHMM. In the codon model, the Insert (I) states are altered so that they insert three nucleotides instead of one amino acid, but the transition probabilities that govern indel likelihood do not change.
Fig. 1.
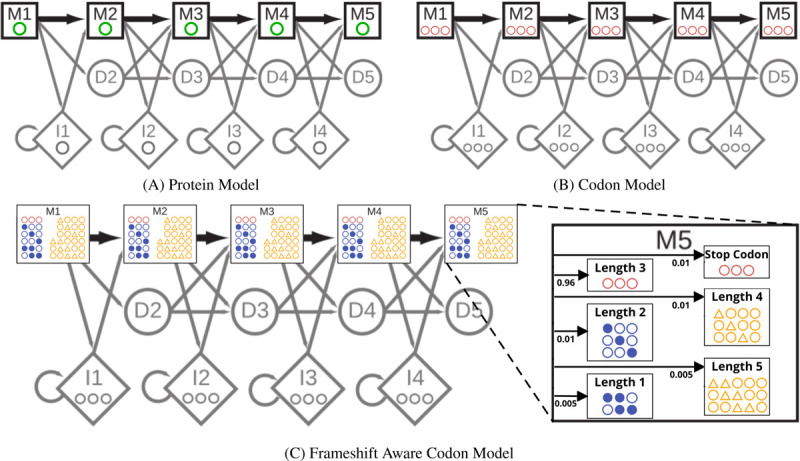
Comparison between the core model states of (A) a protein pHMM with each match state emitting a single amino acid (green), (B) a non-frameshift aware (non-FA) codon pHMM with each match state emitting a 3-nucleotide codon (red), and (C) a frameshift aware (FA) codon pHMM with each match state emitting a quasi-codon with length ranging from 1 to 5 (yellow). The protein model (A) is representative of the one used in HMMER3. The (non-FA) codon model is only an intermediary step in converting the protein model into the FA codon model in (C) which is the pHMM used by bathsearch’s FA algorithms.
To convert the codon pHMM to a FA codon pHMM (Figure 1C) additional emission probabilities need to be determined for both quasi-codons and stop codons. To identify stop codons that may arise as the result of nucleotide substitutions, bathsearch precomputes a per-state probability of emitting each stop codon s: for match state Mi, and for each s, the codon c with the highest probability at Mi is identified among those that are at most one substitution from s, and the probability of s at Mi is set to that of c, multiplied by a stop-codon penalty (default 0.01).
Bathsearch allows quasi-codons of length 1, 2, 4, & 5 and assigns their emission probabilities using a similar procedure to the one it uses for stop codons. For each possible quasi-codon q, bathsearch considers the full set of codons C that could be made from q by either removing the extra nucleotides or adding back in the missing ones. From this set, bathsearch assigns q to the codon c ∈ C (and to its amino acid translation a) with the highest emission probability at each Mi state, and sets the emission probability of q to that of its assigned codon (which is the same as that of a from the original protein pHMM) multiplied by a frameshift penalty (default 0.01 for length 2 or 4 quasi-codons and 0.005 for length 1 or 5 quasi-codons). The full set of emissions probabilities is then normalized (all codon emissions probabilities (except for stop codon) are multiplied by 1 minus the sum of all stop codon and quasi-codon penalties.
For both stop codons and quasi-codons, the final emissions probabilities are dominated by the penalties, but small differences caused by the mapped amino acid probabilities support reasonable choices of frameshift placement in case of ambiguity. The penalties, and the normalization of the non-penalized codons, can also be thought of as another set of transition probabilities inside each match state, as illustrated in the magnified M5 state of Figure 1C. These transition probabilities serve to model the more random distribution of mutations that arise from sequencing errors or relaxed selective pressures, whereas the amino acid derived emissions probabilities and the unchanged transition probabilities between the states maintain the specific distribution of mutations seen within the query family and among related protein sequences at large. The FA codon model also differs from the HMMER3 protein model in the so-called “special states” outside the core model, see Supplementary Figure S1 for details.
Avoiding redundant computation.
Figure 2 shows a simplified representation of the alignment paths available to a protein alignment model and the FA codon model. The corresponding dynamic programming recurrence for the FA Forward recurrence contains more than 3.6x the calculations of the standard protein Forward recurrence due to the additional transition options and large number of new potential emissions. Straightforward implementation leads to excessive memory requirements and run time, as reported with the heuristic implementation in (37). This is also true of algorithms downstream in the pipeline (e.g. Backward and posterior decoding). BATH reduces the impact of this extra complexity by only using the full set of FA algorithms on target-query pairs that (according to the P-value comparison) are more probable under an FA model of homology. Even so, straightforward implementation of the full FA model is slow. Fortunately, many of the expensive calculations are repeated identically in consecutive passes through the recurrence, so that it is possible to amortize the cost of the calculation by memoizing values after their first calculation, and re-using those values until they are no longer needed (see pseudocode in Supplementary Figure S2). This reduces the number of calculations to just 1.6x that of the standard protein pHMM Forward recurrence.
Fig. 2.
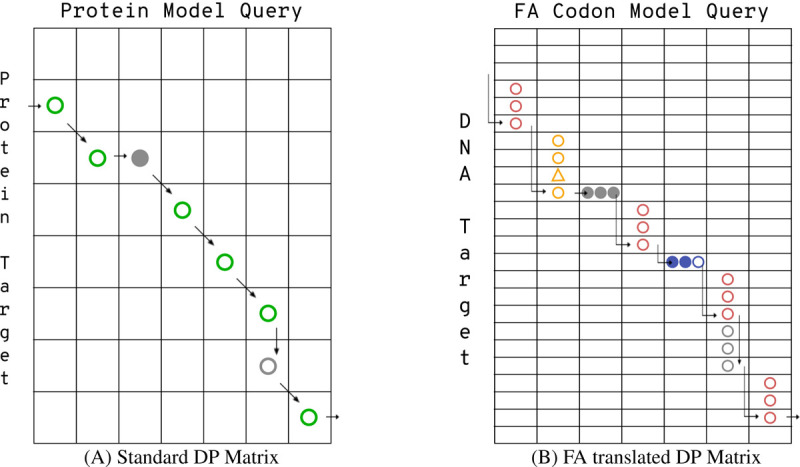
Comparison of toy DP matrices for (A) a protein alignment and (B) a frameshift-aware (FA) codon alignment. Profile HMM alignments are made up of pairings between states in the query model and residues in the target. For a protein-to-protein alignment (A), there are only three types of pairings: a match state to an amino acid (shown as a green circle) a delete state to a gap (filled grey circle), or an insert state to an amino acid (open grey circle). A codon alignment (B) has an analogous set of pairings: a match state to a codon (shown as three pink circles), a delete state to a gap (three filled grey circles), or an insert state to a codon (three open gray circles). BATH’s FA alignment also includes pairings between match states and quasi-codons (yellow circles and triangles for quasi-codons with insertions or blue filled and open circles for quasi-codons with deletions).
Post-processing and E-value calculation.
Once the target-query pair has been fully analyzed, bathsearch reports a final alignment, score, and E-value for each match that passes a user-provided E-value cutoff. Each bathsearch alignment shows the original target DNA split into (quasi-)codons, the amino acid translation of those codons, and the consensus sequence of the query protein pHMM. The final score S is computed in a second run of the appropriate Forward algorithm after alignment boundaries have been identified (removing non-homologous prefix and suffix regions of the target). In computing the P-value of a hit, scores are assumed to follow an exponential distribution, and parameters of the exponential are fit as described in (16). In HMMER, an E-value (indicating the expected number of false positives at a threshold of score S) is computed as a Bonferroni correction of the P-value, multiplying the P-value by the number of tested pairs for a given query. When aligning to long chromosomes, there are many overlapping candidates for alignment; in HMMER3’s DNA-to-DNA search tool, nhmmer (17), the number of tested pairs is determined by first identifying value L such that only 1e-7 sequences emitted by the query model will exceed length L, then dividing the length of the target DNA (x2 if searching both strands) by L. BATH does essentially the same thing, except it multiplies the length of the target DNA an additional x3 to count each possible translation frame.
Construction of benchmark.
To evaluate BATH in the context of other tools, we developed a benchmark that is derivative of the profmark benchmark used to evaluate HMMER in (14). The new benchmark, which we call transmark, was initiated with all the protein seed alignments from Pfam v.27 (42). Each protein sequence in those alignments was mapped to the encoding DNA using the UniProtKB IDMAPPING resource (43). As a result, transmark contains both the protein and the DNA multiple sequence alignments (MSAs) for the Pfam families. Though several dozen individual sequences could not be mapped to their source DNA (apparently due to naming error or a mismatch in notated position), DNA source sequences could be located for at least one sequence in 14,724 of the 14,831 protein families in Pfam v.27.
Since most translated alignment software, including bathsearch, assumes a single NCBI codon translation Table (44) for all sequences in the target database, we eliminated all the DNA sequences that could not be correctly translated using the standard translation table (i.e. the translation did not match the Pfam protein sequence), leaving 14,569 families with at least on representative sequence. Each remaining family was then divided into a test set and a train set such that no sequence in the test set was more than 60% identical to any sequence in the train set (at the DNA level), no sequence in the test set was more than 70% identical to any other sequence in the test set, and both sets had at least ten DNA sequences. A final list of 2,673 families (202,837 test sequences and 97,497 training sequences) could be split to meet these criteria; these form the set of families and sequences used to construct a transmark benchmark.
The transmark benchmarks used here were built by selecting 1,500 of the 2,673 families at random and limiting each of those families to a maximum of 30 train sequences and 20 test sequences. The train sets were stored as MSAs (both as DNA and as proteins) to be used as queries, and the test set DNA sequences were embedded into 10 pseudo-chromosomes, of length 100MB each, to be used as targets. These pseudo-chromosomes were simulated using a 15-state HMM that was trained on real genomic sequences selected from archaeal, bacterial, and eukaryotic genomes, as in (17, 45).
In addition to 28,369 embedded test sequences, each transmark benchmark used here also embeds an additional set of 50,000 “decoy” protein-coding open reading frames into the pseudo-chromosomes. Each decoy was produced by sampling protein sequences from Pfam v.27, shuffling the amino acids to eliminate any true homology, and then using a codon lookup table to reverse translate the amino acids into DNA.
For frameshift tests, transmark benchmarks were also created with simulated indels in the embedded test sequences. Nucleotide indels were added to each test sequence at a parameterized frequency, prior to embedding into the pseudo-chromosomes. For example, at test-specific frequency of 0.02, each nucleotide position in the test sequence will serve as the starting point of an indel with 2% probability, with an equal probability of being an insertion or a deletion. An initiated indel will extend with 50%. Thus a r = 2% indel rate would result in an indel roughly every 17 codons, with ~85.7% of those indels being not a multiple of 3 and therefore resulting in a frameshift.
Results
To investigate the utility of BATH, we evaluated it alongside other tools for annotating protein-coding DNA. Evaluations include measurement of sensitivity when seeking true protein-coding DNA embedded within a simulated ‘genomic’ background, assessment of sensitivity in the presence of simulated frameshifts, analysis of alignment coverage and risk of alignment over-extension, and application to real bacterial sequence containing pseudogenized proteins.
Benchmark overview.
Though most evaluated tools follow a generally similar strategy involving sequence alignment of protein query to target genomic DNA, differences in implementation details lead to differences in performance, both speed and accuracy. All evaluated tools accelerate search by quickly filtering away regions deemed unlikely to contain homology, leaving only potentially homologous regions, called seeds. These seed-selection strategies may be overly restrictive, limiting the final set of matches while achieving their aim of increasing speed. Meanwhile, some of the evaluated tools are designed to produce a profile (MMseqs2) or profile hidden Markov models (pHMMs: nhmmer, BATH) based on the available per-family multiple sequence alignments (MSAs). Profiles and pHMMs capture position-specific character frequency, and provide expected gains in sensitivity. Additionally, different frameshift models (or lack thereof) will yield different performance on sequences containing frameshifts, and other implementation details may impact the length of identified matches. We developed a series of benchmarks to explore these outcomes.
The first simulated benchmark for our analyses, transmark00 was built as described in the Methods section as a translated search benchmark. It contains 28,369 sequences in the target set and 30,979 sequences in the query set from 1,500 Pfam families. The target DNA sequences, along with 50,000 decoy ORFs, were embedded in simulated chromosomes; insertion positions were recorded, and query sequences were retained as MSAs in both DNA and amino acid alphabets. The ‘00’ in transmark00 alludes to the fact that the simulated frameshift insertion rate is 0% (there are no injected indels.
Some of the evaluated tools (BATH, MMseqs2, and nhmmer) are designed to produce profiles/pHMMs based on the available per-family MSAs, and then to perform sequence annotation with those profiles/pHMMs; other tools (tblastn, LAST, and DIAMOND) implement only straightforward sequence-to-sequence annotation. To accommodate these differences we created two variants of the transmark00 benchmark. For the first variant, each tool is given the full contingent of sequences from each family as query input; tools that can produce and use a profile or pHMM are allowed to do so, while other tools perform search with each family member in turn, adjudicating between competing matches based on best E-value. The one-search-per-sequence strategy is referred to as family pairwise search (46). To explore performance when per-family information is limited, a variant of the same analysis was performed using only a single query sequence per protein family (using the consensus sequence derived from the query MSAs). These variants of transmark00, with the full set of each family’s query sequences or with just a single consensus sequence per family, are called transmark00-all and transmark00-cons respectively.
Tools.
Analysis includes tools that are likely to be applied in current analytical pipelines for sensitive annotation of protein-coding DNA. We omit read mappers (47) as these are designed for small levels of divergence, and also ignore older tools (37, 38, 41) that are very slow, under-maintained, and are outperformed by other presented tools in our experience. For all tools, default parameters were used except where noted. E-value thresholds were set to 100 to enable analysis of tradeoffs between recall and false discovery. We include two variants of our tool:
BATH is capable of utilizing a provided protein MSA to produce a pHMM and uses that pHMM to label target DNA sequence. It may alternatively label DNA with a single protein sequence. BATH implements a frameshift model.
BATH --nofs is identical to BATH, except that the frameshift model is disabled. The results from this variant are essentially the same as those produced by translating the target genomic sequence into all peptides of length ≥ 20, then running HMMER3’s hmmsearch or phmmer tool (except that BATH provides positional and E-value bookkeeping).
The following tools also perform protein-to-DNA alignment. The final three tools are all faster than tblastn, and self-report BLAST-level sensitivity (at least in sensitive settings):
tblastn is the relevant translated search tool from BLAST (7), and performs sequence-to-sequence comparison. This approach is not frameshift aware.
LAST (31) produces frameshift-aware alignments based on sequence-to-sequence comparison. As instructed in the userguide, we used last-train to create a separate codon and frameshift scoring scheme for each benchmark.
DIAMOND (11) produces frameshift-aware alignments based on sequence-to-sequence protein-to-DNA comparison. Because our primary focus is on accuracy (rather than speed), DIAMOND was run with ‘-F 15 –ultra-sensitive’.
MMseqs2 (10) can produce a profile from a family MSA. This enables family-to-sequence alignment with family-specific per-position scores (though note that MMseqs2 does not implement the Forward algorithm that provides much of the sensitivity seen in HMMER3 (14)). MMseqs2 is not frameshift aware.
Finally, we included one tool for DNA-to-DNA alignment, to evaluate the accuracy of searching protein-coding DNA with the DNA that encodes query proteins as opposed to the translated alignment approaches used by the other tools.
nhmmer (17) performs DNA-to-DNA alignment, and can construct a pHMM from a family MSA if one is available. In general, this is expected to perform with less sensitivity than a method based on amino-acid to codon alignment, but it will also be less impacted by frameshift-inducing indels, since there is no ‘frame’ to shift at the nucleotide level. Other DNA-to-DNA tools were evaluated, but are omitted for clarity since nhmmer was the most sensitive of the group (in agreement with (17, 48)).
Recovery of protein-coding DNA planted in simulated genomes.
Using the transmark00 benchmarks (all and cons) described above, we evaluated each tool’s ability to recover true positives while avoiding false positives. A true positive is defined as a hit that covers > 50% of an embedded target sequence that belongs to the same family as the query. A false positive is defined as a hit where > 50% of the alignment is to either an embedded decoy or the simulated chromosome. Hits are ignored when > 50% of the alignment is between a query from one family and an embedded test sequence from a different family, as it is not possible to discern whether true homology exists between these two instances.
Figure 3 shows that BATH (using pHMM search) identifies a larger fraction of the planted family instances, at any false positive rate, than other tools. In principle, the frameshift model of BATH could cause an increase in the score of decoys, leading to an increase in false positives; in practice, this seems not to be a concern, with the default (frameshift aware) variant producing nearly identical results to the --nofs variant. Before the first false positive, MMseqs2 (pHMM) and tblastn (family pairwise) show similar recall, but tblastn shows additional sensitivity gain with minor increases in false discovery rate. Other tools demonstrate poorer sensitivity. Despite reports of blast-like sensitivity, DIAMOND (ultrasensitive) shows much lower sensitivity in our test; surprisingly, sensitivity on this frameshift-free benchmark is improved when DIAMOND’s frameshift mode (-F) is enabled – we suspect that this is because that mode leads to slightly different parameterization of the early filter stages, which appear to be responsible for substantial reduction in sensitivity. Notably, despite ignorance of the protein-coding signal of the query, nhmmer shows sensitivity that is competitive with some of the translated alignment tools.
The results in Figure 3B for transmark00-cons show that BATH still outperforms the other tools even when there is only a single sequence available per query family. The relative results are similar to those seen in family pairwise search. In this single-sequence search paradigm, performance of MMseqs2 and LAST improves relative to tblastn, while nhmmer and DIAMOND see a relative degradation in performance.
The analysis shown here diverges from a recent trend of reporting recall before the first false positive (RBFFP, as in (10, 11)). We believe that reporting only RBFFP provides only partial information, as it gives no insight into the trade-off between sensitivity and false positive rates among hits in the marginal range. Furthermore, we note that the score of the first false positive is expected to grow as the number of tested decoy sequences increases. The use of large decoy sets can therefore obscure sensitivity differences in the range of moderate but important E-values. Even so, the measure is a convenient summary statistic that enables the display of the distribution of recall values across a set of queries; we present such an analysis in Supplementary Figure S3, in which BATH shows substantially greater mean and median (per query family) RBFFP. Another deviation from recent trends is that our benchmark does not make use of reversed protein sequences; this is because strings contain approximate palindromes at surprisingly high frequency, resulting in inappropriately high estimates of false discovery (49).
Our analysis is not primarily focused on speed, and the tests are not designed to explore relative performance on massive scale search, but a simple run time analysis (Table 1) shows that BATH’s frameshift model does not dramatically increase search run time relative to other methods implementing the full Forward algorithm on pHMMS (BATH --nofs & nhmmer). The table also provides a reminder that the relative sensitivity losses of MMseqs2, LAST, and DIAMOND are offset by significant gains in speed, so that researchers primarily interested in speed should consider these as preferable options (though note that the speed of DIAMOND is under-represented in this analysis, since we used DIAMOND’s slowest settings).
Table 1.
Run times (in hours) for all tools run on the transmark benchmark using both the family pairwise and consensus sequence query formats. All tests were run with 16 threads on a system with a 94-core Penguin Altus XE2242 @ 2.4GHz, and 512 GB RAM.
| Run Times (hours) | |||||||
|---|---|---|---|---|---|---|---|
| Query type | BATH | BATH (--nofs) | tblastn | MMseqs2 | nhmmer | LAST | DIAMOND |
| family pairwise | 4.34 | 1.69 | 2.31 | 0.05 | 2.20 | 0.04 | 0.22 |
| consensus sequence | 3.54 | 1.64 | 0.06 | 0.04 | 2.08 | 0.03 | 0.13 |
Recovery of protein-coding DNA containing simulated frameshifts.
Insertions and deletions (indels), whether due to sequencing error or true biological processes, can interrupt open reading frames in protein-coding DNA. The resulting frameshifts can reduce search sensitivity by depriving alignment tools of full-length peptide-coding sequences for alignment and annotation. To explore the impact of frameshift frequency, and the ability of BATH’s frameshift model to recover lost signal, we created three more transmark benchmarks with various rates of simulated frameshifts; transmark01 has a 1% indel rate, transmark02 has a 2% indel rate, and transmark05 has a 5% indel rate. For more information on transmark indel rates see Methods. All of these benchmarks use the full set of query sequences (rather than a single consensus sequence).
Figure 4 mirrors the accuracy analysis of the previous section, for indel rates r ∈ {1%, 2%, 5%}. Unsurprisingly, tools with frameshift models (BATH, LAST, DIAMOND) see smaller degradation in performance than do the other translated search tools. BATH results demonstrate the advantage of combining pHMMs with a frameshift model. As expected, nhmmer shows only modest sensitivity loss, since it is only comparing sequences at the nucleotide level.
Fig. 4.

ROC plots showing sensitivity (true positives) vs specificity (false positives) for all tested tools on three translated search benchmarks with varying levels of simulated frameshift indels. (A) transmark01 has a 1% indel rate; (B) transmark02 has a 2% indel rate; (C) transmark05 has a 5% indel rate.
We also analyzed the distribution across query families of recall before the first false positive (RBFFP), as shown in Supplementary Figure S3. These results indicate that some families experience total loss in sensitivity, but that in general, BATH produces substantially greater mean and median (per query family) recall than do other tools.
Coverage and Overextension.
The above assessments are measures of general recall: was a planted sequence identified, or not? Another perspective on annotation accuracy is evaluation of the extent to which the annotation accurately identifies the full length of the planted sequence – this is particularly important when target sequences are likely to be identified as fragments, as is expected for sequences containing frameshifting indels. We measure this accuracy in two ways: coverage and overextension.
To measure coverage, we identified the set of planted target sequences that were matched with E-value 1e-5 or better by all tested tools (restricting to this common set ensures that a tool is not penalized for finding a partial hit that other tools do not find at all). We computed the fraction of nucleotides in these targets that are captured in alignments produced by each tool. Table 2 presents coverage values for benchmarks contaiing 0% and 2% simulated frameshifts. In the frameshift-free test, transmark00-all, protein-to-DNA tools produce superior coverage to the DNA-to-DNA tool (nhmmer), and BATH’s coverage is slightly better than the others. In the frameshifted variant (transmark02, which also provides tools with all query sequences for a family), the tools with no frameshift model (tblastn, MMseqs2, BATH --nofs) show reduced coverage due to target fragmentation. In the case of frameshifted target sequences, Table 3 shows that frameshift-aware tools (and codon-oblivious nhmmer) generally match the target sequence with a single alignment, while other tools are forced to match targets with multiple shorter fragmented matches. The summary interpretation of these results, along with those in the previous section, is that BATH find more hits (Figures 3 and 4) and also produces better coverage of the hits that it finds.
Table 2.
For two of the transmark benchmarks (transmark00-all which has no simulated frameshifts and transmark02 which has a 2% indel rate) we calculated average alignment coverage for a set of shared true positives (the set of test sequences correctly annotated by all tools). For transmark00-all the set includes 10,582 hits and for transmark02 the set includes 6,620 hits. For each sequence in these sets, we compared the true embedding coordinates of the test sequence to the alignment coordinates reported by each tool. To calculate alignment coverage, we record the percentage of all the test sequence nucleotides that are included in a true positive alignment. If there is more than one true positive alignment per tool, we combine the coverage of those alignments, being sure to only count each nucleotide once, to get the maximum coverage across all alignments.
| Average Alignment Coverage on Shared True Positives | |||||||
|---|---|---|---|---|---|---|---|
| Benchmark | BATH | BATH --nofs | tblastn | MMseqs2 | nhmmer | LAST | DIAMOND |
| transmark00 | 95.2% | 95.2% | 93.9% | 94.9% | 81.3% | 88.6% | 94.7% |
| transmark02 | 91.7% | 63.5% | 78.3% | 61.9% | 79.9% | 85.6% | 89.8% |
Table 3.
The average number of alignments needed for each tool to get the coverage is seen in Table 2.
| Average Number of Alignments Used to Achieve Coverage | |||||||
|---|---|---|---|---|---|---|---|
| Benchmark | BATH | BATH --nofs | tblastn | MMseqs2 | nhmmer | LAST | DIAMOND |
| transmark00 | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 |
| transmark02 | 1.0 | 2.1 | 2.1 | 2.1 | 1.1 | 1.3 | 1.0 |
Increased coverage could be the product of a simple tendency to generate long alignments. A risk created by such a tendency is that alignments may extend beyond the bounds of the true instance and into flanking non-homologous sequence, a problem known as ‘homologous overextension’ (48, 50, 51). To assess this risk, we created an overextension variant of the transmark00-all and transmark02 benchmarks. In these variants (transmark00-over and transmark02-over), only the middle 50% of each family instance was embedded into the simulated DNA background. The result is that each full-length query match should produce an alignment to a half-length planted sequence, and any extension beyond those boundaries is a case of overextension. As in the coverage evaluation, we identified the set of planted target sequences that were matched with E-value 1e-5 or better by all tested tools, then computed measures of overextension on those hits.
Table 4 shows, for each tool, the percent of hits for which the alignment extends at least 4 nucleotides (more than a single amino acid) beyond the bounds of the true planted instance. Table 5 shows the mean length of these overextensions. Coverage and overextension are expected to correlate since a tool with low coverage will often fail to reach the end of a matched instance (incomplete coverage), and overextension is only possible after true boundaries have been reached. In line with this expectation, BATH shows a slightly elevated frequency of overextension on transmark00-over, though the average length of BATH’s overextensions is less than half that of the average overextension length of both tblastn and DIAMOND. BATH produces longer, and slightly more frequent, overextension on transmark02-over than on transmark00-over. This is due to the BATH opting to use frameshift-aware translation over standard translation far more frequently for transmark02-over than for transmark00-over.
Table 4.
For each benchmark we first found the set of shared true positives found by all tools - 7678 hits for transmark00-over and 4115 hits for transmark02-over. From this set, we calculated the percent hits that had overextension longer than 3 nucleotides.
| Percent of Shared True Positives with Overextended Alignments longer than 3 nucleotides | |||||||
|---|---|---|---|---|---|---|---|
| Benchmark | BATH | BATH --nofs | tblastn | MMseqs2 | nhmmer | LAST | DIAMOND |
| transmark00-over | 53.3% | 53.3% | 51.8% | 33.6% | 22.5% | 28.1% | 49.8% |
| transmark02-over | 56.8% | 28.7% | 39.0% | 18.6% | 18.7% | 38.6% | 43.0% |
Table 5.
The average length of all overextensions that were at least 4 nucleotides long, taken from the set of shared true positives.
| Average Length of Overextensions (longer than 3 nucleotides) on Shared True Positives | |||||||
|---|---|---|---|---|---|---|---|
| Benchmark | BATH | BATH --nofs | tblastn | MMseqs2 | nhmmer | LAST | DIAMOND |
| transmark00-over | 14.1 | 14.1 | 28.5 | 17.9 | 13.2 | 13.2 | 30.2 |
| transmark02-over | 25.6 | 14.5 | 28.5 | 18.9 | 13.3 | 20.7 | 33.5 |
Accuracy of E-values.
Sequence annotation depends on accurate estimation of the significance of an identified match, usually provided by alignment tools with an E-value. For search of a given query against a given target genome G, producing an alignment with score S, the E-value of an alignment gives the number of alignments expected to meet or exceed the score S if G consisted exclusively of unrelated (random) sequences. Empirical studies demonstrate that HMMER3 E-value predictions are reasonably accurate for search against randomly generated sequences of nucleotides or amino acids (16), but these analyses do not extend to a frameshift model. To evaluate E-value reliability, we created a target set by generating 10 million random amino acid sequences of length 400 and then reverse-translated them into DNA sequences of length 1,200, ensuring each of these non-homogous decoy sequences has at least one full-length open reading frame. For the query set, we randomly selected 150 pHMMs from Pfam. We then searched these targets and queries using BATH’s three modes: --nofs which uses only standard translation, --fsonly which uses only frameshift-aware translation, and default BATH which lets the algorithm choose which form of translation to use.
Figure 5 demonstrates that estimated E-values are reasonably accurate for all three modes. The black line shows the expected occurrence of E-values less than or equal to the value of x-axis, when aligning to non-homologous sequence. The three red lines show the actual occurrence of those E-values from BATH’s three modes. The small difference seen between the lines from BATH --nofs (dotted line) and BATH --fsonly (dashed line) is explained by the impact of stop codons on the length of hits. While each of the random target sequences was generated to have at least one full-length ORF (frame 1), stop codons are often present in the other frames (frames 2 through 6). Since the standard translation used by --nofs cannot align stop codons, alignments to frames 2 to 6 will often be abbreviated compared to the same alignment from --fsonly. E-value parameterization for BATH models only the chance that a given random protein sequence will reach a target score; it does not model the chance that random DNA sequence will contain sufficiently-long ORF in all frames to produce an appropriately long random protein. When BATH is allowed to incorporate frameshifts, it overcomes the modelling challenges created by this data fragmentation, and can properly model P-values produced though both the standard and frameshift-aware Forward implementations.
Fig. 5.
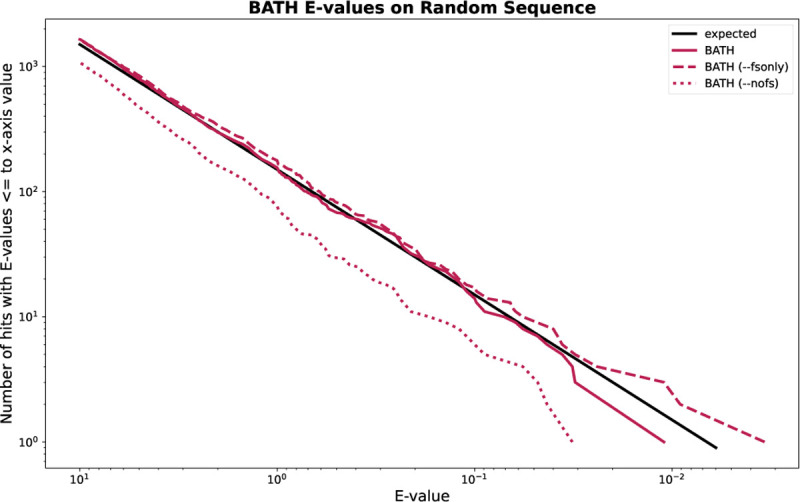
Expected and actual occurrence of E-values for BATH when running 150 randomly selected Pfam pHMMs against 10M randomly generated target sequences.
Annotation of pseudogenes – a case study.
Pseudogenes are segments of DNA that resemble functional genes but have been rendered non-functional due to the accumulation of mutations (including frameshift inducing indels), and usually result from gene duplication or reverse transcription of an mRNA transcript.
Here, we explore the utility of BATH in annotating pseudogenes within the genomes of bacterial strains of Canditatus hodgkinia, which live as obligate endosymbionts in the cells of periodical cicadas (Magicicada) (53). The genome instability common in endosymbionts, combined with the unusual life cycle of Magicicada, has led to extreme lineage splitting among the hodgkinia in all Magicicada species. Whereas the ancestral form of Canditatus hodgkinia had only one circular chromosome, as many as 42 unique hodgkinia chromosomes have been found in a single Magicicada species. Large-scale deletions and pseudogenization have reduced genome annotation coverage from nearly 100% for single chromosome hodgkinia in other cicada species, to just 25.3% (20.2% protein-coding and 5.1% RNAs) across the hodgkinia chromosomes of Magicicadas (53, 54). The remaining sequence landscape presents a useful challenge for a tool such as BATH, since essentially all unannotated sequence is expected to be made up of pseudogenes resulting from gene loss enabled by lineage splitting and community complementation (54).
We searched 231 Canditatus hodgkinia chromosomes identified in seven Magicicada species using a curated set of 165 query protein families from hodgkinia in several other cicada species [Matt Campbell, personal comm.]. Annotation was performed with tblastn, BATH (default), and BATH --nofs. Table 6 shows the total coverage of those genomes (percent of all nucleotides involved in some annotation), produced by each tool, along with the coverage due to manual curation, as captured in GenBank. BATH (default) demonstrates clear gains in coverage, particularly in full-length hits (hits that cover at least 66% of the query length). An example of one of the Canditatus hodgkinia chromosomes (from the species Magicicada tredecim) is shown in Figure 6A, and provides examples of both novel matches (not found in GenBank or by tblastn) and improved continuity of matches that were fragmented by tblastn and BATH --nofs.
Table 6.
Percent of nucleotides annotated by either the GenBank annotations, or by one of the three methods tested (BATH, BATH --nofs, and tblastn) across 231 Canditatus hodgkinia chromosomes from 7 Magicicada species. Only annotations with an E-value of less than 1e-5 were included in these percentages. Full-length hits are defined as hits that cover more than 66% of the length of the query. The 66% cutoff was selected based on the shortest query coverage seen in the GenBank annotations (66.67%).
| Coverage of protein-coding genes across all Canditatus hodgkinia chromosomes identified in Magicicada | ||||
|---|---|---|---|---|
| GenBank | BATH | BATH --nofs | tblastn | |
| all hits | 20.2% | 37.2% | 25.8% | 30.4% |
| full-length hits | 20.2% | 33.6% | 18.6% | 19.8% |
Fig. 6.
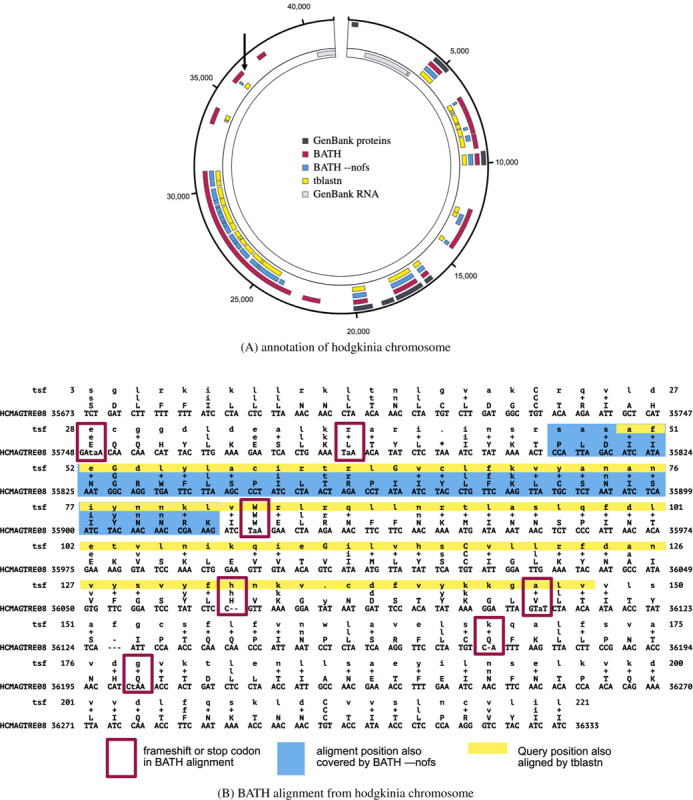
Example annotations from GenBank, BATH, BATH --nofs, and tblastn of (A) a single hodgkinia chromosome (image generated using SODA (52)) and (B) a single alignment from that chromosome. The GenBank annotations were crafted by Campbell et al. (53) with expert knowledge and a custom-built pipeline. The three tool annotations show hits with E-values less than 1e-5. The arrow in the upper left quadrant of (A) points to the location of the BATH alignment seen in (B). Each line of the BATH alignment consists of four rows. The top and bottom rows show the residues of the query (in amino acids) and target (in nucleotides), respectively. The row second from the bottom shows the amino acid translations of the target codon or quasi-codon below, and the line second from the top shows whether the match between the target and query was positive scoring (showing either the amino acid in the case of an exact match or a ‘+’ in the case of a positive scoring mismatch) or negative scoring (left blank).
Figure 6B shows a specific example of a frameshifted alignment produced by BATH, along with the abbreviated annotations from tblastn and BATH --nofs (corresponding to the annotation indicated by the arrow in the upper left section of Figure 6A). By aligning through frameshifts, BATH is able to join high-scoring regions in different frames into a single alignment. The BATH --nofs alignment is shorter than tblastn’s because tblastn’s standard translation allows for alignment to stop codons which BATH (--nofs) does not.
False Frameshifts – a case study.
One concern with the use of frameshift-aware translation is the potential that a tool will infer frameshifts that are not truly present in the sequence. We examined the risk of such “false frameshifts” by computing the rate at which BATH calls frameshifts in the true positives from transmark00-all and transmark00-cons. Of all the true positives found in transmark00-all fewer than 0.2% had frameshifts in their alignments and for transmark00-cons the occurrence of false frameshifts in true positives was just 0.4%.
Even these low false frameshift rates may overstate the true risk. The results are based on the assumption that the Pfam-sourced test sequences are correctly translated from their source DNA, but manual inspection of the highest-scoring “false frameshift” alignments from BATH all showed evidence that the protein in Pfam is translated from genomic DNA containing one or more frameshifts. One example is phosphatase CheZ from Sodalis glossinidius, a Gammaproteobacteria. The DNA that encodes this protein was aligned by BATH with a single frameshift (see Figure 7 for the alignment) not seen in the canonical translation of this protein found in Pfam. The frameshift seems plausible as it allows two high-identity regions in different frames to be stitched through a single nucleotide deletion, whereas the Pfam protein (initially sourced from Uniprot) produces a low-quality alignment in the 5’ segment. Using ESMFold (55), we predicted the structure of both the Pfam-sourced sequence and the protein sequence suggested by frameshifted alignment. Figure 8 shows a predicted disordered structure on the 5’ end of the Pfam variant that is well structured in the BATH-informed variant. Further investigation is needed to determine if this frameshift (along with others predicted by BATH during these analyses) is the result of a sequencing error or if it is a true mutation in the sequenced Sodalis glossinidius genome.
Fig. 7.
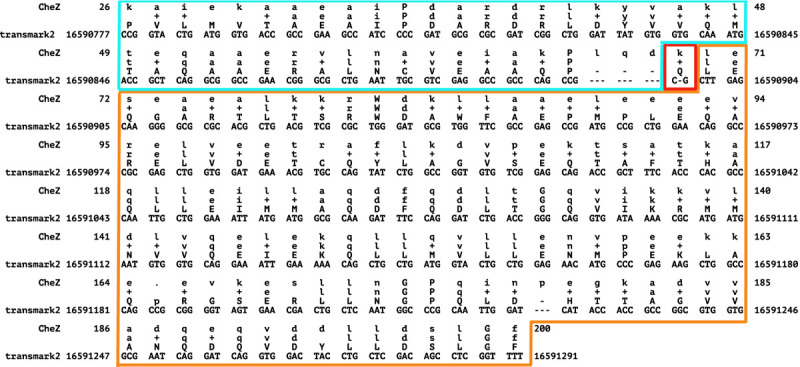
BATH alignment for the DNA encoding the CheZ protein (Uniprot:Q2NR86) from Sodalis glossinidius to the benchmark model derived from Pfam family PF04344. BATH found a single frameshift in this sequence - outlined in red. The translation after this frameshift (outlined in orange) is in the same frame as the Pfam translation, but the section before the frameshift (outlined in blue) is in a different frame and therefore has a different translation than Pfam. The BATH-predicted alignment shows high identity with the query pHMM, with 93% of the matches being positive scoring, providing strong support for the validity of the BATH translation.
Fig. 8.
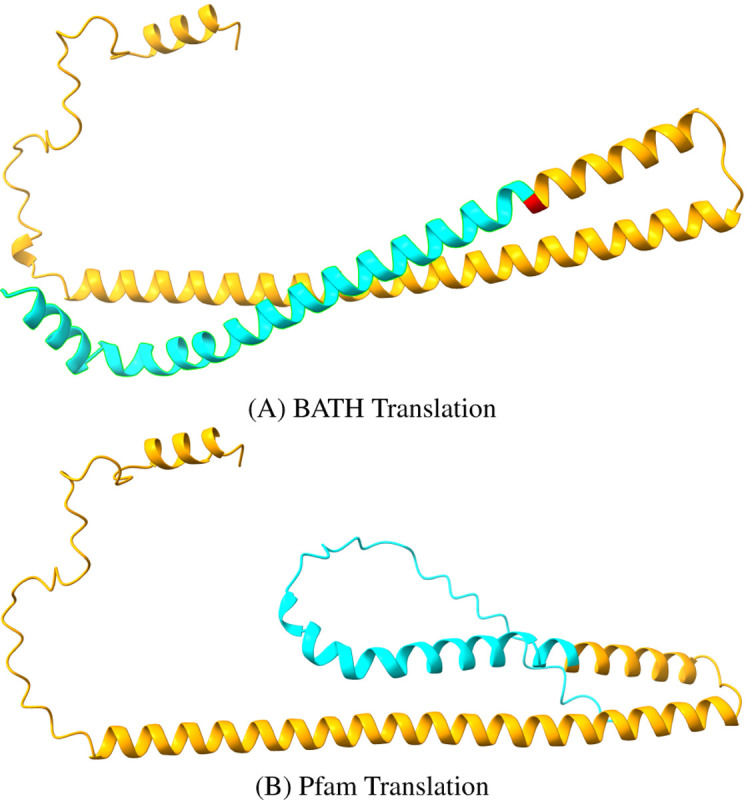
Structure Predictions for competing translations of the DNA encoding the CheZ protein (Uniprot:Q2NR86, Pfam:PF04344) from Sodalis glossinidius from (A) BATH and (B) Pfam. The orange regions show where the translations agree and the blue regions. The single red amino acid is identified as a quasi-codon by BATH, and serves as the bridge between two reading frames (see previous Figure). The blue regions differ at the level of predicted amino acid, and the Pfam translation is predicted to be disordered while the BATH translation is predicted to form an alpha helix structure.
Considering the very low rate at which BATH called any frameshifts in the transmark00 benchmarks, combined with the fact that at least some of these frameshifts seem credible, the risk of false frameshifts from BATH appears negligible. Instead, we find that BATH’s ability to find frameshifts even where we did not expect them can lead to improved translation that could benefit existing databases.
Conclusions
BATH provides superior sensitivity for the annotation of protein-coding DNA with or without the presence of frameshifts. It achieves this sensitivity by applying pHMMs and the Forward algorithm to the challenge of translated search and by modifying the pHMMs and alignment algorithms to be frameshift-aware. BATH also provides excellent alignment coverage and accurate E-values. Run-times are similar to other tools implementing the Forward algorithm (nhmmer) but significantly higher than other less sensitive tools (MMseqs, LAST, and DIAMOND). Future work on BATH will focus on improving run times.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to John McCutcheon, Matt Campbell, and Arkadiy Garber for sharing examples of clearly pseudogenized genomic sequence, discussions surrounding pseudogene and error detection, and aid in developing early versions of some annotation graphics. We thank Jack Roddy and Daniel Olson for thoughtful feedback on both implementation and visualization. We thank Dario Copetti, Neha Sontakke, Conner Copeland, and Simon Roux for performing substantial test runs with BATH, and providing error reports and usability/documentation feedback, and also thank Sean Eddy and Nick Carter for their insightful feedback on matters of usability and formatting. We also gratefully acknowledge the computational resources and expert administration provided by the University of Montana’s Griz Shared Computing Cluster (GSCC) and the high performance computing (HPC) resources supported by the University of Arizona TRIF, UITS, and Research, Innovation, and Impact (RII) and maintained by the UArizona Research Technologies department. GK and TJW were supported during development of BATH by NIH NIGMS P20GM103546 and R01GM132600, NIH NHGRI R15HG009570 and U24HG010136, and DOE DE-SC0021216.
Bibliography
- 1.Sayers Eric W., Bolton Evan E., Brister J. Rodney, Canese Kathi, Chan Jessica, Comeau Donald C., Connor Ryan, Funk Kathryn, Kelly Chris, Kim Sunghwan, Madej Tom, Marchler-Bauer Aron, Lanczycki Christopher, Lathrop Stacy, Lu Zhiyong, Thibaud-Nissen Francoise, Murphy Terence, Phan Lon, Skripchenko Yuri, Tse Tony, Wang Jiyao, Williams Rebecca, Trawick Barton W., Pruitt Kim D., and Sherry Stephen T.. Database resources of the national center for biotechnology information. Nucleic Acids Research, 50(D1):D20–D26, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apweiler Rolf, Bairoch Amos, Wu Cathy H, Barker Winona C, Boeckmann Brigitte, Ferro Serenella, Elisabeth Gasteiger, Hongzhan Huang, Lopez Rodrigo, Magrane Michele, Martin Maria J, Natale Darren A, Donovan Claire O, Redaschi Nicole, and Yeh Lai-su L. UniProt: the Universal Protein knowledgebase. Nucleic Acids Research, 32:D115–D119, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olaf R. P. Bininda-Emonds. TransAlign: Using amino acids to facilitate the multiple alignment of protein-coding DNA sequences. BMC Bioinformatics, 6:1–6, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernersson Rasmus and Pedersen Anders Gorm. RevTrans: Multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Research, 31(13):3537–3539, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler David L, Barrett Tanya, Benson Dennis A, Bryant Stephen H, Canese Kathi, Chetvernin Vyacheslav, Church Deanna M, DiCuccio Michael, Edgar Ron, Federhen Scott, et al. Database resources of the national center for biotechnology information. Nucleic acids research, 35(suppl_1):D5–D12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uniprot: the universal protein knowledgebase in 2021. Nucleic acids research, 49(D1): D480–D489, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camacho Christiam, Coulouris George, Avagyan Vahram, Ma Ning, Papadopoulos Jason, Bealer Kevin, and Madden Thomas L.. BLAST+: Architecture and applications. BMC Bioinformatics, 10:1–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyatt Doug, Chen Gwo-Liang, LoCascio Philip F, Land Miriam L, Larimer Frank W, and Hauser Loren J. Prodigal: prokaryotic gene recognition and translation initiation site identification. Nature Communications, 6(1):1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwengers Oliver, Jelonek Lukas, Marius Alfred Dieckmann Sebastian Beyvers, Blom Jochen, and Goesmann Alexander. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microbial Genomics, 7(11), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinegger Martin and Söding Johannes. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nature Biotechnology, 35(11):1026–1028, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Buchfink Benjamin, Reuter Klaus, and Drost Hajk-Georg. Sensitive protein alignments at tree-of-life scale using diamond. Nature methods, 18(4):366–368, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krogh Anders, Brown Michael, Mian I. Saira, Sjölander Kimmen, and Haussler David. Hidden Markov Models in computational biology: Applications to protein modeling. Journal of Molecular Biology, 235(5):1501–1531, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Durbin Richard, Eddy Sean R, Krogh Anders, and Mitchison Graeme. Biological sequence analysis: probabilistic models of proteins and nucleic acids. Cambridge university press, 1998. [Google Scholar]
- 14.Eddy Sean R.. Accelerated profile HMM searches. PLoS Computational Biology, 7(10), 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett C, Chothia C, Haussler D, Park J, Karplus K, Hughey R, and Hubbard T. Sequence comparisons using multiple sequences detect three times as many remote homologues as pairwise methods. Journal of Molecular Biology, 284(4):1201–10, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Eddy Sean R.. A probabilistic model of local sequence alignment that simplifies statistical significance estimation. PLoS Computational Biology, 4(5), 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler Travis J and Eddy Sean R. nhmmer: DNA homology search with profile HMMs. Bioinformatics, 29(19):2487–2489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawrocki Eric P. and Eddy Sean R.. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics, 29(22):2933–2935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell Alex L., Attwood Teresa K., Babbitt Patricia C., Blum Matthias, Bork Peer, Bridge Alan, Brown Shoshana D., Hsin Yu Chang Sara El-Gebali, Fraser Matthew I., Gough Julian, Haft David R., Huang Hongzhan, Letunic Ivica, Lopez Rodrigo, Luciani Aurélien, Madeira Fabio, Aron Marchler-Bauer Huaiyu Mi, Natale Darren A., Necci Marco, Nuka Gift, Orengo Christine, Pandurangan Arun P., Typhaine Paysan-Lafosse Sebastien Pesseat, Potter Simon C., Qureshi Matloob A., Rawlings Neil D., Redaschi Nicole, Richardson Lorna J., Rivoire Catherine, Salazar Gustavo A., Sangrador-Vegas Amaia, Sigrist Christian J.A., Sillitoe Ian, Sutton Granger G., Thanki Narmada, Thomas Paul D., Tosatto Silvio C.E., Yong Siew Yit, and Finn Robert D.. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Research, 47(D1):D351–D360, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sara El-Gebali Jaina Mistry, Bateman Alex, Eddy Sean R., Luciani Aurélien, Potter Simon C., Qureshi Matloob, Richardson Lorna J., Salazar Gustavo A., Smart Alfredo, Sonnhammer Erik L.L., Hirsh Layla, Paladin Lisanna, Piovesan Damiano, Tosatto Silvio C.E., and Finn Robert D.. The Pfam protein families database in 2019. Nucleic Acids Research, 47 (D1):D427–D432, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubley Robert, Finn Robert D., Clements Jody, Eddy Sean R., Jones Thomas A., Bao Weidong, Smit Arian F.A., and Wheeler Travis J.. The Dfam database of repetitive DNA families. Nucleic Acids Research, 44(D1):D81–D89, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carradec Quentin, Pelletier Eric, Silva Corinne Da, Alberti Adriana, Seeleuthner Yoann, Blanc-Mathieu Romain, Lima-Mendez Gipsi, Rocha Fabio, Tirichine Leila, Labadie Karine, et al. A global ocean atlas of eukaryotic genes. Nature communications, 9(1):373, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin Eli Levy, Mirdita Milot, and Söding Johannes. Metaeuk—sensitive, high-throughput gene discovery, and annotation for large-scale eukaryotic metagenomics. Microbiome, 8: 1–15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modha Sejal, Robertson David L, Hughes Joseph, and Orton Richard J. Quantifying and cataloguing unknown sequences within human microbiomes. Msystems, 7(2):e01468–21, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov Ivaylo P. and Atkins John F.. Ribosomal frameshifting in decoding antizyme mRNAs from yeast and protists to humans: Close to 300 cases reveal remarkable diversity despite underlying conservation. Nucleic Acids Research, 35(6):1842–1858, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arumugam Krithika, Bağcı Caner, Bessarab Irina, Beier Sina, Buchfink Benjamin, Górska Anna, Qiu Guanglei, Huson Daniel H, and Williams Rohan BH. Annotated bacterial chromosomes from frame-shift-corrected long-read metagenomic data. Microbiome, 7(1):1–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amarasinghe Shanika L., Su Shian, Dong Xueyi, Zappia Luke, Ritchie Matthew E., and Gouil Quentin. Opportunities and challenges in long-read sequencing data analysis. Genome Biology, 21(1):1–16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuhrman Jed A. Microbial community structure and its functional implications. Nature, 459 (7244):193–199, 2009. [DOI] [PubMed] [Google Scholar]
- 29.de Cena Jéssica Alves, Zhang Jianying, Deng Dongmei, Damé-Teixeira Nailê, and Do Thuy. Low-abundant microorganisms: the human microbiome’s dark matter, a scoping review. Frontiers in cellular and infection microbiology, 11:689197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaulaurier John, Luo Elaine, Eppley John M, Uyl Paul Den, Dai Xiaoguang, Burger Andrew, Turner Daniel J, Pendelton Matthew, Juul Sissel, Harrington Eoghan, et al. Assembly-free single-molecule sequencing recovers complete virus genomes from natural microbial communities. Genome Research, 30(3):437–446, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sergey L Sheetlin Yonil Park, Martin C Frith, and John L Spouge. Frameshift alignment: statistics and post-genomic applications. Bioinformatics, 30(24):3575–3582, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.States D J and Botstein D. Molecular sequence accuracy and the analysis of protein coding regions. Proceedings of the National Academy of Sciences of the United States of America, 88(13):5518–5522, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claverie Jean-Michel. Detecting Frame Shifts by Amino Acid Sequence Comparison. Journal of Molecular Biology, 234:1140–1157, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Rho Mina, Tang Haixu, and Ye Yuzhen. Fraggenescan: predicting genes in short and error-prone reads. Nucleic acids research, 38(20):e191–e191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackl Thomas, Trigodet Florian, Eren A Murat, Biller Steven J, Eppley John M, Luo Elaine, Burger Andrew, DeLong Edward F, and Fischer Matthias G. proovframe: frameshift-correction for long-read (meta) genomics. bioRxiv, pages 2021–08, 2021. [Google Scholar]
- 36.Richardson Lorna, Allen Ben, Baldi Germana, Beracochea Martin, Bileschi Maxwell L, Burdett Tony, Burgin Josephine, Caballero-Pérez Juan, Cochrane Guy, Colwell Lucy J, et al. Mgnify: the microbiome sequence data analysis resource in 2023. Nucleic Acids Research, 51(D1):D753–D759, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y. and Sun Y.. HMM-FRAME: Accurate protein domain classification for metagenomic sequences containing frameshift errors. BMC Bioinformatics, 12(198), 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson William R., Wood Todd, Zhang Zheng, and Miller Webb. Comparison of DNA sequences with protein equences. Genomics, 36(46):24–36, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Birney Ewan, Clamp Michele, and Durbin Richard. GeneWise and Genomewise. Genome Research, 14(5):988–995, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheetlin Sergey L., Park Yonil, Frith Martin C., and Spouge John L.. Frameshift alignment : Statistics and post-genomic applications. Bioinformatics, 30(24):3575–3582, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slater Guy St C and Birney Ewan. Automated generation of heuristics for biological sequence comparison - exonerate. BMC Bioinformatics, 6(31):1–11, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sara El-Gebali Jaina Mistry, Bateman Alex, Eddy Sean R., et al. The Pfam protein families database in 2019. Nucleic Acids Research, 47(D1):D427–D432, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UniProtKB. Universal Protein Resource - idmapping. https://ftp.uniprot.org/pub/databases/uniprot/current_release/knowledgebase/idmapping/,2023.
- 44.Elzanowski Andrzej and Ostell Jim. The genetic codes, 2019.
- 45.Nawrocki Eric P. and Eddy Sean R.. Query-dependent banding (QDB) for faster RNA similarity searches. PLoS Computational Biology, 3(3):0540–0554, 2007. doi: 10.1371/journal.pcbi.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grundy William Noble. Family-based homology detection via pairwise sequence comparison. In Proceedings of the Second Annual International Conference on Computational Molecular Biology, pages 94–100, 1998. [Google Scholar]
- 47.Li Heng. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics, 34(18): 3094–3100, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheeler Travis J, Clements Jody, Eddy Sean R, Hubley Robert, Jones Thomas A, Jurka Jerzy, Smit Arian FA, and Finn Robert D. Dfam: a database of repetitive dna based on profile hidden markov models. Nucleic acids research, 41(D1):D70–D82, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glidden-Handgis George and Wheeler Travis J. Was it a match i saw? approximate palindromes lead to overstated false match rates in benchmarks using reversed sequences. bioRxiv, pages 2023–06, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez Mileidy W and Pearson William R. Homologous over-extension: a challenge for iterative similarity searches. Nucleic acids research, 38(7):2177–2189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frith Martin C, Park Yonil, Sergey L Sheetlin, and John L Spouge. The whole alignment and nothing but the alignment: the problem of spurious alignment flanks. Nucleic acids research, 36(18):5863–5871, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roddy Jack W, Lesica George T, and Wheeler Travis J. Soda: a typescript/javascript library for visualizing biological sequence annotation. NAR Genomics and Bioinformatics, 4(4): lqac077, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell Matthew A., Łukasik Piotr, Simon Chris, and McCutcheon John P.. Idiosyncratic Genome Degradation in a Bacterial Endosymbiont of Periodical Cicadas. Current Biology, 27(22):3568–3575.e3, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Łukasik Piotr, Nazario Katherine, Van Leuven James T., Campbell Matthew A., Meyer Mariah, Michalik Anna, Pessacq Pablo, Simon Chris, Veloso Claudio, and McCutcheon John P.. Multiple origins of interdependent endosymbiotic complexes in a genus of cicadas. Proceedings of the National Academy of Sciences of the United States of America, 115(2):E226–E235, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Zeming, Akin Halil, Rao Roshan, Hie Brian, Zhu Zhongkai, Lu Wenting, Smetanin Nikita, Verkuil Robert, Kabeli Ori, Shmueli Yaniv, et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science, 379(6637):1123–1130, 2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


