Abstract
Yersinia enterocolitica is an important enteric pathogen which has well-defined virulence determinants that allow the bacteria to become established in their hosts and overcome host defenses. A number of strains obtained from patients with diarrhea, however, lack these genes. Accordingly, the mechanisms by which they cause disease are uncertain. Most of these isolates belong to biotype 1A. Strains of this biotype are also frequently isolated from a variety of nonclinical sources, such as food, soil, water, and healthy animals, and there is evidence that some of these strains are avirulent. In this study we investigated 111 strains of Y. enterocolitica biotype 1A, 79 from symptomatic humans and 32 from nonclinical sources, for virulence-associated characteristics. DNA hybridization studies showed that none of the strains carried sequences homologous with pYV, the ∼70-kb Yersinia virulence plasmid. Some strains hybridized with DNA probes for one of the following chromosomal virulence-associated genes: ail (7.2%), myfA (11.7%), ystA (0.9%), and ystB (85%). In addition, 33 strains (29.7%) produced an enterotoxin that was reactive in infant mice. However, the frequencies of these virulence-associated properties in clinical and nonclinical isolates were similar. Clinical isolates invaded HEp-2 cells and Chinese hamster ovary cells to a significantly greater extent than nonclinical strains (P ≤ 0.002). In addition, clinical strains colonized the intestinal tracts of perorally inoculated mice for significantly longer periods than nonclinical isolates (P ≤ 0.01). Light and electron microscopic examination of tissue culture cells incubated with invasive yersiniae revealed that the bacteria invaded selected cells in large numbers but spared others, suggesting that biotype-1A strains of Y. enterocolitica may invade cells by a novel mechanism. These results indicate that some clinical isolates of Y. enterocolitica which lack classical virulence markers may be able to cause disease via virulence mechanisms which differ from those previously characterized in enteropathogenic Yersinia species.
Yersinia enterocolitica is an important human pathogen which causes a variety of disorders, ranging from nonspecific diarrhea to invasive disease such as mesenteric lymphadenitis, hepatosplenic abscesses, and septicemia (5, 10, 37). The heterogenous nature of Y. enterocolitica, including differences in virulence, has led to the division of the species into subgroups based upon biochemical behavior and lipopolysaccharide O antigens (5, 37). At present, six biotypes are recognized, of which biotype 1B and biotypes 2 through 5 are regarded as including primary pathogens (5, 22, 26, 37, 40). The primary pathogenic strains of Y. enterocolitica are recognized in part by their ability to invade tissue culture cells in large numbers (7, 27, 29, 35). Genes which contribute this ability include the inv and ail genes on the bacterial chromosome and yadA, which is borne on a ca. 70-kb virulence plasmid known as pYV (4, 7, 27). Interestingly, however, other pYV-borne genes impede bacterial penetration of epithelial cells and macrophages, with the result that Y. enterocolitica is located extracellularly in infected animals (9, 15). Chromosomal genes other than inv and ail which may also contribute to virulence include yst (also known as ystA), which encodes a heat-stable enterotoxin (YST-a), myf, which encodes the production of fibrillae (Myf), and the urease gene complex (11, 12, 21).
Biotype-1A strains of Y. enterocolitica, which generally are considered to be avirulent, are highly heterogenous, and include a large number of O serogroups (5, 37). They occur throughout the world in a wide range of environments and generally lack the genotypic and phenotypic markers associated with virulence of classical invasive strains of Y. enterocolitica, such as pYV, ail, myf, ystA, or a functional inv gene (12, 21, 28, 35, 40, 41, 45). Moreover, biotype-1A strains of environmental origin do not colonize the gastrointestinal tracts of experimentally inoculated animals (33, 42, 48). Despite these observations, some biotype-1A strains have been implicated as a cause of gastrointestinal disease. For example, a nosocomial outbreak of gastroenteritis in Canada involving nine patients was attributed to a strain of Y. enterocolitica biotype 1A, serogroup O:5 (36). In several countries, moreover, including Australia, Canada, The Netherlands, New Zealand, the Republic of Georgia, South Africa, Switzerland, and the United States of America, a significant proportion of Y. enterocolitica isolates obtained from patients with diarrhea belong to biotype 1A (3, 6, 17, 32, 34, 39, 46, 47). In addition, a prospective case control study with Chilean children showed that biotype-1A strains were significantly associated with diarrhea (30), and a clinical study in Switzerland demonstrated that the illness associated with biotype-1A strains of Y. enterocolitica was indistinguishable from that due to classical virulent biotypes (6).
If biotype-1A strains of Y. enterocolitica are able to cause disease, their pathogenic mechanisms are not clear because they lack the well-established virulence markers of primary pathogenic strains of Y. enterocolitica. Some Y. enterocolitica strains produce variants of YST-a, known as YST-b and YST-c (20, 52, 53), but their prevalence and contribution to disease are not known. In addition, a biotype-1A strain of serogroup O:6 was reported to produce a novel heat-stable enterotoxin, termed YST-II. This toxin differs from YST-a in a number of respects, including its mechanism of action, which does not appear to involve activation of guanylate cyclase (41). Other putative virulence determinants in this or other biotype-1A strains have not been investigated or reported.
As biotype-1A strains of Y. enterocolitica are so heterogenous and occupy such a diverse range of environmental niches, we hypothesized that there may be a subset of these bacteria which are capable of causing disease but which lack the classical virulence markers of Yersinia species and therefore cannot be identified by assays for these markers. The aim of this study was to test this hypothesis by examining a collection of biotype-1A strains of clinical and nonclinical origins for virulence-associated determinants.
MATERIALS AND METHODS
Bacteria.
One hundred eleven strains of Y. enterocolitica biotype 1A, isolated from diverse sources in widespread geographic areas, were provided by a number of investigators and selected from our culture collection to represent a variety of serogroups. Seventy-nine strains were obtained from the feces of humans who displayed symptoms consistent with intestinal yersiniosis. Twenty-four strains were of food or environmental origin; of these, 9 were from milk, 10 were from other foods (predominantly vegetables), 3 were from water, 1 was from soil, and 1 was from hay. Another eight strains were isolated from animals which did not exhibit any clinical evidence of infection. In this report, bacteria isolated from food or environmental sources and from healthy animals are collectively referred to as nonclinical strains, whereas isolates from symptomatic humans are designated clinical strains. All bacteria were maintained at −70°C in brain heart infusion (BHI) broth (Oxoid, Basingstoke, Hampshire, England) containing 30% glycerol. Before testing, their identity was confirmed as Y. enterocolitica biotype 1A by standard biochemical techniques (2, 51).
Two invasive strains of Y. enterocolitica, A2635 (biotype 1B, serogroup O:8) and W22703c (biotype 2, serogroup O:9), were used as controls (40). Both of these strains carry the inv, ail, and ystA genes. In addition, A2635 carries pYV, while W22703c does not. Y. enterocolitica JP273 and YE2v, which carry mutations in the inv and ail genes, respectively (27), were obtained from V. L. Miller, Washington University, St. Louis, Mo., who derived them from Y. enterocolitica 8081 (biotype 1B, serogroup O:8). pYV-cured derivatives of these strains, referred to as JP273c and YE2c, respectively, were obtained by passaging the bacteria three times on BHI agar containing 20 mM sodium oxalate and 20 mM MgCl2 at 37°C (18). Escherichia coli HB101(pVM101) and E. coli HB101(pVM103), which express the inv and ail genes of Y. enterocolitica, respectively, were also provided by V. L. Miller. For all studies, strains of Y. enterocolitica were grown on BHI agar or in BHI broth at 28°C, whereas E. coli strains were grown in the same media at 37°C.
DNA methods and plasmids.
DNA extractions, restriction enzyme digestions, ligations, and transformations into E. coli were performed by standard techniques (1, 43). DNA probes for pYV, ail, inv, and ystA were prepared and used to examine colony blots of the test bacteria at high stringency as described previously (40, 41). Oligonucleotide DNA probes for the ystB and ystC variants of the ystA gene were designed to correspond to the variable regions of the respective genes. The ystB probe was 5′-ACTCAGACCCCATCGCCTTCAGAA-3′, corresponding to nucleotides 232 to 255 of the ystB gene (GenBank accession no. D88145), whereas the ystC probe was 5′-GTTGGTGATGTATCATCGTCAACAATAGCT-3′, corresponding to nucleotides 79 to 108 of the published sequence (20). Oligonucleotide probes were end labeled with [γ-32P]dATP (DuPont, New York, N.Y.) by using T4 polynucleotide kinase (Pharmacia, Uppsala, Sweden). A DNA probe for myfA was prepared by PCR amplification of a 280-bp fragment of myfA from strain W22703c using the primers and conditions described previously (21). The resultant fragment was cloned into the SmaI site of pBluescript (SK−; Stratagene, La Jolla, Calif.) to create the plasmid pMyf2. Sequence analysis confirmed that pMyf2 contained the PCR product derived from myfA. The myfA gene probe was then prepared by digesting pMyf2 with EcoRV and BamHI, purifying the 280-bp fragment, and labeling it with [α-32P]dATP (DuPont) by random priming (16). The ymoA gene was sought in 20 biotype-1A isolates (10 clinical and 10 nonclinical) by performing PCR on 1 μl of an overnight culture of each test strain that had been resuspended in distilled water and boiled for 5 min. DNA was amplified by using AmpliTaq DNA polymerase (Roche, Branchburg, N.J.) and primers ymoA-C (5′-GACTTTTCTCAGGGGAATAC-3′) and ymoA-D (5′-GCTCAACGTTGTGTGTCT-3′) under the following conditions: 94°C for 60 s (melting), 50°C for 60 s (annealing), and 72°C for 60 s (extension), repeated for 35 cycles.
Assay for mouse-reactive enterotoxin.
The ability of bacteria to produce enterotoxin was assessed in infant mice as described previously (41). Briefly, bacteria were cultured with shaking in Trypticase soy broth (BBL, Cockeysville, Md.) containing 0.6% (wt/vol) yeast extract (Oxoid) at 28°C for 48 h. Bacterial cells were removed by centrifugation, and 0.1 ml of the supernatant was administered by gavage to 2- to 3-day-old BALB/c mice in groups of three. After 2 h, mice were killed and the mean ratio of intestinal weight to the remaining body weight was determined. Ratios greater than 0.09 were considered indicative of enterotoxin production.
Bacterial adhesion to and invasion of tissue culture cells.
Quantitative assays of bacterial adhesion to and invasion of tissue culture cells were performed as described previously (38). Briefly, HEp-2 (human pharyngeal epithelial) cells were cultured until almost confluent in 24-mm-diameter plastic wells (Nunc, Roskilde, Denmark) containing minimal Eagle’s medium (MEM) supplemented with 5% heat-inactivated (at 56°C for 30 min) fetal calf serum (CSL, Melbourne, Victoria, Australia) and 20 mM HEPES (Boehringer, Mannheim, Germany). Chinese hamster ovary (CHO) cells were cultured in α-MEM containing 10% heat-inactivated fetal calf serum, 2 mM glutamine, and 20 mM HEPES. Twenty million CFU of each test strain was incubated for 3 h with tissue culture cells, which were then washed three times with phosphate-buffered saline (PBS) to remove nonadherent bacteria. For the adhesion assay, cells were immediately lysed with 0.1% digitonin (Sigma, St. Louis, Mo.) and the number of cell-associated bacteria was determined on agar plates (38). For the invasion assay, cells were incubated for a further 90 min in tissue culture medium containing 100 μg of gentamicin/ml, after which the gentamicin was removed by two washes with PBS. Tissue culture cells were then lysed with digitonin, and the number of intracellular bacteria was determined as described above. In a modification of the above assay, designed to determine the extent to which bacteria replicated in tissue culture cells, medium containing 100 μg of gentamicin/ml was replaced with medium containing 10 μg of gentamicin/ml, after which the bacteria and tissue culture cells were incubated for a further 18 h. Tissue culture cells were then lysed with digitonin, and the bacteria were counted as described above.
Microscopic examination of bacterium-cell interactions.
For transmission electron microscopy (TEM), HEp-2 or CHO cells were grown to approximately 80% confluency in 50-mm-diameter plastic petri dishes (Becton Dickinson, Plymouth, England), after which 108 bacteria were incubated with the tissue culture cells for 3 h at 37°C. The cells were then washed three times with PBS to remove nonadherent bacteria and were fixed in 2.5% glutaraldehyde for 1 h. After further washing, the cell monolayer was scraped off with a rubber policeman. Cells were then centrifuged to form a pellet, which was postfixed in 2.5% osmium tetroxide for 1 h, dehydrated through a graded acetone series, and embedded in Epon-araldite epoxy resin. Thin sections were cut and stained with 10% uranyl acetate and 2.5% lead citrate before viewing under a Philips CM12 electron microscope at 60 kV.
For light microscopic examination, 2 × 107 CFU was incubated at 37°C for 3 h with HEp-2 or CHO cells cultured on glass coverslips (50). Nonadherent bacteria were removed by three washes with PBS, and the cells were fixed with 100% methanol for 10 min. Cells were then stained with Giemsa stain (BDH, Dorset, England) and viewed by light microscopy.
Colonization of mice with Y. enterocolitica.
Six-week-old BALB/c mice were inoculated by gavage with 100 μl of a 10% solution of sodium bicarbonate, followed 30 min later by approximately 109 CFU of a test strain suspended in 200 μl of PBS. For each bacterial strain, three mice infected with the strain were killed by CO2 inhalation on days 2, 3, 4, 7, 10, 15, and 23 after inoculation. Portions of ileum, cecum, colon, mesenteric lymph node, liver, spleen, and femoral bone marrow were removed aseptically from each animal. Each sample was then weighed and diluted 1/10 (wt/vol) in PBS. The samples were then homogenized with a Polytron homogenizer (Kinematica, Lucerne, Switzerland), and the number of viable bacteria was determined on duplicate Yersinia selective agar plates (CIN; Oxoid).
Statistical analysis.
Data were analyzed by the Mann-Whitney test (two-tailed), the log-rank test, or Fisher’s exact test as appropriate. A critical P value of 0.05 was used for all analyses.
RESULTS
Examination of biotype-1A strains for virulence-associated genes of Yersinia species.
Although all 111 strains of Y. enterocolitica biotype 1A hybridized with the DNA probe prepared from the inv gene of Y. enterocolitica, thus confirming their identity as Yersinia species (40), no strain was recognized by probes prepared from pYV. Eight strains, four of human origin and four nonclinical isolates, were recognized by the probe for the ail gene (Table 1). Six of these strains (two clinical and four nonclinical) showed weak hybridization compared to the positive control. The myfA probe hybridized with 13 strains, 11 of which were isolated from humans and 2 of which were nonclinical. Sequences homologous to ystA were detected in only one strain—a clinical isolate. By contrast, 95 (85.6%) strains were recognized by the ystB probe, 69 of which were clinical isolates, and 26 of which were nonclinical. No strain was recognized by the ystC probe. The differences in the frequency of carriage of virulence-associated genes by clinical and nonclinical isolates were not significant (Table 1). All 20 biotype-1A strains (10 clinical and 10 nonclinical) examined for ymoA by PCR yielded a product of the expected size and nucleotide sequence, suggesting that the ymoA gene occurs in all Y. enterocolitica strains regardless of potential virulence.
TABLE 1.
Prevalence of virulence-associated genes of Y. enterocolitica and enterotoxin activity in Y. enterocolitica strains of biotype 1A
| Origin (n) | No. of strains positive for:
|
||||||
|---|---|---|---|---|---|---|---|
| pYVa | aila | myfAa | ystAa | ystBa | ystCa | Enterotoxin activityb | |
| Clinicalc (79) | 0 | 4 | 11 | 1 | 69 | 0 | 20 |
| Nonclinical (32) | 0 | 4 | 2 | 0 | 26 | 0 | 13 |
Determined by DNA hybridization.
Measured in suckling mice.
Differences between clinical and nonclinical isolates are not significant (P > 0.05 by Fisher’s exact test).
Production of enterotoxin.
In an earlier study, we showed that a clinical isolate of Y. enterocolitica biotype 1A produced a novel enterotoxin, which we termed YST-II (41). To determine if the ability to produce enterotoxin is a feature of clinical isolates of biotype-1A strains in general, we examined culture supernatants of Y. enterocolitica biotype-1A strains for enterotoxin(s) that was reactive in infant mice. Positive results were obtained with 20 of 79 clinical isolates and with 13 of 32 strains isolated from other sources (P = 0.17 by two-tailed Fisher’s exact test) (Table 1). Although none of these strains hybridized with the ystA or ystC gene probe, 27 (17 clinical and 10 nonclinical) hybridized with the ystB probe, suggesting that YST-b may contribute to the enterotoxigenicity of these bacteria. Six enterotoxin-secreting strains (three clinical and three nonclinical) were not recognized by the probes for ystA, ystB, or ystC, suggesting that these bacteria may produce an unidentified enterotoxin. Since YST-II has not been purified and the gene encoding its production has not been identified, we were unable to determine if the enterotoxigenicity of these bacteria was due to the presence of YST-II or to that of another, as yet uncharacterized, enterotoxin.
Adherence to and invasion of tissue culture cells.
The ability of Y. enterocolitica to invade epithelial cells is an important correlate of virulence (28, 44). One reason that strains of biotype 1A are generally regarded as avirulent is that they invade tissue culture cells to a lesser extent than Y. enterocolitica strains of virulent biotypes (25, 44). In this study, we investigated biotype-1A strains of clinical and nonclinical origins to determine if they differed in terms of their ability to adhere to or invade cultured epithelial cells.
Thirty-seven strains, twenty-five of clinical origin and twelve from other sources, were examined in a quantitative assay of adherence to HEp-2 cells (38). Most strains in the two groups adhered in large numbers, and there was no significant difference between strains of clinical and nonclinical origins (P = 0.31 by two-tailed Mann-Whitney test) (Fig. 1A).
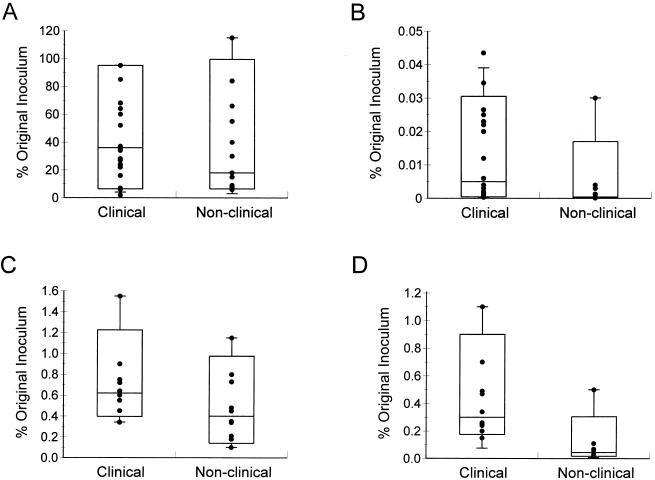
FIG. 1.
Abilities of clinical and nonclinical strains of Y. enterocolitica biotype 1A to adhere to (A) and invade (B) HEp-2 cells and to adhere to (C) and invade (D) CHO cells. Approximately 107 CFU was incubated with epithelial cells for 3 h before nonadherent bacteria were removed by three washes. To determine the number of adhesive bacteria, some epithelial cells were lysed and the cell-associated bacteria were enumerated. To determine the number of intracellular bacteria, other epithelial cells were incubated in fresh tissue culture medium containing 100 μg of gentamicin/ml for 90 min before epithelial cells were lysed and bacteria were enumerated. Data are expressed as percentages of the original inoculum and are means from at least two separate experiments using duplicate wells. In the box-and-whisker plots, the horizontal line within the box is the median value, the limits of the box are the 10th and 90th percentiles, and the whiskers are the 5th and 95th percentiles. The difference in adhesion between the two groups of isolates is not significant (P = 0.3 for HEp-2 cells and P = 0.14 for CHO cells by the Mann-Whitney test). The difference in invasion between the two groups of isolates is significant (P = 0.002 for HEp-2 cells and P < 0.001 for CHO cells).
Despite their high levels of adhesion, strains of biotype 1A invaded HEp-2 cells relatively poorly, with less than 0.05% of the original inoculum recovered (Fig. 1B). In contrast, the control strains, W22703c and 8081c, exhibited levels of invasion of 15 and 21% respectively. Interestingly, however, clinical isolates of biotype 1A penetrated HEp-2 cells to a significantly greater extent than nonclinical isolates (P = 0.002) (Fig. 1B). To determine if environmental factors influenced invasive capacity, we repeated the assay after growing the bacteria at 28 or 37°C, under aerobic or anaerobic conditions, and in MEM tissue culture medium, but none of these treatments significantly altered their invasive ability (data not shown). Consequently, we examined 10 clinical and 10 nonclinical isolates (the same strains investigated for the presence of ymoA) for their ability to invade CHO cells. The numbers of bacteria in the two groups of strains which adhered to CHO cells were similar (P = 0.14 by the Mann-Whitney test) (Fig. 1C) but approximately 50-fold less than the number which adhered to HEp-2 cells. Despite their relatively poor adherence, however, clinical isolates invaded CHO cells in numbers 10- to 100-fold greater than those which penetrated HEp-2 cells (Fig. 1D). The bacteria recovered from these cells ranged between 0.15 and 1.1% of the original inoculum for clinical strains and between 0.02 and 0.5% for nonclinical isolates (P < 0.001) (Fig. 1D). By comparison, the control strains, W22703c and 8081c, invaded CHO cells efficiently, with 21 and 29% of the original inoculum recovered, respectively (data not shown). Isolates which possessed sequences homologous to ail or myfA did not invade HEp-2 or CHO cells more efficiently than those which lacked these sequences (data not shown).
Microscopic examination of epithelial cells.
The interaction of Y. enterocolitica biotype-1A strains with HEp-2 and CHO cells was investigated by TEM and by light microscopy. TEM examination of HEp-2 cells inoculated with 10 different biotype-1A strains revealed bacteria loosely associated with the tissue culture cells (data not shown). Although occasional bacteria were identified within some HEp-2 cells, cells containing more than two bacteria were rarely observed and the vast majority of cells were uninfected (data not shown). Light microscopic examination of these cells showed that the bacteria did not exhibit any characteristic pattern of adherence analogous to the diffuse, localized, or aggregative phenotypes of some strains of diarrheagenic E. coli (31).
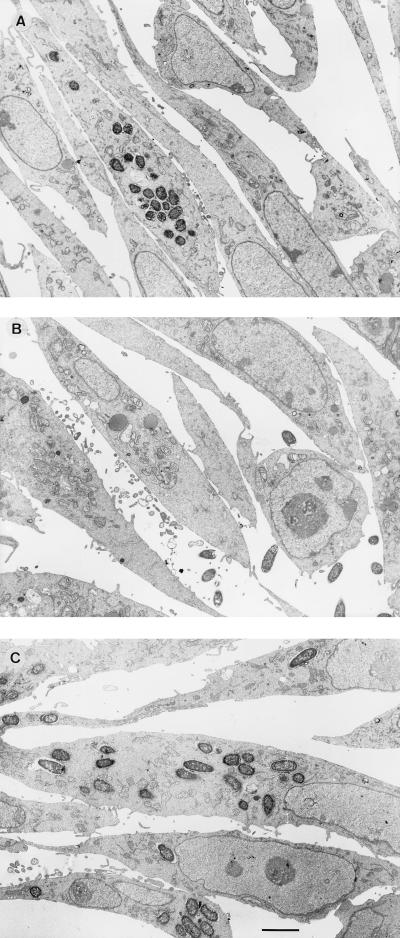
TEM examination of CHO cells incubated with Y. enterocolitica 937 (biotype 1A, serogroup O:6) revealed that some cells contained 20 to 30 internalized bacteria, although most cells were not infected (Fig. 2A). The impression that the majority of cells were not infected was verified by examination of serial sections above and below that shown in Fig. 2A. Some intracellular bacteria appeared to be replicating. This suspicion was confirmed by quantitative culture of internalized bacteria in CHO cells which had been incubated for various times in medium containing gentamicin (Table 2). Gentamicin was used because it kills extracellular (but not internalized) bacteria and hence would prevent any bacteria which may have escaped from the tissue culture cells during the course of the assay from reentering these cells (19).
FIG. 2.
TEM of CHO cells incubated for 3 h with representative strains of Y. enterocolitica: 937 (biotype 1A, invasive) (A), AM5 (biotype 1A, noninvasive) (B), and W22703c (biotype 2, invasive) (C). Findings similar to those illustrated in panels A and B were observed with eight other strains of Y. enterocolitica biotype 1A, five invasive and three noninvasive. Bar = 1 μm.
TABLE 2.
Effect of prolonged incubation on the number of internalized bacteria recovered from CHO cellsa
| Strain | Biotype | Origin | Internalized bacteriab after:
|
Fold increasec | |
|---|---|---|---|---|---|
| 4.5 h | 22.5 h | ||||
| Y. enterocolitica | |||||
| W22703c | 2 | Clinical | 22.5 ± 7.4 | 25.5 ± 2.2 | 1.1 |
| 937 | 1A | Clinical | 0.24 ± 0.11 | 1.0 ± 0.60 | 4.3 |
| T83 | 1A | Clinical | 0.38 ± 0.11 | 1.33 ± 0.06 | 3.5 |
| AM5 | 1A | Nonclinical | 0.03 ± 0.01 | 0.11 ± 0.08 | 4.3 |
| IP2222 | 1A | Nonclinical | 0.03 ± 0.02 | 0.21 ± 0.14 | 6.1 |
| E. coli HB101 | 0.01 ± 0.01 | 0.01 ± 0.02 | 1.5 | ||
This experiment was performed as described in Materials and Methods, “Bacterial adhesion to and invasion of tissue culture cells.” The counts at 4.5 and 22.5 h measured invasion of and replication in tissue culture cells, respectively.
Percent of original inoculum; mean ± standard deviation from three independent determinations.
Calculated from raw data.
To determine if this pattern of invasion of CHO cells is a characteristic of other biotype 1A strains, three clinical isolates (Y. enterocolitica AC12 [serogroup O:6,30], T83 [serogroup O:5], and 61525 [serogroup O:6,30]) and the two most invasive nonclinical isolates, CIDC 2064 (potato [serogroup O:5]) and CIDC 7134 (hay [serogroup O:6,31]), were chosen for further investigation by TEM. The results of these studies showed that these bacteria invaded CHO cells in a manner similar to that of strain 937. By contrast, CHO cells infected with poorly invasive biotype-1A strains of nonclinical origin, including two milk isolates, Y. enterocolitica AM5 (serogroup O:6) (Fig. 2B) and Y. enterocolitica AM8 (serogroup O:13,15), and two water isolates, IP2222 (serogroup O:36) and Eco88 (serogroup O:29), revealed no more than two bacteria per cell, with the great majority of cells being uninfected.
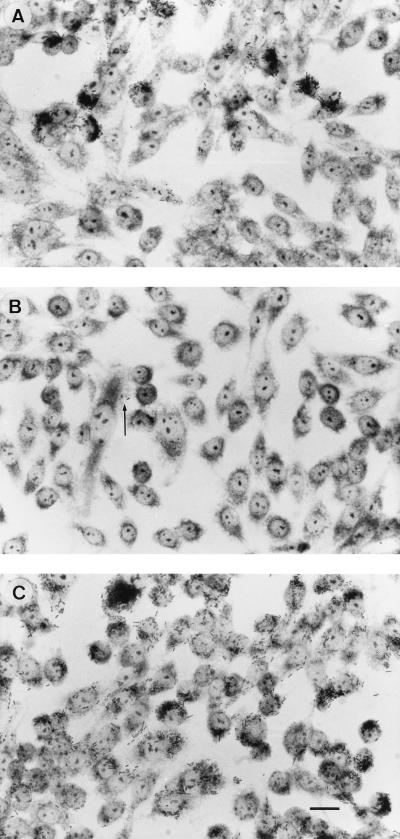
The patterns of adhesion and invasion of biotype-1A Y. enterocolitica were compared with those of strains W22703c and 8081c, which bear classical chromosomally encoded virulence markers but were cured of pYV. Although both of these strains lack the plasmid-encoded adhesin YadA, they adhered strongly to and invaded CHO cells with a more even distribution than that observed with biotype-1A yersiniae (Fig. 2C). To determine if the difference in the patterns of adhesion and invasion between biotype-1A yersiniae and other biotypes was due to invasin or Ail, Y. enterocolitica JP273c (Inv−) and YE2c (Ail−) and E. coli HB101(pVM101) (Inv+) and HB101(pVM103) (Ail+) were investigated in the CHO cell assay and examined by TEM. These studies showed that the Y. enterocolitica mutants and E. coli strains all displayed a far more even distribution of host cells invaded than invasive strains of biotype 1A (data not shown). To confirm the observation that invasive strains of yersiniae biotype 1A invade only some CHO cells, we repeated the assay with CHO cells grown on glass coverslips. Light-microscopic examination of these cells confirmed that invasive strains of biotype 1A examined by TEM adhered to and/or invaded selected CHO cells, while sparing the majority (Fig. 3A). This pattern of adherence and invasion was not observed with poorly invasive strains of environmental origin (Fig. 3B) or with the control strain W22703c (serogroup O:9, biotype 2) (Fig. 3C).
FIG. 3.
CHO cells incubated for 3 h with representative strains of Y. enterocolitica: 937 (biotype 1A, invasive) (A), AM5 (biotype 1A, noninvasive) (B), and W22703c (biotype 2, invasive) (C). Large numbers of strain 937 are associated with groups of five or more CHO cells, whereas only a few bacteria (arrows) of strain AM5 are associated with these cells. Strain W22703c demonstrates a more even pattern of association with the CHO cells. Findings similar to those illustrated in panels A and B were observed with eight other strains of Y. enterocolitica biotype 1A, five invasive and three noninvasive. Giemsa stain was used. Bar = 5 μm.
Colonization of mice by Y. enterocolitica biotype 1A.
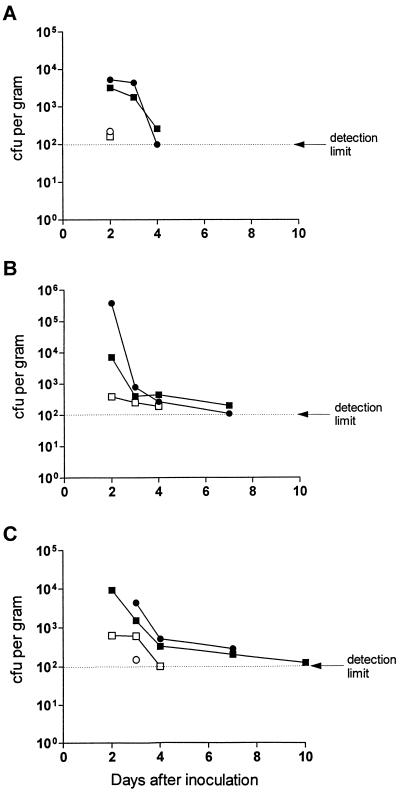
To investigate the ability of biotype-1A Y. enterocolitica to colonize the gastrointestinal tracts of mice, two clinical isolates, Y. enterocolitica 937 and 61525, and two which were not of clinical origin, Y. enterocolitica AM5 and IP2222, were administered perorally to adult BALB/c mice, which were then examined for the presence of the bacteria in the small and large intestines and in selected abdominal organs. The results showed that, compared with nonclinical strains, bacteria of clinical origin were detected for longer periods in the ileum (median, 5.5 versus 2.5 days; P = 0.003 by the log-rank test), cecum (7 versus 2 days; P = 0.01), and colon (10 versus 4 days; P = 0.003) in infected mice (Fig. 4). None of the strains tested was detected in the liver, spleen, or bone marrow at any time after infection, although Y. enterocolitica 937 and the control strain, W22703c, were cultured from mesenteric lymph nodes on the 2nd and 3rd days, respectively (data not shown).
FIG. 4.
Colonization of BALB/c mice by strains of Y. enterocolitica. Mice were inoculated by gavage with one of four strains of biotype-1A Y. enterocolitica, namely, clinical isolate 61525 (•) or 937 (▪) or nonclinical isolate IP2222 (○) or AM5 (□). Each point represents the mean CFU obtained from the ilea (A), ceca (B), or colons (C) of three mice at the times indicated after inoculation. The duration of colonization by clinical isolates in all three sites was significantly longer than that for the nonclinical strains (P ≤ 0.01).
DISCUSSION
Although biotype-1A Y. enterocolitica strains were once regarded as avirulent, the findings of several clinical and epidemiological studies suggest that these bacteria produce gastrointestinal symptoms similar to those caused by pYV-bearing strains of biotypes 1B and 2 through 5 (6, 30). Despite this evidence of pathogenicity, however, there are few reported studies of the pathogenic properties of biotype-1A yersiniae. The aim of this investigation was to identify virulence-associated markers of these bacteria.
To achieve this, we compared the virulence-associated characteristics of 79 biotype-1A isolates from symptomatic humans with those of 32 strains obtained from healthy animals, food, and the environment. The hypothesis underlying this approach was that biotype-1A strains of Y. enterocolitica may differ in pathogenicity, with clinical isolates more likely to display virulence-associated properties than bacteria obtained from other sources. In choosing the strains for this study, we took care to ensure that the bacteria came from a variety of sources and represented a wide range of serogroups.
In agreement with previous studies, we found that strains of biotype 1A did not carry pYV and possessed other classical Yersinia virulence factors only infrequently (13, 40). A possible exception to this was YST-b, which was detected by DNA hybridization in 85% of clinical and nonclinical strains. However, the frequencies of YST-b in clinical and nonclinical isolates were similar (P > 0.2). Moreover, the role of this toxin in the pathogenicity of Y. enterocolitica in general is unproven.
Some strains possessed sequences homologous to ail and myfA, which previously were considered to be restricted to highly invasive strains (21, 28, 40). The presence of these sequences was not associated with increased adhesion or invasion in vitro, however, suggesting that these genes may not be expressed in these bacteria, as is the case with inv (35, 40). The general lack of classical virulence genes in biotype-1A strains of Y. enterocolitica indicates clear differences between the virulence mechanisms of these bacteria and those of Y. enterocolitica strains of other biotypes.
The key novel finding of the present study was that biotype-1A strains of clinical origin invaded epithelial cells, especially CHO cells, in significantly greater numbers than bacteria obtained from other sources (Fig. 1). The finding that biotype-1A Y. enterocolitica invaded CHO cells more efficiently than HEp-2 cells is reminiscent of invasion mediated by Ail (27), but there was no correlation between invasive capacity and the presence of ail-homologous sequences, suggesting that biotype-1A yersiniae penetrate cells by a novel mechanism. In keeping with published observations (25, 44), biotype-1A yersiniae invaded tissue culture cells, including CHO and other polarized epithelial-cell lines, such as CaCo-2 and T84 cells (data not shown), less efficiently than the plasmidless biotype-2 strain W22703c. However, as classical pathogenic strains of Y. enterocolitica carry pYV, the products of which impede bacterial invasion of epithelial cells, these bacteria remain extracellular in host tissues (9, 15). Thus, it is conceivable that in infected tissues, more biotype-1A yersiniae (which never carry pYV) are located intracellularly than are classical pathogenic strains. The finding that individual isolates in the clinical and nonclinical groups overlapped in terms of their invasive capacities was not surprising, given that some clinical isolates may be nonpathogens which were obtained from patients whose symptoms were attributable to another cause and that some strains isolated from nonclinical sources were pathogens, given that the source of such bacteria is likely to be food, milk, water, or domestic animals (37).
The mechanisms which strains of biotype 1A used to invade epithelial cells is not known. The observation that bacteria penetrated selected tissue culture cells within a monolayer, while sparing others, suggests that biotype-1A strains invade cells by a novel process. The alternative hypothesis that clinical strains replicated more rapidly within the cells they had penetrated than nonclinical strains was discounted by the demonstration that strains 937 and T83 (clinical) and strains AM5 and IP2222 (nonclinical), which exhibited two distinct patterns of invasion (Fig. 2 and 3), grew at comparable rates within CHO cells they had invaded (Table 2). Interestingly, however, all four biotype-1A strains replicated more efficiently in CHO cells than the highly invasive biotype-2 strain or the E. coli control (Table 2). This observation points to another feature of biotype-1A strains which could be explored as a means to elucidate their virulence mechanisms.
The tendency for bacteria to associate with selected cells could be due to the presence of specific receptors at certain phases of the cell cycle, as has been suggested for E. coli, Clostridium difficile, and Listeria monocytogenes (14, 23, 24, 49). It is possible that invasion is not a virulence determinant of these bacteria but simply an in vitro marker of virulent strains. However, the capacity of biotype-1A yersiniae to penetrate tissue culture cells corresponded with an ability to colonize the intestinal tracts of orally inoculated mice. These two properties are likely to be linked, because bacteria which penetrate the intestinal epithelium would persist in the intestinal tract for longer periods than less-invasive strains. Studies to examine the invasive capacity of biotype-1A strains in vivo are currently in progress.
We previously suggested that the ability of biotype-1A Y. enterocolitica to cause diarrhea may be linked to its ability to produce the heat-stable enterotoxin YST-II (41). In this study, however, we found that only some of the clinical isolates we studied produced a mouse-reactive enterotoxin and that the proportions of enterotoxigenic bacteria were similar among clinical and nonclinical isolates. Interpretation of these findings is complicated by the facts that Y. enterocolitica seems capable of producing a variety of mouse-reactive enterotoxins, including YST-a, YST-b, YST-c, and YST-II, and that carriage of a yst gene does not invariably correspond to the presence of the toxin in culture supernatants. This may be because the genes are not functional or because their expression is reduced in vitro, possibly by ymoA, which modulates expression of the ystA gene in classical pathogenic strains of Y. enterocolitica (8). The finding that six toxin-secreting strains did not hybridize with any of the probes for YST-a, YST-b, or YST-c, suggests that these bacteria may produce an alternative toxin(s), possibly YST-II. This matter should be clarified when a probe for YST-II becomes available.
In conclusion, we have shown that Y. enterocolitica strains of biotype 1A associated with disease are able to invade epithelial cells and to colonize the intestines of mice. The mechanism by which these bacteria cause diarrhea is uncertain but may be due to the production of enterotoxin or of other, as yet unknown, virulence determinants. Current work in our laboratory is directed towards identifying these determinants.
ACKNOWLEDGMENTS
We are grateful to the many investigators who provided the strains that were the focus of this study, in particular S. Bell, Prince of Wales Hospital, Sydney, Australia; S. Fenwick, Massey University, Palmerston North, New Zealand; J. G. Morris, Jr., and A. Sulakvelidze, University of Maryland, Baltimore; and G. Wauters, Catholic University of Louvain, Louvain, Belgium. We are also indebted to V. L. Miller for the gift of Y. enterocolitica JP273 and YE2v, and E. coli HB101(pVM101) and HB101(pVM103).
This work was supported in part by grants from the Australian National Health and Medical Research Council and the Royal Children’s Hospital Research Foundation.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 2.Barrow G I, Feltham R K A. Cowan and Steel’s manual for the identification of medical bacteria. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 3.Bissett M L, Powers C, Abbott S L, Janda J M. Epidemiologic investigations of Yersinia enterocolitica and related species: sources, frequency, and serogroup distribution. J Clin Microbiol. 1990;28:910–912. doi: 10.1128/jcm.28.5.910-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska J B, Copass M C, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottone E J. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnens A P, Frey A, Nicolet J. Association between clinical presentation, biogroups and virulence attributes of Yersinia enterocolitica strains in human diarrhoeal disease. Epidemiol Infect. 1996;116:27–34. doi: 10.1017/s0950268800058921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis G, Laroche Y, Balligand G, Sory M-P, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis G, Sluiters C, Delor I, Geib D, Kaninga K, Lambert de Rouvroit C, Sory M-P, Vanooteghem J-C, Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol Microbiol. 1991;5:1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 10.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 11.de Koning-Ward T F, Robins-Browne R M. Contribution of urease to acid tolerance in Yersinia enterocolitica. Infect Immun. 1995;63:3790–3795. doi: 10.1128/iai.63.10.3790-3795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delor I, Cornelis G R. Role of Yersinia enterocolitica YST toxin in experimental infection of young rabbits. Infect Immun. 1992;60:4269–4277. doi: 10.1128/iai.60.10.4269-4277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delor I, Kaeckenbeeck A, Wauters G, Cornelis G R. Nucleotide sequence of yst, the Yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic yersiniae. Infect Immun. 1990;58:2983–2988. doi: 10.1128/iai.58.9.2983-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eveillard M, Fourel V, Barc M C, Kernéis S, Coconnier M-H, Karjalainen T, Bourlioux P, Servin A L. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol Microbiol. 1993;7:371–381. doi: 10.1111/j.1365-2958.1993.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 15.Fällman M, Persson C, Wolf-Watz H. Yersinia proteins that target host cell signalling pathways. J Clin Invest. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg A P, Vogelstein B. Addendum. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 17.Fenwick S G, McCarthy M D. Yersinia enterocolitica is a common cause of gastroenteritis in Auckland. N Z Med J. 1995;108:269–271. [PubMed] [Google Scholar]
- 18.Higuchi K, Smith J L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homes B, Quie P G, Windhorst W B. Protection of phagocytized bacteria from the killing action of antibiotics. Nature (London) 1966;210:1131–1142. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Yoshino K, Nakao H, Takeda T. Nucleotide sequence of a gene encoding the novel Yersinia enterocolitica heat-stable enterotoxin that includes pro-region-like sequence in its mature toxin molecule. Microb Pathog. 1997;22:89–97. doi: 10.1006/mpat.1996.0094. [DOI] [PubMed] [Google Scholar]
- 21.Iriarte M, Vanooteghem J C, Delor I, Diaz R, Knutton S, Cornelis G R. The Myf fibrillae of Yersinia enterocolitica. Mol Microbiol. 1993;9:507–520. doi: 10.1111/j.1365-2958.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 22.Kandolo K, Wauters G. Pyrazinamidase activity in Yersinia enterocolitica and related organisms. J Clin Microbiol. 1985;21:980–982. doi: 10.1128/jcm.21.6.980-982.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerneis S, Bilge S S, Fourel V, Chauviere G, Coconnier M-H, Servin A L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991;59:4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kernéis S, Chauvière G, Darfeuille-Michaud A, Aubel D, Coconnier M-H, Joly B, Servin A L. Expression of receptors for enterotoxigenic Escherichia coli during enterocyte differentiation of human polarized intestinal epithelial cells in culture. Infect Immun. 1992;60:2572–2580. doi: 10.1128/iai.60.7.2572-2580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee W H, McGrath P P, Carter P B, Eide E L. The ability of some Y. enterocolitica strains to invade HeLa cells. Can J Microbiol. 1977;23:1714–1722. doi: 10.1139/m77-247. [DOI] [PubMed] [Google Scholar]
- 26.Miller I, Maskell D, Hormaeche C, Johnson K, Pickard D, Dougan G. Isolation of orally attenuated Salmonella typhimurium following TnphoA mutagenesis. Infect Immun. 1989;57:2758–2763. doi: 10.1128/iai.57.9.2758-2763.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller V L, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller V L, Farmer III J J, Hill W E, Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989;57:121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller V L, Finlay B B, Falkow S. Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol. 1988;138:15–39. [PubMed] [Google Scholar]
- 30.Morris J G, Jr, Prado V, Ferreccio C, Robins-Browne R M, Bordun A-M, Cayazzo M, Kay B A, Levine M M. Yersinia enterocolitica isolated from two cohorts of young children in Santiago, Chile: incidence of and lack of correlation between illness and proposed virulence factors. J Clin Microbiol. 1991;29:2784–2788. doi: 10.1128/jcm.29.12.2784-2788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Noble M A, Barteluk R L, Freeman H J, Subramaniam R, Hudson J B. Clinical significance of virulence-related assay of Yersinia species. J Clin Microbiol. 1987;25:802–807. doi: 10.1128/jcm.25.5.802-807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pai C, Mors V, Seemayer T. Experimental Y. enterocolitica enteritis in rabbits. Infect Immun. 1980;28:238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pham J N, Bell S M, Lanzarone J Y M. Biotype and antibiotic sensitivity of 100 clinical isolates of Yersinia enterocolitica. J Antimicrob Chemother. 1991;28:13–18. doi: 10.1093/jac/28.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Pierson D E, Falkow S. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect Immun. 1990;58:1059–1064. doi: 10.1128/iai.58.4.1059-1064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratnam S, Mercer E, Picco B, Parsons S, Butler R. A nosocomial outbreak of diarrheal disease due to Yersinia enterocolitica serotype O:5, biotype 1. J Infect Dis. 1982;145:242–247. doi: 10.1093/infdis/145.2.242. [DOI] [PubMed] [Google Scholar]
- 37.Robins-Browne R M. Yersinia enterocolitica. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C: American Society for Microbiology; 1997. pp. 192–215. [Google Scholar]
- 38.Robins-Browne R M, Bennett-Wood V. Quantitative assessment of the ability of Escherichia coli to invade cultured animal cells. Microb Pathog. 1992;12:159–164. doi: 10.1016/0882-4010(92)90119-9. [DOI] [PubMed] [Google Scholar]
- 39.Robins-Browne R M, Jacobs M R, Koornhof H J, Mauff A C. Yersinia enterocolitica biotype 1 in South Africa. S Afr Med J. 1979;55:1057–1060. [PubMed] [Google Scholar]
- 40.Robins-Browne R M, Miliotis M D, Cianciosi S, Miller V L, Falkow S, Morris J G., Jr Evaluation of DNA colony hybridization and other techniques for detection of virulence in Yersinia species. J Clin Microbiol. 1989;27:644–650. doi: 10.1128/jcm.27.4.644-650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins-Browne R M, Takeda T, Fasano A, Bordun A-M, Dohi S, Kasuga H, Fang G, Prado V, Guerrant R L, Morris J G., Jr Assessment of enterotoxin production by Yersinia enterocolitica and identification of a novel heat-stable enterotoxin produced by a noninvasive Y. enterocolitica strain isolated from clinical material. Infect Immun. 1993;61:764–767. doi: 10.1128/iai.61.2.764-767.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robins-Browne R M, Tzipori S, Gonis G, Hayes J, Withers M, Prpic J K. The pathogenesis of Yersinia enterocolitica infection in gnotobiotic piglets. J Med Microbiol. 1985;19:297–308. doi: 10.1099/00222615-19-3-297. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Schiemann D A, Devenish J A. Relationship of HeLa cell infectivity to biochemical, serological, and virulence characteristics of Yersinia enterocolitica. Infect Immun. 1982;35:497–506. doi: 10.1128/iai.35.2.497-506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shayegani M, DeForge I, McGlynn D M, Root T. Characteristics of Yersinia enterocolitica and related species isolated from human, animal, and environmental sources. J Clin Microbiol. 1981;14:304–312. doi: 10.1128/jcm.14.3.304-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stolk-Engelaar V M M, Meis J F G M, Mulder J A, Loeffen F L A, Hoogkamp-Korstanje J A A. In-vitro antimicrobial susceptibility of Yersinia enterocolitica isolates from stools of patients in The Netherlands from 1982-1991. J Antimicrob Chemother. 1995;36:839–843. doi: 10.1093/jac/36.5.839. [DOI] [PubMed] [Google Scholar]
- 47.Sulakvelidze A, Dalakishvili K, Barry E, Wauters G, Robins-Browne R, Imnadze P, Morris J G., Jr Analysis of clinical and environmental Yersinia isolates in the Republic of Georgia. J Clin Microbiol. 1996;34:2325–2327. doi: 10.1128/jcm.34.9.2325-2327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Une T. Studies on the pathogenicity of Y. enterocolitica. I. Experimental infection in rabbits. Microbiol Immunol. 1977;21:349–363. [PubMed] [Google Scholar]
- 49.Velge P, Bottreau E, Kaeffer B, Pardon P. Cell immortalization enhances Listeria monocytogenes invasion. Med Microbiol Immunol. 1994;183:145–158. doi: 10.1007/BF00196049. [DOI] [PubMed] [Google Scholar]
- 50.Vial P A, Mathewson J J, Guers L, Levine M M, DuPont H L. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J Clin Microbiol. 1990;28:882–885. doi: 10.1128/jcm.28.5.882-885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wauters G, Kandolo K, Janssens M. Revised biogrouping scheme of Yersinia enterocolitica. Contrib Microbiol Immunol. 1987;9:14–21. [PubMed] [Google Scholar]
- 52.Yoshino K, Huang X, Miyachi M, Hong Y-M, Takao T, Nakao H, Takeda T, Shimonishi Y. Amino acid sequence of a novel heat-stable enterotoxin produced by a yst gene-negative strain of Yersinia enterocolitica. Lett Pept Sci. 1994;1:95–105. [Google Scholar]
- 53.Yoshino K, Takao T, Huang X, Murata H, Nakao H, Takeda T, Shimonishi Y. Characterization of a highly toxic, large molecular size heat-stable enterotoxin produced by a clinical isolate of Yersinia enterocolitica. FEBS Lett. 1995;362:319–322. doi: 10.1016/0014-5793(95)00267-d. [DOI] [PubMed] [Google Scholar]