Abstract
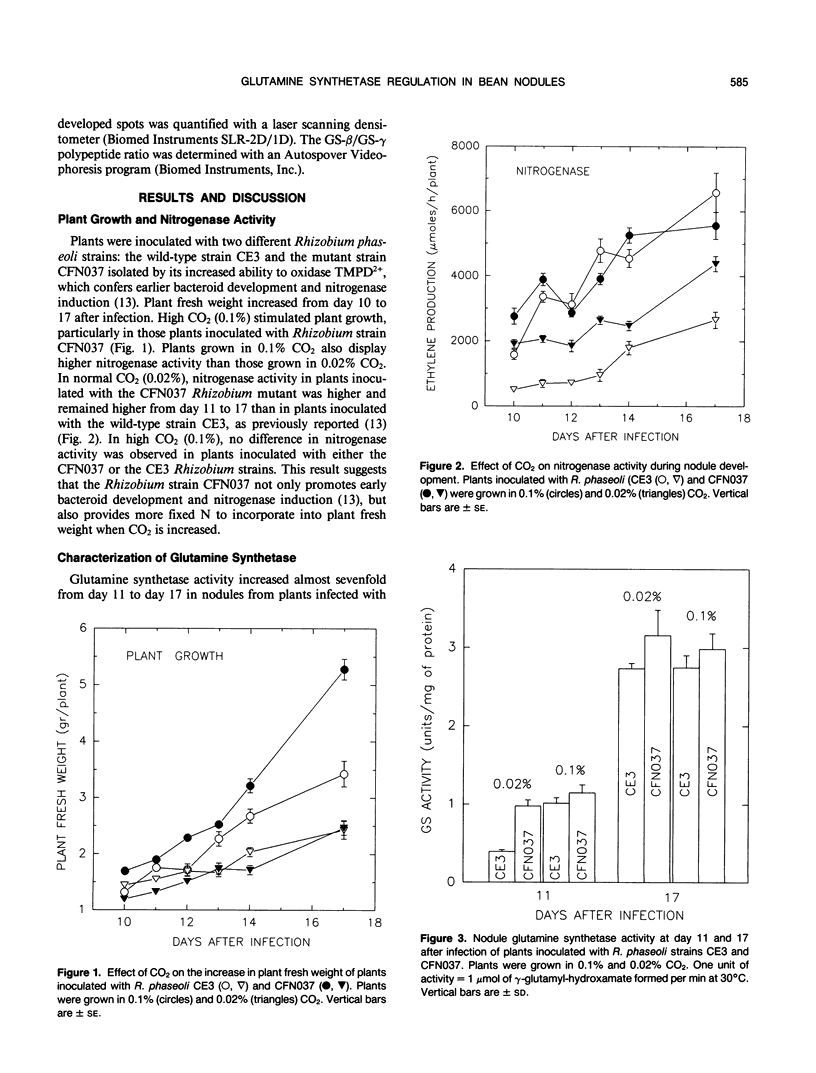
Nodulated bean (Phaseolus vulgaris) plants were grown for 17 days after infection in normal (0.02%) CO2 and from day 8 to 17 in high (0.1%) CO2 in order to increase nitrogen fixation and define how nodule glutamine synthetase (GS) isoforms are regulated by the ammonia derived from the bacteroid. Nitrogenase activity was detected by day 10, and by day 17 activity was over twofold higher in 0.1% of CO2 compared with plants grown in 0.02% CO2 and inoculated with Rhizobium wild-type strain CE3. Likewise, plant fresh weight increased in response to increased CO2, particularly in plants inoculated with the Rhizobium phaseoli mutant strain CFN037. Glutamine synthetase specific activity increased 2.5- to 6.5-fold from day 11 to 17. However, increased CO2 did not appear to have an effect on GS specific activity. Analysis of the nodule GS polypeptide composition revealed that the γ polypeptide was significantly reduced in response to high CO2, whereas the β polypeptide was not affected. The significance of this result in relation to the regulation of GS isoforms and their role in the assimilation of ammonia in the nodule is discussed in this paper.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Egli M. A., Griffith S. M., Miller S. S., Anderson M. P., Vance C. P. Nitrogen Assimilating Enzyme Activities and Enzyme Protein during Development and Senescence of Effective and Plant Gene-Controlled Ineffective Alfalfa Nodules. Plant Physiol. 1989 Nov;91(3):898–904. doi: 10.1104/pp.91.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. R., Sims A. P. The regulation of glutamine metabolism in Candida utilis: the role of glutamine in the control of glutamine synthetase. J Gen Microbiol. 1974 Jan;80(1):159–171. doi: 10.1099/00221287-80-1-159. [DOI] [PubMed] [Google Scholar]
- Lara M., Porta H., Padilla J., Folch J., Sánchez F. Heterogeneity of Glutamine Synthetase Polypeptides in Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):1019–1023. doi: 10.1104/pp.76.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao G. H., Hirel B., Marsolier M. C., Ridge R. W., Verma D. P. Ammonia-regulated expression of a soybean gene encoding cytosolic glutamine synthetase in transgenic Lotus corniculatus. Plant Cell. 1991 Jan;3(1):11–22. doi: 10.1105/tpc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Soberón M., Membrillo-Hernández J., Aguilar G. R., Sánchez F. Isolation of Rhizobium phaseoli Tn5-induced mutants with altered expression of cytochrome terminal oxidases o and aa3. J Bacteriol. 1990 Mar;172(3):1676–1680. doi: 10.1128/jb.172.3.1676-1680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



