Abstract
The cGAS-STING pathway detects cytosolic DNA and activates a signaling cascade that results in a type I interferon (IFN) response. The endoplasmic reticulum (ER)-associated exonuclease TREX1 suppresses cGAS-STING by eliminating DNA from the cytosol. Mutations that compromise TREX1 function are linked to autoinflammatory disorders, including systemic lupus erythematosus (SLE) and Aicardi-Goutières syndrome (AGS). Despite key roles in regulating cGAS-STING and suppressing excessive inflammation, the impact of many disease-associated TREX1 mutations - particularly those outside of the core catalytic domains - remains poorly understood. Here, we characterize a recessive AGS-linked TREX1 P61Q mutation occurring within the poorly characterized polyproline helix (PPII) motif. In keeping with its position outside of the catalytic core or ER targeting motifs, neither the P61Q mutation, nor aggregate proline-to-alanine PPII mutation, disrupt TREX1 exonuclease activity, subcellular localization, or cGAS-STING regulation in overexpression systems. Introducing targeted mutations into the endogenous TREX1 locus revealed that PPII mutations destabilize the protein, resulting in impaired exonuclease activity and unrestrained cGAS-STING activation. Overall, these results demonstrate that TREX1 PPII mutations, including P61Q, impair proper immune regulation and lead to autoimmune disease through TREX1 destabilization.
Keywords: cGAS, STING, TREX1, Aicardi-Goutières syndrome
INTRODUCTION
Type I interferonopathies, such as the monogenic disease Aicardi-Goutières syndrome (AGS), often involve chronic systemic and neurological autoinflammation and high levels of type I interferon (IFN) activity in the blood and cerebrospinal fluid (Crow and Stetson, 2022). AGS can result from loss-of-function (or specific dominant-negative) mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR1, gain-of-function mutations in IFIH1 (Lehtinen et al., 2008; Rice et al., 2007a). Mutations in TREX1 are among the most common in AGS, accounting for nearly one-quarter of all AGS-linked mutations (Crow et al., 2015; Rice et al., 2007b).
TREX1 is a 3′→5′ exonuclease that degrades cytosolic DNA to act as a nucleolytic antagonist of the cGAS-STING pathway (Ablasser et al., 2014; Gray et al., 2015; Grieves et al., 2015; Mazur and Perrino, 2001; Stetson et al., 2008; Wolf et al., 2016). Binding to cytosolic DNA stimulates cGAS catalytic activity and the production of the 2′3′-cyclic GMP-AMP (cGAMP) second messenger (Ablasser et al., 2013; Diner et al., 2013; Gao et al., 2013). cGAMP engagement with its downstream receptor STING ultimately results in activation of the transcription factor IRF3 and the expression of type I IFNs and other immunomodulatory proteins (Ablasser and Chen, 2019).
Mouse models of TREX1 dysfunction recapitulate hallmarks of AGS and related disorders, including familial chilblain lupus. Trex1-deficient mice exhibit multi-organ inflammation and decreased survival (Grieves et al., 2015; Stetson et al., 2008). Replacement of the wild-type Trex1 gene in mice with the nuclease-deficient Trex1 D18N mutant results in a lupus-like disease (Grieves et al., 2015). The health and viability of Trex1-deficient animals are restored by deletion of Cgas, Sting1, Irf3 and Ifnar, indicating that unchecked DNA sensing is responsible for the observed pathologies (Ablasser et al., 2014; Ahn et al., 2014; Gao et al., 2015; Gray et al., 2015; Stetson et al., 2008).
Specific dominant-negative mutations in TREX1 include D18N, D200N, and H195Y, which disrupt key catalytic residues, and more frequently observed recessive alleles, e.g. R114H and R97H, that occur in the dimerization surface and hinder requisite homodimerization of TREX1 (Lehtinen et al., 2008; Rice et al., 2015). Other less-common mutations have been proposed to impede TREX1 function by altering phase separation or by destabilizing the protein (Zhou et al., 2022, 2021). The mechanisms associated with many disease-linked TREX1 mutations are poorly understood.
Outside of its catalytic core, TREX1 possesses a single-pass transmembrane helix at its C-terminus that anchors the protein in the ER and positions the nuclease domain in the cytosol (Lee-Kirsch et al., 2007; Mazur and Perrino, 2001; Mohr et al., 2021; Wolf et al., 2016). Deleting this C-terminal extension ablates TREX1 ER localization but does not affect its catalytic activity (De Silva et al., 2007; Lee-Kirsch et al., 2007). TREX1 mutations that truncate the C-terminus disrupt TREX1-ER association while preserving nucleolytic activity, and are associated with a distinct clinical disease referred to as retinal vasculopathy with cerebral leukoencephalopathy (RVCL) (Crow and Manel, 2015; Yan, 2017). RVCL is inherited in an autosomal dominant manner and lacks clear links to excessive type I IFN production (Rodero et al., 2017).
The non-repetitive proline-rich region termed the polyproline II helix (PPII) is another unique motif present in TREX1, but not found in other nucleases within the larger DnaQ family, including the closely related TREX2 homolog (Brucet et al., 2007; De Silva et al., 2007). Like the TREX1 C-terminal extension, the positioning of the PPII helix distal to the TREX1 active site and its absence from the otherwise closely related, catalytically active TREX2 nuclease suggest that it is also unlikely to participate in catalysis or DNA binding. The functional significance of this domain is not known.
Here, we report that TREX1 P61Q mutations located in the PPII motif are linked with AGS and show how these mutations destabilize TREX1 without directly affecting nucleolytic activity or subcellular localization. We demonstrate that TREX1 P61Q instability causes overactive cGAS-STING signaling, ultimately resulting in cGAMP overproduction and excessive levels of type I IFN expression. Thus, these results indicate that the TREX1 P61Q mutations cause AGS through TREX1 protein destabilization and suggest that protein destabilization may account for a subset of AGS patients with TREX1 mutations.
RESULTS
TREX1 PPII mutations are associated with AGS
We identified proline-to-glutamine (P61Q) point mutations in TREX1 in two patients from two families presenting with features of AGS (Fig. 1A; AGS972: c.182C>A p.Pro61Gln homozygote; AGS1583: p.Pro61Gln/Arg114His compound heterozygote) (Rice et al., 2017). Since R114H renders the allele null by disrupting obligate dimerization of TREX1 (Lehtinen et al., 2008), these findings suggest a recessive, loss-of-function nature of the P61Q mutation. Indeed, calculation of IFN scores, derived by measuring the expression of six IFN stimulated genes (ISGs) using quantitative polymerase chain reaction, revealed a significant upregulation of IFN signaling relative to persons considered to be controls, thus placing both individuals within the type I interferonopathy spectrum (Crow and Manel, 2015; Rice et al., 2017).
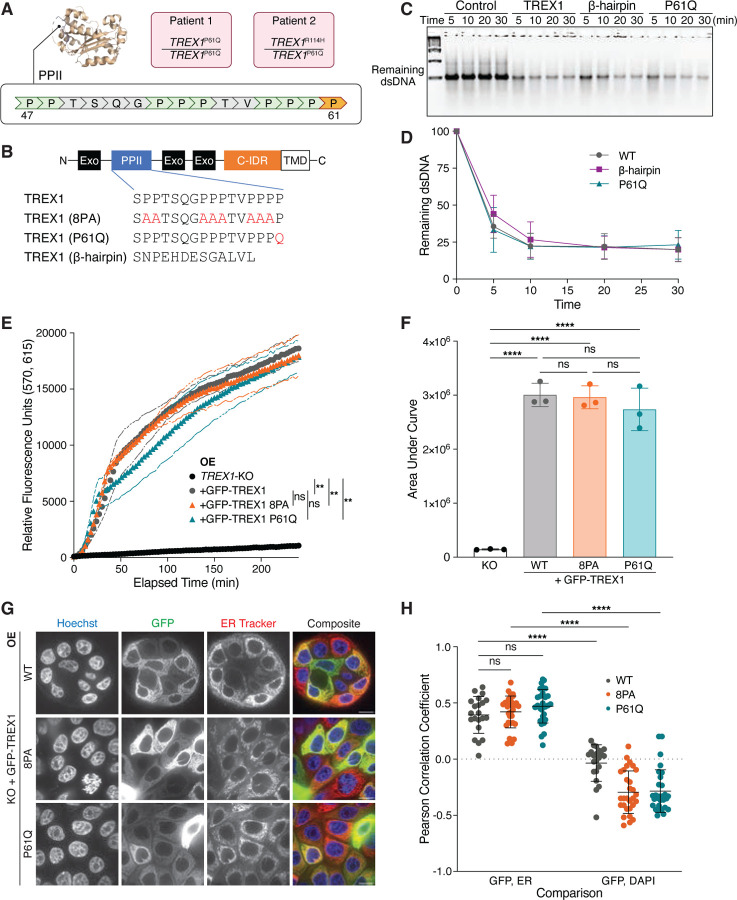
Figure 1. Mutations in PPII are linked to AGS but do not compromise intrinsic functions of overexpressed TREX1.
A. Location of PPII and P61 (orange) within TREX1. Genotypes of two AGS patients harboring the P61Q mutation are shown in pink. B. Schematic of GFP-TREX1 mutants used to reconstitute MCF10A TREX1 KO cells via lentiviral overexpression. Exo = exonuclease domain; C-IDR = C-terminal intrinsically disordered region; TMD = transmembrane domain responsible for TREX1-ER linkage. C. Representative DNA gel from in vitro nuclease assay. A dsDNA substrate was co-incubated with purified TREX1 mutant protein for the indicated duration. Control = no TREX1 added; β-hairpin = TREX1 with PPII replaced with TREX2 β-hairpin occurring at corresponding position as TREX1. P61Q = TREX1 P61Q D. Quantification of the in vitro nuclease assay in (C); mean ± s.d., n = 3, two-way ANOVA (interaction p = 0.5020). E. Time course fluorescence reading of the lysate-based nuclease assay. Briefly, a dsDNA substrate labeled with adjacent TEX615 fluorophore and Iowa Black quencher was co-incubated with whole cell lysates. 3′→5′ exonuclease activity eliminates the quencher, liberating TEX615 fluorescence; mean ± s.d., n = 3, **p < 0.01, ns = not significant, two-way ANOVA (interaction p < 0.0001, time p < 0.0001, genotype p < 0.0001). F. Definite integral values from t = 0 min to t = 240 min for each time course sample in (E); mean ± s.d., n = 3, ****p < 0.0001, ns = not significant, one-way ANOVA (p < 0.0001). G. Live-cell images of GFP-TREX1 (green) in TREX1-KO cells. DNA was stained with Hoechst 33342 (blue) and ER was stained with ER Tracker Red (red). Scale bars = 10 μm. H. Pearson correlation coefficients of the indicated cells as in Fig. 1G; mean ± s.d., n = 5 experiments, ****p < 0.0001, ns = not significant, two-way ANOVA (interactions p < 0.0001, comparison pair p < 0.0001, genotype p = 0.0027).
Pro-61 lies in a proline-rich tract termed the polyproline II (PPII) helix (Fig. 1A,B) (Brucet et al., 2007; De Silva et al., 2007). PPII positioning distal to the TREX1 active site and its absence from other catalytically proficient enzymes of the DnaQ family, including TREX2, suggest it is likely to be dispensable for nucleolytic activity. To test this directly, we purified the N-terminal enzymatic domain of TREX1 proteins, including human TREX1, a TREX1 P61Q mutant, and a TREX1 PPII>β-hairpin chimera, in which the TREX1 PPII helix is replaced by the β-hairpin found in the corresponding position within TREX2 (Fig. 1B). As expected, in vitro nuclease assays using purified proteins demonstrated that TREX1 P61Q and β-hairpin mutants digested dsDNA with efficiencies comparable to the wild-type enzyme with >50% of substrate degraded within the first 5 minutes of incubation (Fig. 1C,D).
To further investigate the potential impact of TREX1 PPII mutations we assayed TREX1 exonuclease activity in cell lysates. In brief, lysates were incubated with a dsDNA substrate possessing a fluorescent label at one 5′ end closely positioned next to a 3′ quencher (Methods). TREX1 3′→5′ exonuclease activity is predicted to liberate the fluorescent dye from the 3′ quencher and thus result in the acquisition of fluorescence. Cell lysates were prepared from TREX1-deficient MCF10A cells stably transduced with GFP-TREX1-WT, GFP-TREX1-P61Q, and GFP-TREX1-8PA, in which eight prolines in PPII - excluding P61 - are mutated to alanine (Fig. 1B). Lentiviral transduction of these constructs into TREX1-deficient MCF10A cells yielded stable overexpression of GFP-tagged mutant proteins, with no significant differences in protein levels between the three genotypes (Fig. S1A and S1B). As expected, incubation of the dsDNA probe with lysates prepared from MCF10A TREX1 KO cells reconstituted with GFP-TREX1-WT resulted in the rapid acquisition of fluorescence (Fig. 1E,F). In contrast, TREX1 deletion severely diminished the acquisition of fluorescence, confirming the specificity of this assay for TREX1 exonuclease activity (Fig. 1E,F). Similar to results obtained using isolated proteins, measurement of GFP-TREX1-8PA and GFP-TREX1-P61Q activities exhibited no significant differences from GFP-TREX1-WT (Fig. 1E,F). Taken together, these data indicate that targeted mutations within the PPII helix do not directly interfere with TREX1 exonuclease activity and suggest that the PPII helix is dispensable for TREX1 exonuclease activity against dsDNA.
We previously demonstrated that TREX1 association with the ER is critical for processing a subset of cytosolic DNA substrates including nuclear aberrations like micronuclei (Mohr et al., 2021). Positioning of the PPII within the catalytic core and distal to the ER transmembrane domain at the C-terminus of TREX1 suggested that the PPII domain is likely dispensable for ER association. To test this possibility directly, we performed live-cell imaging of cells overexpressing GFP-TREX1 mutants to characterize their subcellular localization. As previously reported (Mohr et al., 2021; Stetson et al., 2008; Wolf et al., 2016), GFP-TREX1-WT was excluded from the nucleus and its localization significantly overlapped with the ER, as indicated by staining with an ER tracker dye (Fig. 1G,H). GFP-TREX1-8PA and GFP-TREX1-P61Q subcellular localizations could not be distinguished from that of the wild-type enzyme, suggesting that the PPII is dispensable for directing TREX1 ER association (Fig. 1G,H; Figure S1C,D). Together, these data indicate that PPII mutations are unlikely to cause TREX1 dysfunction by interfering with its ER localization.
Overexpressed TREX1 PPII mutants suppress cGAS-STING signaling
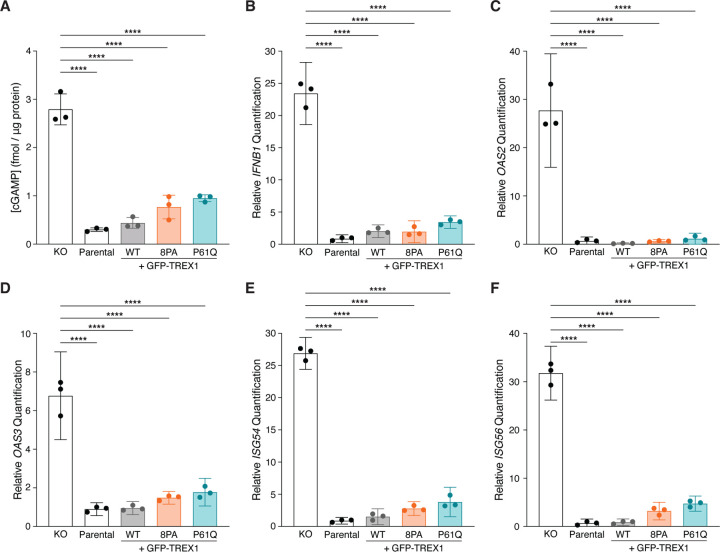
To test whether PPII mutations affect cGAS activation, we quantified intracellular cGAMP via ELISA (Fig. 2A). MCF10A cells lack high levels of cytosolic DNA and do not show strong cGAS activity at baseline, even upon TREX1 deletion (Mohr et al., 2021; Zhou et al., 2021). We therefore stimulated cGAS activation by herring testes (HT-) DNA transfection. ELISA analysis revealed low to undetectable amounts of cGAMP (0.3005±0.03630 s.d. fmol/μg protein) in MCF10A cells after HT-DNA stimulation (Fig. 2A). As expected, cGAMP levels increased dramatically in TREX1 KO cell lysates following HT-DNA transfection (2.792±0.3207 s.d. fmol/μg protein) (Fig. 2A). Reconstitution of MCF10A TREX1 KO cells by overexpressing GFP-TREX1-WT diminished cGAMP to levels observed in the parental cell line (0.4398±0.1119 s.d. fmol/μg protein) (Fig. 2A). In keeping with their catalytic proficiency and normal ER localization, GFP-TREX1-8PA and GFP-TREX1-P61Q overexpression led to cGAMP reductions that were comparable to the wild-type GFP-TREX1 transgene (0.7678±0.2449 s.d. fmol/μg protein for GFP-TREX1-8PA; 0.9510±0.06908 s.d. fmol/μg protein for GFP-TREX1-P61Q).
Figure 2. Overexpressed TREX1 mutants can suppress cGAS-STING signaling.
A. ELISA analysis of cGAMP production in the indicated cells following the transfection of 4 μg HT-DNA; mean ± s.d., n = 3, ****p < 0.0001, one-way ANOVA (p < 0.0001). B–F. RT-qPCR of IFNB1, OAS2, OAS3, ISG54, and ISG56 expression in the indicated MCF10A cells following the transfection of 4 μg HT-DNA; mean ± s.d., n = 3, ****p < 0.0001, one-way ANOVA (p < 0.0001 for IFNB1, p < 0.0001 for OAS2, p < 0.0001 for OAS3, p < 0.0001 for ISG54, and p < 0.0001 for ISG56).
We next sought to determine if TREX1 PPII mutations impacted the downstream cGAS-STING response by using RT-qPCR to measure expression of IFNB1 and interferon-stimulated genes (ISGs) such as OAS2, OAS3, ISG54, and ISG56 (Fig. 2B–F). As expected, RT-qPCR revealed strong increases in IFNB1 and ISG mRNA levels in TREX1 KO MCF10A cells upon HT-DNA stimulation relative to parental controls (Fig. 2B–F). In line with our cGAMP ELISA results, GFP-TREX1-WT, GFP-TREX1-8PA and GFP-TREX1-P61Q suppressed IFNB1 and ISG expression to similar degrees upon overexpression in TREX1 KO cells (Fig. 2B–F). Thus, counter to expectations based on the association between the TREX1 P61Q mutations and AGS (Fig. 1A), these results indicate that TREX1 PPII mutants are functionally proficient to suppress cGAS activation and downstream ISG expression upon overexpression in MCF10A cells.
TREX1 PPII mutations destabilize the protein
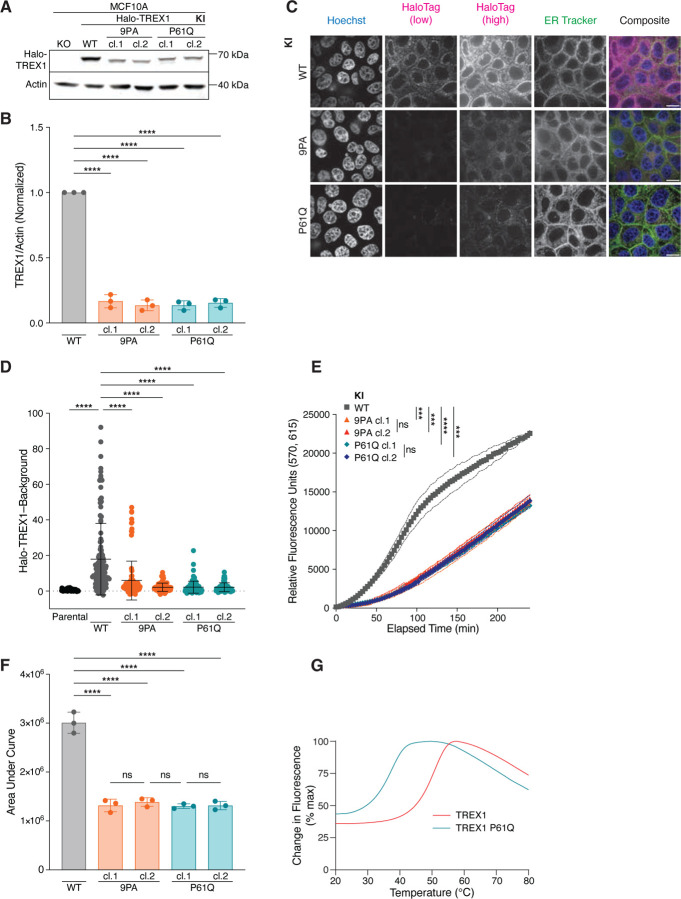
We reasoned that strong TREX1 overexpression resulting from lentiviral delivery (Fig. S1A and S1B) may obscure defects associated with PPII mutation. We therefore used CRISPR-Cas9 gene editing to endogenously introduce an N-terminal HaloTag concurrently with a PPII edit—proline-to-alanine mutation of all nine prolines in PPII (9PA) or P61Q - into the diploid MCF10A cell line (Fig. S2A). The remaining, unedited allele was deleted, yielding Halo-TREX1/Δ genotypes for all subsequent experiments (Fig. S2A). All gene edits were validated by Sanger sequencing and PCR screening (Fig. S2B–D). Immunoblotting with anti-TREX1 antibodies further confirmed successful insertion of the HaloTag into the endogenous TREX1 locus. (Fig. 3A).
Figure 3. PPII mutations destabilize TREX1 and reduce TREX1 exonucleolytic activity.
A. Immunoblot of MCF10A knock-in (KI) cell lines using anti-TREX1 and anti-actin antibodies. B. Quantification of TREX1 immunoblot signal normalized to actin; mean ± s.d., n = 3, ****p < 0.0001, one-way ANOVA (p < 0.0001). For each replicate, the WT TREX1/Actin signal was set to one. C. Live-cell images Halo-TREX1 (magenta) in MCF10A knock-in cell lines. DNA was stained with Hoechst 33342 (blue) and ER was stained with ER Tracker Green (green). Halo-TREX1 images are shown using two lookup tables in order to highlight differences in fluorescence signal (HaloTag low) and to depict ER localization (HaloTag high). Scale bars = 10 μm. D. Quantification of Halo-TREX1 signal in the indicated MCF10A cells as in (E); mean ± s.d., n = 5 experiments, ****p < 0.0001, one-way ANOVA (p < 0.0001). E. Time course fluorescence reading of lysate-based nuclease assay, using MCF10A knock-in cell lines; mean ± s.d., n = 3, ***p < 0.001, ****p < 0.0001, ns = not significant, two-way ANOVA (interaction p < 0.0001, time p < 0.0001, genotype p < 0.0001). F. Definite integral values from t = 0 min to t = 240 min for each time course sample in (E); mean ± s.d., n = 3, ****p < 0.0001, one-way ANOVA (p < 0.0001). G. Thermal shift assay using purified TREX1 proteins. Tm = 51 °C for TREX1 WT, Tm = 37.5 °C for TREX1 P61Q.
Interestingly, immunoblotting revealed significantly diminished Halo-TREX1-9PA and Halo-TREX1-P61Q signals in multiple, independently isolated subclones relative to the wild-type Halo-TREX1 control (Fig. 3A,B). Live-cell imaging confirmed decreased expression of Halo-TREX1-9PA and Halo-TREX1-P61Q relative to wild-type Halo-TREX1 (Fig. 3C and 3D). As expected, neither mutation compromised the ER localization of TREX1 (Fig. S3A and S3B). Similar to our prior results from GFP-TREX1 overexpression (Fig. 1E and 1F), all Halo-TREX1 lysates retained the ability to digest dsDNA (Fig. 3E and 3F). However, fluorescence increased at a much slower rate in Halo-TREX1-9PA/∆ and Halo-TREX1-P61Q/∆ lysates than Halo-TREX1-wild-type/∆ lysates, with the area under curve values decreased about two-fold. Thus, TREX1-P61Q and TREX1-9PA mutations lead to significant reductions in protein levels that are associated with corresponding decreases in nucleolytic activity.
Observed reductions in TREX1-9PA and TREX1-P61Q protein levels and activity could not be explained by reduced TREX1 mRNA expression (Fig. S3C). Instead, Thermofluor analysis of purified TREX1 and TREX1-P61Q proteins demonstrated a significant 13.5°C difference in protein stability with TREX1 exhibiting a melting temperature (Tm) of 51°C and TREX1-P61Q exhibiting a Tm of 37.5°C (Fig. 3G). These results indicate that TREX1 PPII mutations destabilize the protein, and thus lead to reduced overall protein levels with corresponding decreases in nucleolytic activity.
cGAS-STING signaling is elevated in TREX1 PPII mutant cells
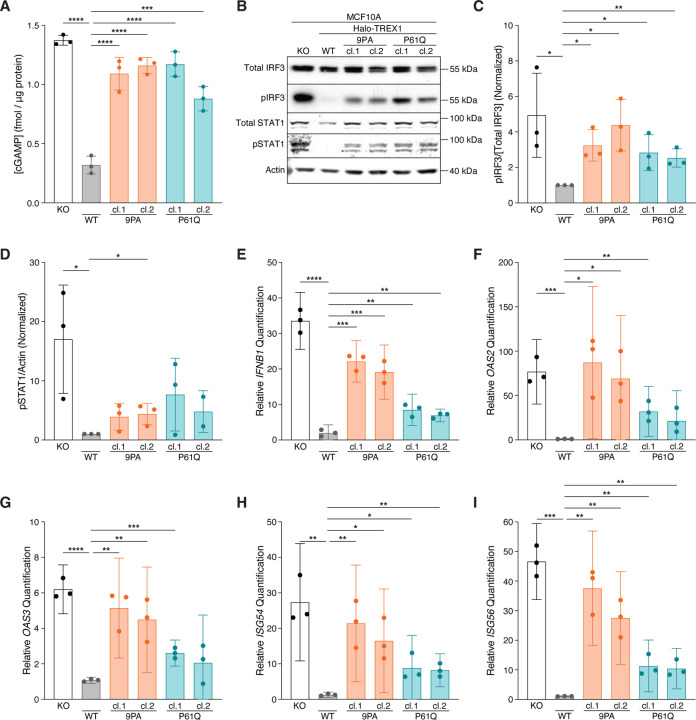
We next asked whether Halo-TREX1-PPII mutations interfere with cGAS-STING regulation. cGAMP ELISA analysis following stimulation by HT-DNA transfection demonstrated significant increases in intracellular cGAMP levels in multiple, independently isolated Halo-TREX1-9PA (1.092±0.1379 s.d. fmol/μg protein for clone 1; 1.161±0.06758 s.d. fmol/μg protein for clone 2) and Halo-TREX1-P61Q (1.171±0.1046 s.d. fmol/μg protein for clone 1; 0.8806±0.1024 s.d. fmol/μg protein for clone 2) mutant cell lines relative to a wild-type Halo-TREX1 control line (0.3194±0.07516 s.d. fmol/μg protein) (Fig. 4A). Indeed, cGAMP levels in lysates prepared from Halo-TREX1-P61Q and Halo-TREX1-9PA mutant cells more closely resembled levels measured in TREX1 KO lysates (1.374±0.04191 s.d. fmol/μg protein). cGAMP levels were unchanged in all cell lines tested, including TREX1 KO lines, following mock transfection, further confirming that MCF10A cells lack sufficient cytosolic dsDNA to activate an immune response under baseline conditions (data not shown).
Figure 4. Mutations in PPII activate cGAS-STING signaling.
A. ELISA analysis of cGAMP production in the indicated cells following the transfection of 4 μg HT-DNA; mean ± s.d., n = 3, ***p < 0.001, ****p < 0.0001, one-way ANOVA with post-hoc pairwise comparisons (p < 0.0001). B. Immunoblot of MCF10A knock-in (KI) cell lines using anti-IRF3, anti-phospho-S386 IRF3, anti-STAT1, anti-phospho-Y701 STAT1, and anti-actin. C. Quantification of phospho-S386 IRF3 immunoblot signal normalized to total IRF3 as in Fig. 4B; mean ± s.d., n = 3, *p < 0.05, **p < 0.01, unpaired two-tailed t-tests. For each replicate, the WT pIRF3/[Total IRF3] signal was set to one. D. Quantification of phospho-Y701 STAT1 immunoblot signal normalized to actin; mean ± s.d., n = 3, *p < 0.05, unpaired two-tailed t-tests. For each replicate, the WT pSTAT1/actin signal was set to one. E–I. RT-qPCR of IFNB1, OAS2, OAS3, ISG54, and ISG56 expression in the indicated cells following the transfection of 4 μg HT-DNA; mean ± s.d., n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, unpaired two-tailed t-tests.
Following cGAS-STING activation, TBK1 phosphorylates the transcription factor IRF3 at multiple residues including S386 and S396, inducing IRF3 dimerization and transcription of type I IFN (Liu et al., 2015). Increased type I IFN signaling results in the phosphorylation and activation of STAT1 (pY701)/STAT2 heterodimers, ultimately culminating in the transactivation of a wide-ranging pro-inflammatory response (Galluzzi et al., 2018). We therefore immunoblotted for phospho-IRF3 (pS386) and phospho-STAT1 (pY701) to assess cGAS-STING signaling downstream of cGAMP production (Fig. 4B–D). Consistent with prior work (Mohr et al., 2021), TREX1 KO cells exhibited significant increases in the phosphorylated forms of IRF3 and STAT1 following HT-DNA stimulation relative to wild-type Halo-TREX1 controls (Fig. 4B–D). Congruent with the observed increase in cGAMP levels, Halo-TREX1-9PA and Halo-TREX1-P61Q mutant cells exhibited increased levels of pIRF3 and pSTAT1 compared to wild-type controls, albeit to a lesser extent than TREX1 KO cells (Fig. 4B–D).
We next performed RT-qPCR to measure IFNB1 and associated ISG mRNA levels to test if increases in cGAMP, and IRF3, and STAT1 phosphorylation are associated with elevated pro-inflammatory gene expression. Indeed, IFNB1, OAS2, OAS3, ISG54, and ISG56 transcripts were elevated across multiple Halo-TREX1-9PA and Halo-TREX1-P61Q mutant subclones relative to wild-type controls to levels that were often indistinguishable from TREX1 KO cells (Fig. 4E–I). Taken together, these results indicate that TREX1 PPII mutations result in defective cGAS regulation and an increased pro-inflammatory transcriptional response, defects most likely stemming from TREX1 protein instability and associated reductions in overall TREX1 protein levels and corresponding decreases in nucleolytic activity.
DISCUSSION
Genetic associations of type I interferonopathies like AGS have been well-characterized, particularly in cases involving TREX1 mutations linked with compromised catalytic activity (Crow and Manel, 2015). Yet, how missense mutations outside of the catalytic site can lead to inflammatory disease has often remained unclear. Here, we identify an AGS-linked P61Q point mutation within the non-catalytic PPII motif of TREX1. Using in vitro biochemical measures of protein stability and endogenous gene editing, we show that TREX1 PPII mutations, including P61Q, destabilize the protein, resulting in significantly decreased TREX1 protein levels, diminished TREX1 exonucleolytic activity, and impaired cGAS-STING regulation. These defects were obscured in lentiviral delivery models where massive overexpression of TREX1 PPII mutants masks reductions in protein stability to maintain effective cGAS inhibition. The distal position of PPII to the catalytic site, along with the lack of differences in the GFP-TREX1 lysate-based nuclease assay, suggests that the nucleolytic defect observed in the endogenous system is due to decreased protein levels, rather than a direct effect of the mutations on catalysis. Thus, our results indicate diminished protein stability and an associated reduction in overall nucleolytic power of TREX1 as a plausible molecular explanation for why TREX1 P61Q mutations lead to severe AGS phenotypes in patients.
Autoinflammatory disease-linked TREX1 missense mutations often affect residues that play direct roles in DNA binding (i.e. R128H, K160R), catalytic activity (D18N/H, H195Y/Q, D200H/N) or dimerization (R97H, R114H). We recently reported that TREX1 mutations may also cause dysfunction by interfering with TREX1 interactions with cGAS-DNA condensates (E198K) (Zhou et al., 2021). Here, the identification of AGS-linked TREX1 P61Q mutations suggests that another class of mutations may compromise TREX1 function by diminishing overall protein stability. Indeed, structural analyses predict that the disease-linked TREX1 T13N, T32R, R185C, and D220G substitutions are likely to diminish protein stability (Zhou et al., 2022). Biochemical experimentation supports this premise as TREX1 T13N, T32R, R185C, and D220G substituted proteins exhibit Tm reductions of 4–8 °C in vitro (Zhou et al., 2022). Thus, TREX1 protein destabilization may be a common defect occurring across multiple AGS-linked TREX1 mutations.
TREX1 P61 is located with the PPII polyproline helix, a proline-rich region containing 9 prolines within a 15 amino acid stretch (Brucet et al., 2007; De Silva et al., 2007). This type of proline-rich segment is a conserved feature of TREX1, as it occurs in all organisms harboring TREX1, including placental mammals and marsupials. The paralog TREX2, as well as the ancient TREX nuclease occurring in non-mammals such as Anopheles and Drosophila, lack a proline-rich motif (Brucet et al., 2007), indicating that PPII likely evolved during the gene duplication event. Interestingly, the emergence of PPII in evolution seems to have coincided with the addition of a long C-terminal intrinsically disordered region. In keeping with structure-based predictions based on the PPII positioning outside of the catalytic core and ER transmembrane domains, our data confirm that the PPII motif is dispensable for TREX1 nucleolytic activity and subcellular localization. The precise function of the PPII motif therefore remains unknown.
The close positioning of the two PPII helices along the same side of the TREX1 dimer interface has been proposed to create a surface that allows for protein-protein interactions without occluding the active sites (Brucet et al., 2007; De Silva et al., 2007). Indeed, their high potential for presenting exposed hydrogen bond donors and acceptors, cause proline-rich motifs to be considered likely protein interaction domains (Adzhubei et al., 2013). The amino acid sequence of PPII matches the binding motif for the WW domain (Brucet et al., 2007), a peptide module characterized by two tryptophan residues (Sudol et al., 1995). Co-immunoprecipitation experiments have previously confirmed that murine TREX1 PPII interacts with the WW domain protein CA150 in vitro (Brucet et al., 2007). Whether human TREX1 also interacts with WW domain proteins and endogenous interactors remains unknown. Outside of a proposed interaction with the nucleosome assembly SET protein (Chowdhury et al., 2006), TREX1 protein partners are largely uncharacterized. Further work is therefore necessary to investigate this exciting hypothesis.
Our study relies heavily on the N-terminal HaloTag for studying the behavior of endogenous TREX1 PPII mutations. We observed an apparent stabilizing effect of the HaloTag on TREX1, as Halo-TREX1(WT)/Δ yielded a stronger immunoblot signal than parental cells (data not shown). This observation is consistent with a prior report, which demonstrated that HaloTags can elicit a significant impact on the detection of proteins by Western blot (Broadbent et al., 2023). Apparent increases of HaloTag protein levels were attributed to enhanced western blot transfer efficiency (Broadbent et al., 2023). Therefore, western blotting analysis may underestimate the full extent of TREX1 P61Q protein instability. A further potential limitation of our study is the use of the non-malignant MCF10A breast epithelial cell line to model AGS-linked TREX1 mutations. MCF10A cells were selected for this study because they possess an intact cGAS-STING-TREX1 pathway (Mohr et al., 2021) and are suitable for facile gene editing. However, it is not clear how well this cell model recapitulates aspects of AGS, a disease that primarily affects the central nervous system. Nevertheless, orthogonal measurements of TREX1 P61Q stability via Thermofluor analysis provide assurance that the P61Q mutation is likely to exert a destabilizing effect across multiple cell types and thus reinforce our proposed mechanism of pathogenesis in patients harboring the TREX1 P61Q mutation.
METHODS
Experimental Model and Subject Details
MCF10A cells were cultured in a 1:1 mixture of F12:DMEM media, supplemented with 5 % horse serum (Thermo Fisher Scientific #26050088), 20 ng/mL human EGF (Sigma Aldrich #E9644-.2mg), 0.5 mg/mL hydrocortisone (Sigma Aldrich), 100 ng/mL cholera toxin (Sigma Aldrich #H0888), 10 μg/mL recombinant human insulin (Sigma Aldrich #I9278-5ml), and 1% penicillin-streptomycin (Thermo Scientific #15140122). All media were supplied by the MSKCC Media Preparation core facility.
For HaloTag insertion and PPII gene editing of endogenous TREX1 in MCF10A cells, an RNP mix was prepared by mixing 10 μg purified SpCas9 and 500 pmol of sgRNA (TREX1_gRNA#1, see Key Resources Table). After a 10-minute incubation at room temperature, 2500 ng of the pUC19-HA-Halo-TREX1 plasmid harboring the desired mutation was added to the RNP mix. The RNP-plasmid mixture was nucleofected using 4D-Nucleofector X Unit (Lonza). Fluorescence-activated cell sorting was used to isolate single-cell clones from the polyclonal cell population. For monoallelic knockout of TREX1, a pUC19-BBsI-CBh-TREX1_gRNA#2-Cas9-T2A-mCherry plasmid (Mohr et al., 2021) was transfected using Lipofectamine 3000 (Invitrogen #L3000075). Single-cell clones were isolated by limiting dilution culture.
KEY RESOURCES TABLE
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| GFP | Santa Cruz Biotechnology | Cat#sc-9996; RRID:AB_627695 |
| IRF3 | Abcam | Cat#ab76409; RRID:AB_1523835 |
| pIRF3 (S386) | Abcam | Cat#ab76493; RRID:AB_1523836 |
| pSTAT1 (Y701) | Cell Signaling Technologies | Cat#9167; RRID:AB_561284 |
| STAT1 | Cell Signaling Technologies | Cat#9176; RRID:AB_2240087 |
| TREX1 | Abcam | Cat#ab185228; RRID:AB_2885196 |
| β-actin (mouse) | Abcam | Cat#ab8224; RRID:AB_449644 |
| β-actin (rabbit) | Abcam | Cat#ab8227; RRID:AB_2305186 |
| Goat anti-mouse IgG Alexa Fluor Plus 680 | Invitrogen | Cat#A32729; RRID:AB_2633278 |
| Goat anti-mouse IgG Alexa Fluor Plus 800 | Invitrogen | Cat#A32730; RRID:AB_2633279 |
| Goat anti-rabbit IgG Alexa Fluor Plus 680 | Invitrogen | Cat#A32734; RRID:AB_2633283 |
| Goat anti-rabbit IgG Alexa Fluor Plus 800 | Invitrogen | Cat#A32735; RRID:AB_2633284 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cholera Toxin | Sigma-Aldrich | Cat#C8052-2mg |
| cOmplete Mini Protease Inhibitor Cocktail | Sigma-Aldrich | Cat#11836153001 |
| ER Tracker Green | Invitrogen | Cat#E34251 |
| ER Tracker Red | Invitrogen | Cat#E34250 |
| Horse Serum | Thermo Fisher Scientific | Cat#26050088 |
| Human EGF | Sigma-Aldrich | Cat#E9644-.2mg |
| Hydrocortisone | Sigma-Aldrich | Cat#H0888 |
| Insulin | Sigma-Aldrich | Cat#I9278-5ml |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | Cat#15140122 |
| FluoroBrite DMEM | Thermo Fisher Scientific | Cat#A1896701 |
| Janelia Fluor HaloTag Ligand 646 | Promega | Cat#GA1120 |
| ER Tracker Green | Thermo Fisher Scientific | Cat#E34251 |
| ER Tracker Red | Thermo Fisher Scientific | Cat#E34250 |
| Lipofectamine 3000 | Thermo Fisher Scientific | Cat#L3000075 |
| Blasticidin S HCl, powder | Thermo Fisher Scientific | Cat#R21001 |
| Amersham Protran 0.45 NC Nitrocellulose Membrane | Cytiva | Cat#10600002 |
| Novex WedgeWell Tris Glycine Mini gels | Invitrogen | Cat#XP08165BOX |
| Intercept T20 (TBS) Antibody Diluent | LI-COR | Cat#927-65001 |
| Intercept Blocking Buffer | LI-COR | Cat#NC1660556 |
| Quick-RNA Miniprep Kit | Zymo Research | Cat#R1055 |
| SYBR Green Master Mix | Applied Biosystems | Cat#A25742 |
| SuperScript IV First-Strand Synthesis System | Invitrogen | Cat#18091200 |
| Critical Commercial Assays | ||
| 2′3′-Cyclic GAMP Direct EIA Kit | Arbor Assays | Cat#K067-H5 |
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat#23227 |
| Pierce BCA Protein Assay Kit, Reducing Agent-compatible | Thermo Fisher Scientific | Cat#23250 |
| Experimental Models: Cell Lines | ||
| MCF10A | Maria Jasin Lab | N/A |
| MCF10A TREX1-KO | this paper | cJM14 |
| MCF10A TREX1-KO + GFP-TREX1 | this paper | cAS1 |
| MCF10A TREX1-KO + GFP-TREX1(8PA) | this paper | cAS2 |
| MCF10A TREX1-KO + GFP-TREX1(P61Q) | this paper | cAS3 |
| MCF10A Halo-TREX1(WT/Δ) | this paper | cAS4 |
| MCF10A Halo-TREX1(9PA/Δ) | this paper | cAS5 |
| MCF10A Halo-TREX1(P61Q/Δ) | this paper | cAS6 |
| Oligonucleotides | ||
| ACTB F for qPCR (ATCTGGCACCACACCTTCTAC) | this paper | N/A |
| ACTB R for qPCR (CAGCCAGGTCCAGACGCAGG) | this paper | N/A |
| GAPDH F for qPCR (CATCACCATCTTCCAGGAGCGA) | this paper | N/A |
| GAPDH R for qPCR (CCTGCTTCACCACCTTCT) | this paper | N/A |
| IFNB1 F for qPCR (TACTGCCTCAAGGACAGGATGAA) | Li et al., 2023 (PMID: 37612508) | N/A |
| IFNB1 R for qPCR (GCATCTCATAGATGGTCAATGCG) | Li et al., 2023 (PMID: 37612508) | N/A |
| OAS2 F for qPCR (GAGCCAGTTGCAGAAAACCAG) | Bakhoum et al., 2018 (PMID: 29342134) | N/A |
| OAS2 R for qPCR (GCATTGTCGGCACTTTCCAA) | Bakhoum et al., 2018 (PMID: 29342134) | N/A |
| OAS3 F for qPCR (GAAGCCCAGGCCTATCATCC) | Bakhoum et al., 2018 (PMID: 29342134) | N/A |
| OAS3 R for qPCR (TCATCCAGTAGGACCGCTGA) | Bakhoum et al., 2018 (PMID: 29342134) | N/A |
| ISG54 F for qPCR (ACGGTATGCTTGGAACGATTG) | Diner et al., 2015 (PMID: 25693804) | N/A |
| ISG54 R for qPCR (AACCCAGAGTGTGGCTGATG) | Diner et al., 2015 (PMID: 25693804) | N/A |
| ISG56 F for qPCR (AAGGCAGGCTGTCCGCTTA) | Diner et al., 2015 (PMID: 25693804) | N/A |
| ISG56 R for qPCR (TCCTGTCCTTCATCCTGAAGCT) | Diner et al., 2015 (PMID: 25693804) | N/A |
| TREX1 F for qPCR (GCATCTGTCAGTGGAGACCA) | Yan et al., 2010 (PMID: 20871604) | N/A |
| TREX1 R for qPCR (AGATCCTTGGTACCCCTGCT) | Yan et al., 2010 (PMID: 20871604) | N/A |
| Oligo 1 for nuclease activity assay (/5TEX615/GCTAGGCAG) | this paper | N/A |
| Oligo 2 for nuclease activity assay (CTGCCTAGC/3IAbRQSp/) | this paper | N/A |
| TREX1; guide RNA #1 (GCAGGTACGTACCCAACCAT) | Umbreit et al., 2020 (PMID: 32299917) | N/A |
| TREX1; guide RNA #2 (GAGCCCCCCCACCTCTC) | this paper | N/A |
| Recombinant DNA | ||
| pLenti-CMV-GFP-TREX1-BLAST | Mohr et al., 2021 (PMID: 33476576) | Addgene #164228 |
| pLenti-CMV-GFP-TREX1(8PA)-BLAST | this paper | |
| pLenti-CMV-GFP-TREX1(P61Q)-BLAST | this paper | |
| pUC19-HA-Halo-TREX1(9PA) | this paper | |
| pUC19-HA-Halo-TREX1(P61Q) | this paper | |
| psPAX2 | gift from Didier Trono | Addgene #12260 |
| pMD2.G | gift from Didier Trono | Addgene #12259 |
Viral Transduction
For lentiviral transduction, open reading frames were cloned into pLenti-CMV-GFP-blast plasmids. Constructs were transfected into 293FT cells together with psPAX2 (Addgene #12260) and pMD2.G (Addgene #12259) using calcium phosphate precipitation. Supernatants containing lentivirus were filtered through a 0.45 μm filter and supplemented with 4 μg/mL polybrene. Successfully transduced cells were selected using 5 μg/mL blasticidin (Thermo Fisher Scientific #R21001).
Nuclease Assay with Recombinant TREX1
In vitro DNA degradation assay was performed as previously described with minor modifications (Zhou et al., 2022). Briefly, 1 μM 100-bp dsDNA (see below for sequence) was incubated with 0.1 μM human TREX1 or TREX1 variants in a 20 μL reaction system (20 mM Tris-HCl pH 7.5, 15 mM NaCl, 135 mM KCl, 5 mM MgCl2, and 1 mg/ml BSA) at 25°C with a time gradient of 5–30 min. DNA degradation was quenched by adding SDS (final concentration at 0.0167% (w/v)) and EDTA (final concentration at 10 mM) and incubating at 75°C for 15 min. The remaining DNA was separated on a 4% agarose gel using 0.5 × TB buffer (45 mM Tris, 45 mM boric acid) as a running buffer. After DNA electrophoresis, the agarose gel was stained with 0.5x TB buffer (containing 10 μg/mL ethidium bromide) at 25°C for 15 min, followed by de-staining with milli-Q water for an additional 45 min. DNA was visualized by ImageQuant 800 Imaging System and quantified using FIJI (Schindelin et al., 2012).
100-bp dsDNA sense:
5′-ACATCTAGTACATGTCTAGTCAGTATCTAGTGATTATCTAGACATACATCTAGTACATGTCTAGTCAGTATCTAGTGATTATCTAGACATGGACTCATCC −3′
100-bp dsDNA anti-sense:
5′-GGATGAGTCCATGTCTAGATAATCACTAGATACTGACTAGACATGTACTAGATGTATGTCTAGATAATCACTAGATACTGACTAGACATGTACTAGATGT −3′
Nuclease Assay in Cell Lysates
dsDNA substrate was prepared by annealing oligo 1 (IDT; /5TEX615/GCTAGGCAG) and oligo 2 (IDT; CTGCCTAGC/3IAbRQSp/) in DNA duplex buffer (100 mM KAc, 30 mM HEPES pH 7.5) at a 1:1.15 ratio.
Whole cell lysates were generated by resuspending 3 million cells in 80 μL of assay buffer containing 25 mM HEPES 7.5, 20 mM KCl, 1 mM DTT, 1% Triton X-100, 0.25 mM EDTA, and 10 mM MgCl2 supplemented with Complete Mini Protease Inhibitor Cocktail (Invitrogen #11836153001). Cells were lysed by passing the cell resuspension through a 28 G syringe (BD #329461) ten times, incubated on ice for 15 minutes, and then were spun down at 14,000 ✕ g, 4°C for 15 minutes to remove pellets. 1:10 dilution of whole cell lysates in assay buffer were used to quantify protein content using Reducing Agent-compatible Pierce BCA Assay Kit (Thermo Fisher Scientific #23250).
2.5 μg (Fig. 1E) or 50 μg (Fig. 3E) of protein was loaded onto a 384-well F-bottom polystyrene microplate (Greiner Bio-One International AG Cat# 784076) with 1 μM dsDNA substrate in assay buffer. The fluorescence intensity (excitation = 570 nm, emission = 615 nm) of the plate was read immediately with Cytation 3 Multi-mode Reader (BioTek) at 25° C for 4 hours every 3 minutes.
Live-cell Imaging
Cells were plated onto 4-well glass-bottom μ-slide dishes (Ibidi #80427) 24 h before imaging. Five minutes before imaging, media in each well was replaced with FluoroBrite DMEM Imaging Media (Thermo Scientific #A1896701) containing 1 μM ER Tracker Red (Thermo Scientific #E34250) or ER Tracker Green (Thermo Scientific #E34251). Live-cell imaging was performed at room temperature using Nikon Eclipse Ti2-E equipped with CSU-W1 SoRa spinning disk super resolution confocal system, Borealis microadapter, Perfect Focus 4, motorized turret and encoded stage, 5-line laser launch [405 (100 mw), 445 (45 mw), 488 (100 mw), 561 (80 mw), 640 (75 mw)], PRIME 95B Monochrome Digital Camera, and CFI Apo TIRF 60× 1.49 NA objective lens. Images were acquired using NIS-Elements Advanced Research Software on a Dual Xeon Imaging workstation. Adjustment of brightness and contrast were performed using Fiji software. Images were cropped and assembled into figures using Illustrator 2024 (Adobe).
Immunoblotting
Whole cell lysates were generated by resuspending 1 million cells in RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1 % NP-40, 1 % sodium deoxycholate, 0.1 % SDS) supplemented with phosphatase inhibitors (10 mM NaF, 20 mM β-glycerophosphate) and 100 μM phenylmethylsulfonyl fluoride. Cells were lysed by sonication for 15 cycles (high, 30 seconds on, 30 seconds off) using Bioruptor Plus (Diagenode). After a 15-minute incubation on ice and centrifugation (21,000 ✕ g, 4 °C for 20 minutes), pellets were removed. 1:10 dilution of whole cell lysates in RIPA were used to quantify protein content using Pierce BCA Assay Kit (Thermo Fisher Scientific #23227). 20 μg protein was loaded per sample into 15-well Novex WedgeWell Tris-Glycine Mini gels (Invitrogen #XP08165BOX). Gels were run at 120 V for 90 minutes and then transferred onto 0.45 μm nitrocellulose membranes (Cytiva #10600002) at 100 V for 60 minutes on ice. Membranes were blocked in Intercept Blocking Buffer (LI-COR #NC1660556). Primary antibodies were diluted (1:4000 for β-actin, 1:1000 for all others) in Intercept T20 (TBS) Antibody Diluent (LI-COR #927-65001) and incubated with membranes overnight at 4 °C on a nutator. Membranes were washed three times in TBST. Secondary antibodies were diluted 1:10,000 in Intercept T20 (TBS) Antibody Diluent and incubated for 1 hour at room temperature on a shaker. After three rounds of washing with TBST and one round of washing with TBS, membranes were scanned using the Odyssey XL infrared imaging scanner (LI-COR).
2′3′-cGAMP Quantification
2 million cells were seeded onto 10-cm dishes 24 hours before transfection. Each plate was either transfected with 4 μg herring testes (HT-) DNA or mock-transfected using Lipofectamine 3000 (Thermo Fisher Scientific #L3000075). 24 hours after transfection, cells were harvested, washed with PBS, pelleted, flash-frozen in liquid nitrogen, and stored at −80° C. To quantify 2′3′-cGAMP levels, 2 million cells were resuspended in 200 μL LP2 lysis buffer (20 mM Tris-HCl pH 7.7, 100 mM NaCl, 10 mM NaF, 20 mM β-glycerophosphate, 5 mM MgCl2, 0.1 % Triton X-100, 5 % glycerol). Cells were lysed by passing the cell resuspension through a 28 G syringe (BD #329461) ten times, incubated on ice for 15 minutes, and then were spun down at 21,300 g, 4° C for 20 minutes to remove pellets. 2′3′-cGAMP levels were quantified using the 2′3′-cGAMP ELISA Kit (Arbor Assays #K067-H5) according to the manufacturer’s instructions. 1:10 dilution of lysates in LP2 buffer were used to quantify protein content using Pierce BCA Assay Kit (Thermo Fisher Scientific #23227). The resulting 2′3′-cGAMP levels were normalized to protein content in each sample.
RT-qPCR
Total RNA was isolated from 1 million cells using Quick RNA Miniprep Kit (Zymo Research #R1055) according to the manufacturer’s instructions. A DNase I digestion step was included prior to eluting the RNA. cDNA was generated from 1000 ng total RNA using the SuperScript IV First-strand Synthesis System (Invitrogen #18091200) with random hexamer and oligo-(dT) priming. Reverse-transcribed samples were treated with RNase H to remove RNA. qPCR was performed with gene-specific primers (see Key Resources Table) and SYBR Green qPCR Master Mix (Applied Biosystems #A25742). qPCR was performed on QuantStudio 6 (Applied Biosystems), using 10 ng of cDNA and 250 nM of each primer on a MicroAmp 384-well reaction plate (Applied Biosystems #4309849). Relative transcription levels were calculated by normalizing to the geometric mean of ACTB and GAPDH cycle threshold values.
Thermal Denaturation Assay
10 μM of purified TREX1 mutant protein and 3✕ SYPRO Orange Protein dye (Life Technologies) were loaded into a 96-well reaction plate, in a 20 μL reaction containing 20 mM Tris-HCl pH 7.5, 75 mM KCl, and 1 mM TCEP. Reactions were incubated with an increasing temperature from 20 to 95° C in a Bio-Rad CFX thermocycler with HEX channel fluorescence measurements taken every 0.5° C, and melting temperature (Tm) was defined as the temperature at which the half of the maximum fluorescence change occurs.
Statistical Analysis
Information regarding biological replicates, sample size, and statistical testing is provided in the figure legends.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Y. Chen and E. Toufektchan for advice on experimental procedures; C. Krumm for the lysate-based in vitro nuclease assay protocol; J. Petrini and J. Tyler for advice; and support from the National Cancer Institute (NCI) (R37CA261183), the Pew Charitable Trusts, the Mary Kay Ash Foundation, and the MSKCC Frank A. Howard Fellowship.
Footnotes
DECLARATION OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
- Ablasser A, Chen ZJ. 2019. cGAS in action: Expanding roles in immunity and inflammation. Science 363. doi: 10.1126/science.aat8657 [DOI] [PubMed] [Google Scholar]
- Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, Hopfner K-P, Ludwig J, Hornung V. 2013. cGAS produces a 2’−5’-linked cyclic dinucleotide second messenger that activates STING. Nature 498:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablasser A, Hemmerling I, Schmid-Burgk JL, Behrendt R, Roers A, Hornung V. 2014. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol 192:5993–5997. [DOI] [PubMed] [Google Scholar]
- Adzhubei AA, Sternberg MJE, Makarov AA. 2013. Polyproline-II helix in proteins: structure and function. J Mol Biol 425:2100–2132. [DOI] [PubMed] [Google Scholar]
- Ahn J, Ruiz P, Barber GN. 2014. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J Immunol 193:4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DG, Barnaba C, Perez GI, Schmidt JC. 2023. Quantitative analysis of autophagy reveals the role of ATG9 and ATG2 in autophagosome formation. J Cell Biol 222. doi: 10.1083/jcb.202210078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucet M, Querol-Audí J, Serra M, Ramirez-Espain X, Bertlik K, Ruiz L, Lloberas J, Macias MJ, Fita I, Celada A. 2007. Structure of the dimeric exonuclease TREX1 in complex with DNA displays a proline-rich binding site for WW Domains. J Biol Chem 282:14547–14557. [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Beresford PJ, Zhu P, Zhang D, Sung J-S, Demple B, Perrino FW, Lieberman J. 2006. The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell 23:133–142. [DOI] [PubMed] [Google Scholar]
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GMA, Gornall HL, Oojageer A, Anderson B, Pizzino A, Helman G, Abdel-Hamid MS, Abdel-Salam GM, Ackroyd S, Aeby A, Agosta G, Albin C, Allon-Shalev S, Arellano M, Ariaudo G, Aswani V, Babul-Hirji R, Baildam EM, Bahi-Buisson N, Bailey KM, Barnerias C, Barth M, Battini R, Beresford MW, Bernard G, Bianchi M, Billette de Villemeur T, Blair EM, Bloom M, Burlina AB, Carpanelli ML, Carvalho DR, Castro-Gago M, Cavallini A, Cereda C, Chandler KE, Chitayat DA, Collins AE, Sierra Corcoles C, Cordeiro NJV, Crichiutti G, Dabydeen L, Dale RC, D’Arrigo S, De Goede CGEL, De Laet C, De Waele LMH, Denzler I, Desguerre I, Devriendt K, Di Rocco M, Fahey MC, Fazzi E, Ferrie CD, Figueiredo A, Gener B, Goizet C, Gowrinathan NR, Gowrishankar K, Hanrahan D, Isidor B, Kara B, Khan N, King MD, Kirk EP, Kumar R, Lagae L, Landrieu P, Lauffer H, Laugel V, La Piana R, Lim MJ, Lin J-PS-M, Linnankivi T, Mackay MT, Marom DR, Marques Lourenço C, McKee SA, Moroni I, Morton JEV, Moutard M-L, Murray K, Nabbout R, Nampoothiri S, Nunez-Enamorado N, Oades PJ, Olivieri I, Ostergaard JR, Pérez-Dueñas B, Prendiville JS, Ramesh V, Rasmussen M, Régal L, Ricci F, Rio M, Rodriguez D, Roubertie A, Salvatici E, Segers KA, Sinha GP, Soler D, Spiegel R, Stödberg TI, Straussberg R, Swoboda KJ, Suri M, Tacke U, Tan TY, te Water Naude J, Wee Teik K, Thomas MM, Till M, Tonduti D, Valente EM, Van Coster RN, van der Knaap MS, Vassallo G, Vijzelaar R, Vogt J, Wallace GB, Wassmer E, Webb HJ, Whitehouse WP, Whitney RN, Zaki MS, Zuberi SM, Livingston JH, Rozenberg F, Lebon P, Vanderver A, Orcesi S, Rice GI. 2015. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 167A:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Manel N. 2015. Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol 15:429–440. [DOI] [PubMed] [Google Scholar]
- Crow YJ, Stetson DB. 2022. The type I interferonopathies: 10 years on. Nat Rev Immunol 22:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva U, Choudhury S, Bailey SL, Harvey S, Perrino FW, Hollis T. 2007. The crystal structure of TREX1 explains the 3′ nucleotide specificity and reveals a polyproline II helix for protein partnering. J Biol Chem 282:10537–10543. [DOI] [PubMed] [Google Scholar]
- Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. 2013. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3:1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vanpouille-Box C, Bakhoum SF, Demaria S. 2018. SnapShot: CGAS-STING Signaling. Cell 173:276–276.e1. [DOI] [PubMed] [Google Scholar]
- Gao D, Li T, Li X-D, Chen X, Li Q-Z, Wight-Carter M, Chen ZJ. 2015. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci U S A 112:E5699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, Others. 2013. Structure-function analysis of STING activation by c [G (2′, 5′) pA (3′, 5′) p] and targeting by antiviral DMXAA. Cell 154:748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Treuting PM, Woodward JJ, Stetson DB. 2015. Cutting Edge: cGAS Is Required for Lethal Autoimmune Disease in the Trex1-Deficient Mouse Model of Aicardi–Goutières Syndrome. The Journal of Immunology 195:1939–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW. 2015. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc Natl Acad Sci U S A 112:5117–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee Y-A, de Silva U, Bailey SL, Witte T, Vyse TJ, Kere J, Pfeiffer C, Harvey S, Wong A, Koskenmies S, Hummel O, Rohde K, Schmidt RE, Dominiczak AF, Gahr M, Hollis T, Perrino FW, Lieberman J, Hübner N. 2007. Mutations in the gene encoding the 3’−5’ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39:1065–1067. [DOI] [PubMed] [Google Scholar]
- Lehtinen DA, Harvey S, Mulcahy MJ, Hollis T, Perrino FW. 2008. The TREX1 double-stranded DNA degradation activity is defective in dominant mutations associated with autoimmune disease. J Biol Chem 283:31649–31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu Y-T, Grishin NV, Chen ZJ. 2015. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347:aaa2630. [DOI] [PubMed] [Google Scholar]
- Mazur DJ, Perrino FW. 2001. Excision of 3′ Termini by the Trex1 and TREX2 3′→ 5′ Exonucleases characterization of the recombinant proteins. J Biol Chem 276:17022–17029. [DOI] [PubMed] [Google Scholar]
- Mohr L, Toufektchan E, von Morgen P, Chu K, Kapoor A, Maciejowski J. 2021. ER-directed TREX1 limits cGAS activation at micronuclei. Mol Cell. doi: 10.1016/j.molcel.2020.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Melki I, Frémond M-L, Briggs TA, Rodero MP, Kitabayashi N, Oojageer A, Bader-Meunier B, Belot A, Bodemer C, Quartier P, Crow YJ. 2017. Assessment of Type I Interferon Signaling in Pediatric Inflammatory Disease. J Clin Immunol 37:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Rodero MP, Crow YJ. 2015. Human disease phenotypes associated with mutations in TREX1. J Clin Immunol 35:235–243. [DOI] [PubMed] [Google Scholar]
- Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, Robins P, Harvey S, Hollis T, O’Hara A, Herrick AL, Bowden AP, Perrino FW, Lindahl T, Barnes DE, Crow YJ. 2007a. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet 80:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, Artuch R, Montalto SA, Bacino CA, Barroso B, Baxter P, Benko WS, Bergmann C, Bertini E, Biancheri R, Blair EM, Blau N, Bonthron DT, Briggs T, Brueton LA, Brunner HG, Burke CJ, Carr IM, Carvalho DR, Chandler KE, Christen H-J, Corry PC, Cowan FM, Cox H, D’Arrigo S, Dean J, De Laet C, De Praeter C, Dery C, Ferrie CD, Flintoff K, Frints SGM, Garcia-Cazorla A, Gener B, Goizet C, Goutieres F, Green AJ, Guet A, Hamel BCJ, Hayward BE, Heiberg A, Hennekam RC, Husson M, Jackson AP, Jayatunga R, Jiang Y-H, Kant SG, Kao A, King MD, Kingston HM, Klepper J, van der Knaap MS, Kornberg AJ, Kotzot D, Kratzer W, Lacombe D, Lagae L, Landrieu PG, Lanzi G, Leitch A, Lim MJ, Livingston JH, Lourenco CM, Lyall EGH, Lynch SA, Lyons MJ, Marom D, McClure JP, McWilliam R, Melancon SB, Mewasingh LD, Moutard M-L, Nischal KK, Ostergaard JR, Prendiville J, Rasmussen M, Rogers RC, Roland D, Rosser EM, Rostasy K, Roubertie A, Sanchis A, Schiffmann R, Scholl-Burgi S, Seal S, Shalev SA, Corcoles CS, Sinha GP, Soler D, Spiegel R, Stephenson JBP, Tacke U, Tan TY, Till M, Tolmie JL, Tomlin P, Vagnarelli F, Valente EM, Van Coster RNA, Van der Aa N, Vanderver A, Vles JSH, Voit T, Wassmer E, Weschke B, Whiteford ML, Willemsen MAA, Zankl A, Zuberi SM, Orcesi S, Fazzi E, Lebon P, Crow YJ. 2007b. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet 81:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, McGlasson SL, Alyanakian M-A, Bader-Meunier B, Barnerias C, Bellon N, Belot A, Bodemer C, Briggs TA, Desguerre I, Frémond M-L, Hully M, van den Maagdenberg AMJM, Melki I, Meyts I, Musset L, Pelzer N, Quartier P, Terwindt GM, Wardlaw J, Wiseman S, Rieux-Laucat F, Rose Y, Neven B, Hertel C, Hayday A, Albert ML, Rozenberg F, Crow YJ, Duffy D. 2017. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med 214:1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. 2008. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Chen HI, Bougeret C, Einbond A, Bork P. 1995. Characterization of a novel protein-binding module--the WW domain. FEBS Lett 369:67–71. [DOI] [PubMed] [Google Scholar]
- Wolf C, Rapp A, Berndt N, Staroske W, Schuster M, Dobrick-Mattheuer M, Kretschmer S, König N, Kurth T, Wieczorek D, Kast K, Cardoso MC, Günther C, Lee-Kirsch MA. 2016. RPA and Rad51 constitute a cell intrinsic mechanism to protect the cytosol from self DNA. Nat Commun 7:11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N. 2017. Immune Diseases Associated with TREX1 and STING Dysfunction. J Interferon Cytokine Res 37:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Mohr L, Maciejowski J, Kranzusch PJ. 2021. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell 81:739–755.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Richmond-Buccola D, Wang Q, Kranzusch PJ. 2022. Structural basis of human TREX1 DNA degradation and autoimmune disease. Nat Commun 13:4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.