Abstract
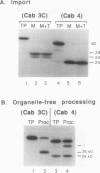
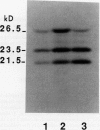
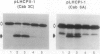
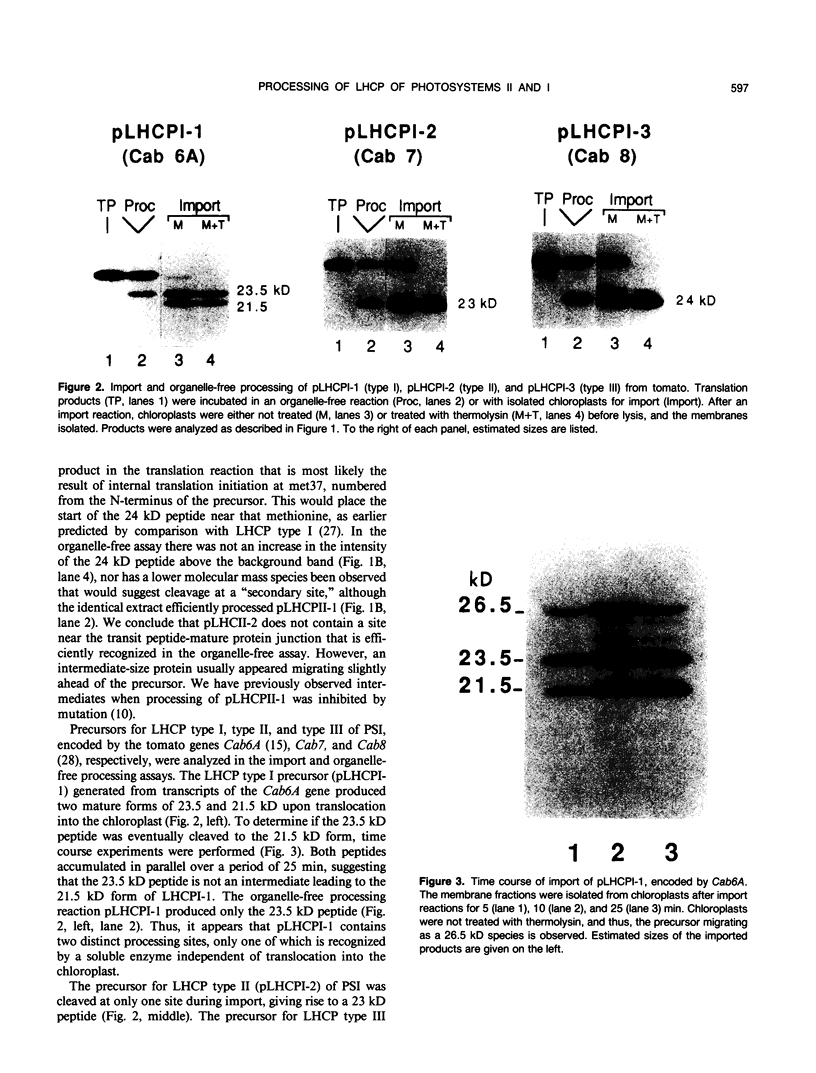
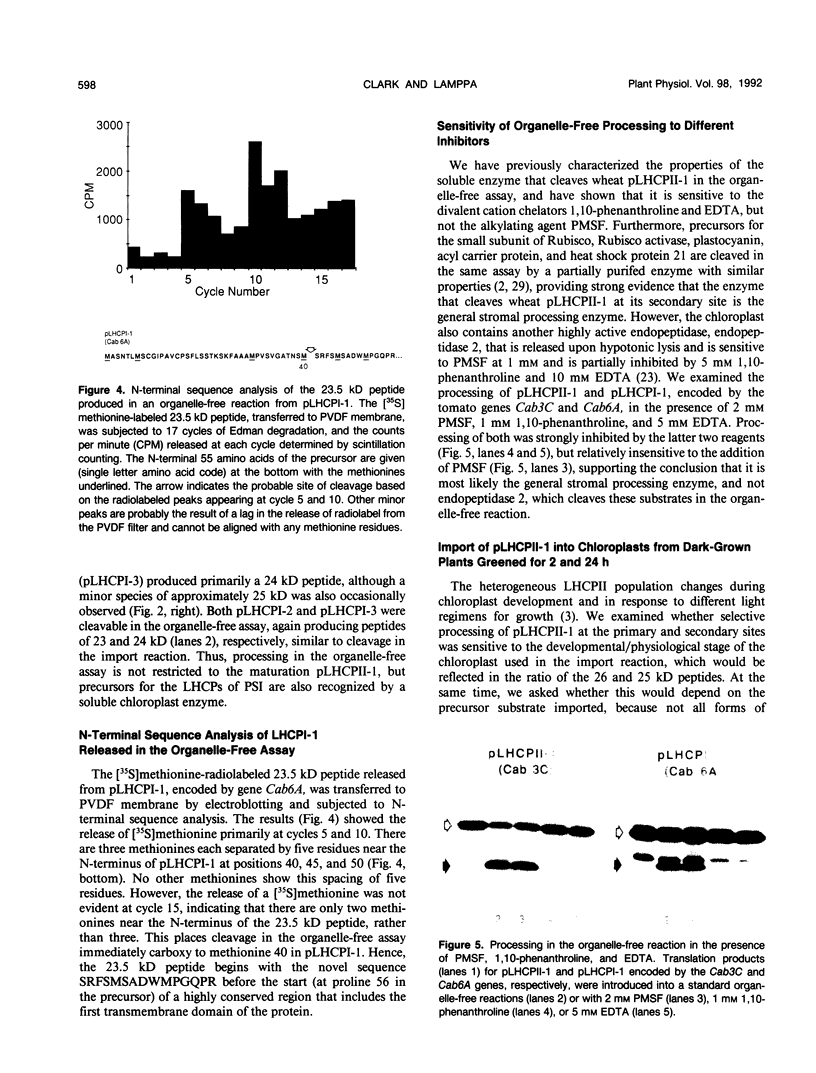
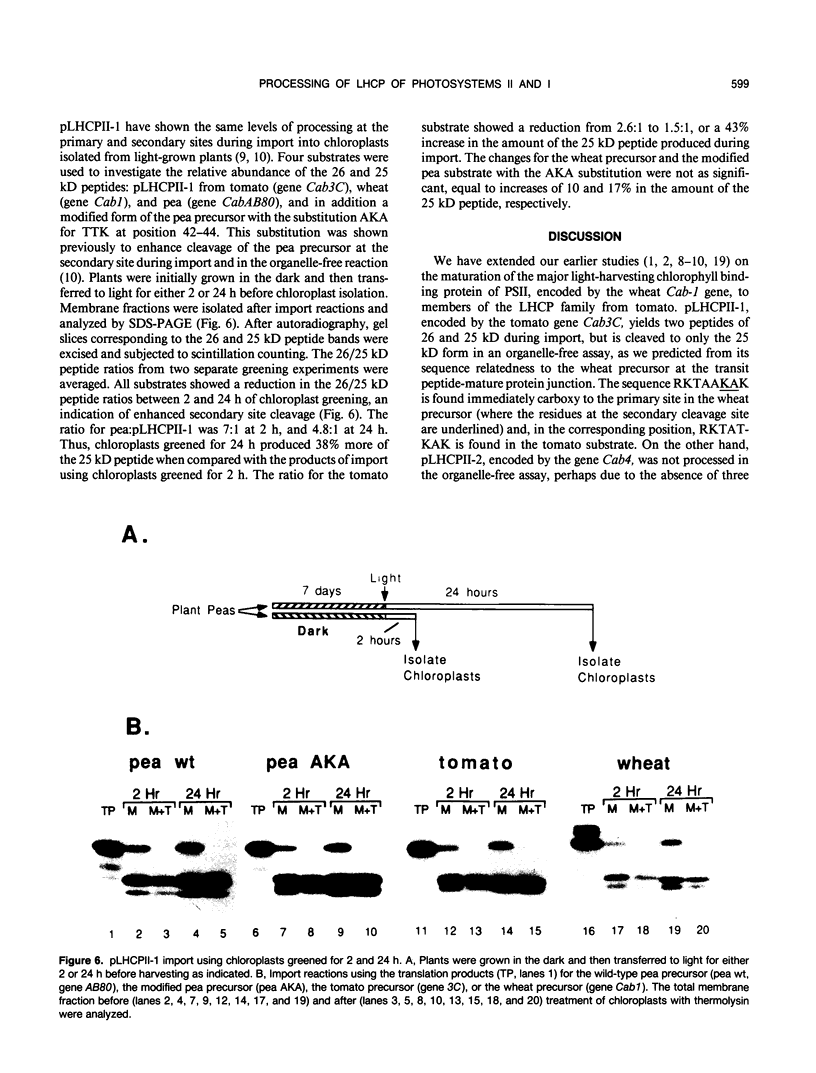
We have investigated whether the precursors for the light-harvesting chlorophyll a/b binding proteins (LHCP) of photosystems II and I (PSII and PSI) are cleavable substrates in an organelle-free reaction, and have compared the products with those obtained during in vitro import into chloroplasts. Representatives from the tomato (Lycopersicon esculentum) LHCP family were analyzed. The precursor for LHCP type I of PSII (pLHCPII-1), encoded by the tomato gene Cab3C, was cleaved at only one site in the organelle-free assay, but two sites were recognized during import, analogous to our earlier results with a wheat precursor for LHCPII-1. The relative abundance of the two peptides produced was investigated during import of pLHCPII-1 into chloroplasts isolated from plants greened for 2 or 24 hours. In contrast to pLHCPII-1, the precursors for LHCP type II and III of PSI were cleaved in both assays, giving rise to a single peptide. The precursor for LHCP type I of PSI, encoded by gene Cab6A, yielded two peptides of 23.5 and 21.5 kilodaltons during import, whereas in the organelle-free assay only the 23.5 kilodalton peptide was found. N-terminal sequence analysis of this radiolabeled peptide has tentatively identified the site cleaved in the organelle-free assay between met40 and ser41 of the precursor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad M. S., Clark S. E., Lamppa G. K. Properties of a Chloroplast Enzyme that Cleaves the Chlorophyll a/b Binding Protein Precursor : Optimization of an Organelle-Free Reaction. Plant Physiol. 1989 May;90(1):117–124. doi: 10.1104/pp.90.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad M. S., Oblong J. E., Lamppa G. K. Soluble Chloroplast Enzyme Cleaves preLHCP Made in Escherichia coli to a Mature Form Lacking a Basic N-Terminal Domain. Plant Physiol. 1991 Aug;96(4):1220–1227. doi: 10.1104/pp.96.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Andersson B. The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci. 1988 Sep;13(9):351–355. doi: 10.1016/0968-0004(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci U S A. 1984 May;81(10):2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., O'Riordan V., Boutry M. Protein transport into mitochondria is conserved between plant and yeast species. J Biol Chem. 1990 Oct 5;265(28):16856–16862. [PubMed] [Google Scholar]
- Clark S. E., Abad M. S., Lamppa G. K. Mutations at the transit peptide-mature protein junction separate two cleavage events during chloroplast import of the chlorophyll a/b-binding protein. J Biol Chem. 1989 Oct 15;264(29):17544–17550. [PubMed] [Google Scholar]
- Clark S. E., Lamppa G. K. Determinants for cleavage of the chlorophyll a/b binding protein precursor: a requirement for a basic residue that is not universal for chloroplast imported proteins. J Cell Biol. 1991 Aug;114(4):681–688. doi: 10.1083/jcb.114.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. E., Oblong J. E., Lamppa G. K. Loss of efficient import and thylakoid insertion due to N- and C-terminal deletions in the light-harvesting chlorophyll a/b binding protein. Plant Cell. 1990 Feb;2(2):173–184. doi: 10.1105/tpc.2.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Fulsom D. R., Viitanen P. V. An imported thylakoid protein accumulates in the stroma when insertion into thylakoids is inhibited. J Biol Chem. 1989 Aug 25;264(24):14225–14232. [PubMed] [Google Scholar]
- Cline K. Light-Harvesting Chlorophyll a/b Protein : Membrane Insertion, Proteolytic Processing, Assembly into LHC II, and Localization to Appressed Membranes Occurs in Chloroplast Lysates. Plant Physiol. 1988 Apr;86(4):1120–1126. doi: 10.1104/pp.86.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Bogorad L. Plastid Development in Pisum sativum Leaves during Greening : II. Post-Translational Uptake by Plastids as an Indicator System to Monitor Changes in Translatable mRNA for Nuclear-Encoded Plastid Polypeptides. Plant Physiol. 1987 Nov;85(3):816–822. doi: 10.1104/pp.85.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. R., Pichersky E., Kloppstech K. Chlorophyll a/b-binding proteins: an extended family. Trends Biochem Sci. 1991 May;16(5):181–186. doi: 10.1016/0968-0004(91)90072-4. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Pichersky E., Malik V. S., Castresana C., Ko K., Darr S. C., Cashmore A. R. A cDNA clone encoding a photosystem I protein with homology to photosystem II chlorophyll a/b-binding polypeptides. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8844–8848. doi: 10.1073/pnas.84.24.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K., Abad M. S. Processing of a wheat light-harvesting chlorophyll a/b protein precursor by a soluble enzyme from higher plant chloroplasts. J Cell Biol. 1987 Dec;105(6 Pt 1):2641–2648. doi: 10.1083/jcb.105.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Musgrove J. E., Elderfield P. D., Robinson C. Endopeptidases in the stroma and thylakoids of pea chloroplasts. Plant Physiol. 1989 Aug;90(4):1616–1621. doi: 10.1104/pp.90.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan L. A., Cline K. A stromal protein factor maintains the solubility and insertion competence of an imported thylakoid membrane protein. J Cell Biol. 1991 Feb;112(4):603–613. doi: 10.1083/jcb.112.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Breidenbach R. B., Kausch A. P., Cashmore A. R. Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-binding proteins in Lycopersicon esculentum (tomato). Gene. 1985;40(2-3):247–258. doi: 10.1016/0378-1119(85)90047-2. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]