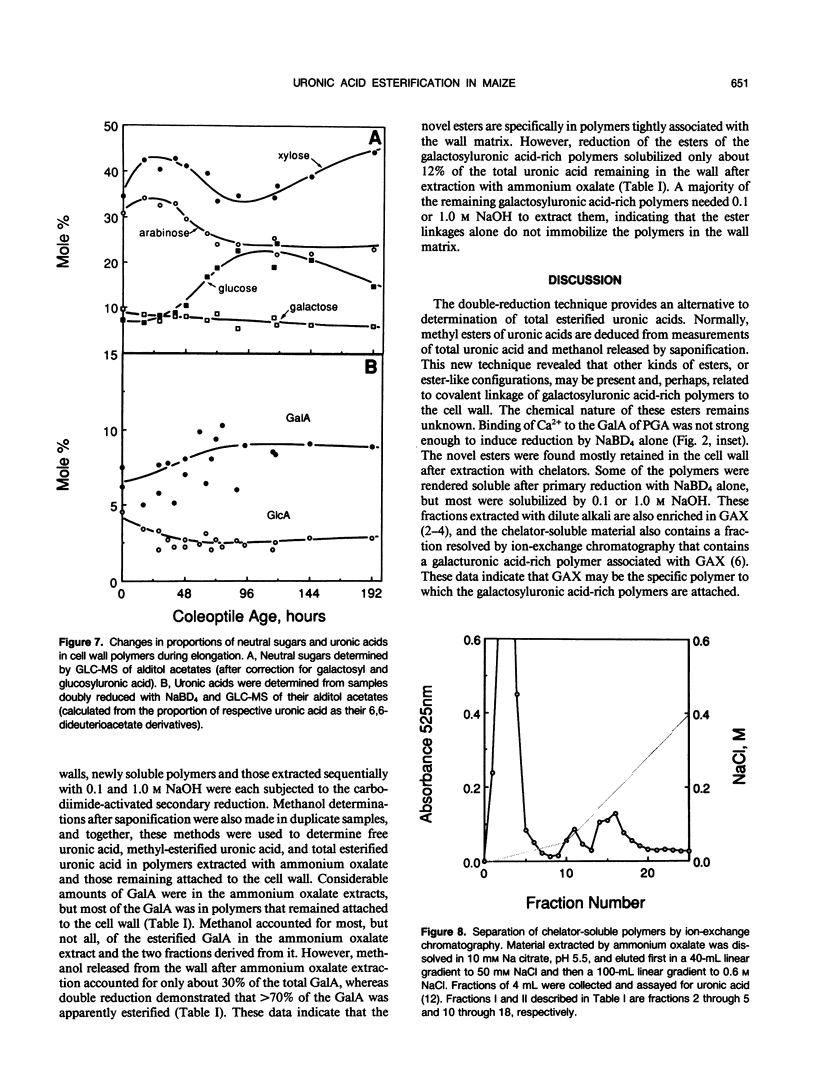
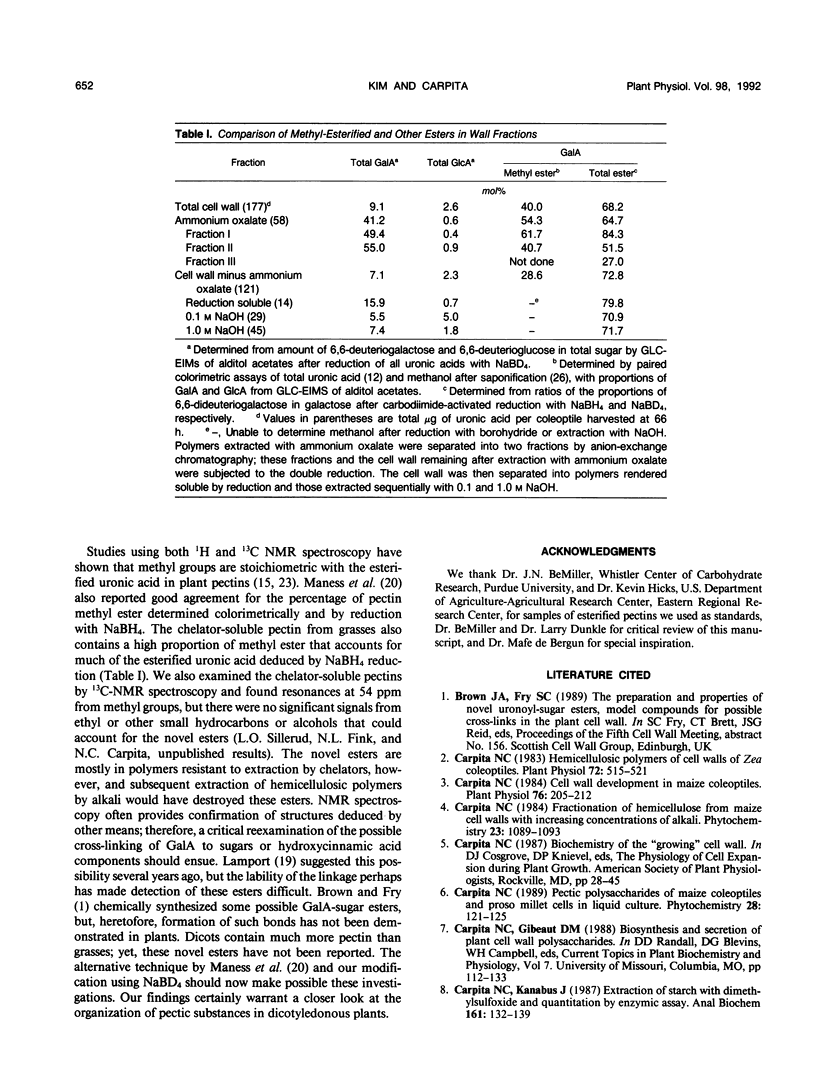
Abstract
Cell walls of grasses have two major polysaccharides that contain uronic acids, the hemicellulosic glucuronoarabinoxylans and the galactosyluronic acid-rich pectins. A technique whereby esterified uronic acid carboxyl groups are reduced selectively to yield their respective 6,6-dideuterio neutral sugars was used to determine the extent of esterification and changes in esterification of these two uronic acids during elongation of maize (Zea mays L.) coleoptiles. The glucosyluronic acids of glucuronoarabinoxylans did not appear to be esterified at any time during coleoptile elongation. The galactosyluronic acids of embryonal coleoptiles were about 65% esterified, but this proportion increased to nearly 80% during the rapid elongation phase before returning to about 60% at the end of elongation. Methyl esters accounted for about two-thirds of the total esterified galacturonic acid in cell walls of unexpanded coleoptiles. The proportion of methyl esters decreased throughout elongation and did not account for the increase in the proportion of esterified galactosyluronic acid units during growth. The results indicate that the galactosyluronic acid units of grass pectic polysaccharides may be converted to other kinds of esters or form ester-like chemical interactions during expansion of the cell wall. Accumulation of novel esters or ester-like interactions is coincident with covalent attachment of polymers containing galactosyluronic acid units to the cell wall.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Hemicellulosic polymers of cell walls of zea coleoptiles. Plant Physiol. 1983 Jun;72(2):515–521. doi: 10.1104/pp.72.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Kanabus J. Extraction of starch by dimethyl sulfoxide and quantitation by enzymatic assay. Anal Biochem. 1987 Feb 15;161(1):132–139. doi: 10.1016/0003-2697(87)90662-2. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Mulligan J. A., Heyser J. W. Hemicelluloses of cell walls of a proso millet cell suspension culture. Plant Physiol. 1985 Oct;79(2):480–484. doi: 10.1104/pp.79.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filisetti-Cozzi T. M., Carpita N. C. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991 Aug 15;197(1):157–162. doi: 10.1016/0003-2697(91)90372-z. [DOI] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Koch J. L., Nevins D. J. Tomato fruit cell wall : I. Use of purified tomato polygalacturonase and pectinmethylesterase to identify developmental changes in pectins. Plant Physiol. 1989 Nov;91(3):816–822. doi: 10.1104/pp.91.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness N. O., Ryan J. D., Mort A. J. Determination of the degree of methyl esterification of pectins in small samples by selective reduction of esterified galacturonic acid to galactose. Anal Biochem. 1990 Mar;185(2):346–352. doi: 10.1016/0003-2697(90)90306-t. [DOI] [PubMed] [Google Scholar]
- Moustacas A. M., Nari J., Diamantidis G., Noat G., Crasnier M., Borel M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 2. The role of pectin methyl esterase in the modulation of electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):191–197. doi: 10.1111/j.1432-1033.1986.tb09476.x. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Diamantidis G., Woudstra M., Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 3. Interplay between limited cell-wall autolysis, pectin methyl esterase activity and electrostatic effects in soybean cell walls. Eur J Biochem. 1986 Feb 17;155(1):199–202. doi: 10.1111/j.1432-1033.1986.tb09477.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer P. E., Doner L. W., Hoagland P. D., McDonald G. G. Molecular interactions with dietary fiber components. Investigation of the possible association of pectin and bile acids. J Agric Food Chem. 1981 May-Jun;29(3):455–461. doi: 10.1021/jf00105a005. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Morris E. R., Gidley M. J., Rees D. A. Conformations and interactions of pectins. II. Influences of residue sequence on chain association in calcium pectate gels. J Mol Biol. 1982 Mar 15;155(4):517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Siddiqui I. R. Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal Biochem. 1971 Feb;39(2):418–428. doi: 10.1016/0003-2697(71)90432-5. [DOI] [PubMed] [Google Scholar]


