Abstract
Aging and neurodegeneration entail diverse cellular and molecular hallmarks. Here, we studied the effects of aging on the transcriptome, translatome, and multiple layers of the proteome in the brain of a short-lived killifish. We reveal that aging causes widespread reduction of proteins enriched in basic amino acids that is independent of mRNA regulation, and it is not due to impaired proteasome activity. Instead, we identify a cascade of events where aberrant translation pausing leads to reduced ribosome availability resulting in proteome remodeling independently of transcriptional regulation. Our research uncovers a vulnerable point in the aging brain’s biology – the biogenesis of basic DNA/RNA binding proteins. This vulnerability may represent a unifying principle that connects various aging hallmarks, encompassing genome integrity and the biosynthesis of macromolecules.
Keywords: brain, aging, proteome, translation, ribosome, proteasome, mitochondria, killifish, protein aggregation, post-translational modification
One-Sentence Summary:
Translation pausing reshapes the aging brain proteome, revealing vulnerabilities in the biogenesis of nucleic-acid protein.
Both aging and neurodegeneration disrupt protein homeostasis, also known as proteostasis, leading to the progressive accumulation of protein aggregates (1, 2). Proteostasis involves mechanisms that regulate the coordination of protein synthesis, degradation, and localization and is essential to ensure an adequate supply of protein building blocks. It also prevents the accumulation of misfolded and “orphan” proteins susceptible to aggregation.
Age-dependent decline in proteostasis coincides with the onset of other aging hallmarks, but a causal connection remains unproven, necessitating integrative analysis to reveal these associations. This knowledge gap remains, at least partially, because these aspects were analyzed separately and in different model systems. Therefore, we conducted a comprehensive analysis of proteostasis in the aging brain of the short-lived killifish Nothobranchius furzeri. We chose killifish because of its accelerated aging and spontaneous emergence of neurodegenerative phenotypes (3–8)
Using this model system, we measured the effects of aging on the transcriptome, translatome, and multiple layers of the proteome, enabling quantification of their relationships. We also established a protocol for long-term partial inhibition of proteasome activity to investigate which age-related brain phenotypes are caused by this specific dysfunction in vivo. Finally, we performed Ribo-Seq to assess the contribution of mRNA translation to proteome alterations. Our work reveals that the biosynthesis of specific proteins rich in basic amino acids becomes perturbed with age due to aberrant translation elongation and pausing. Such alterations lead to the depletion of protein complexes involved in DNA/RNA-binding and protein synthesis, and remodel the proteomes of organelles such as mitochondria. This altered translation dynamic also provides a mechanistic explanation for the highly conserved age-related discrepancies between transcriptome and proteome changes (9,10,11,12,13,14,6), which have been linked to neurodegeneration in humans (15). Thus, our work reveals how the biogenesis of a specific subset of proteins might further enhance vulnerabilities of the proteostasis system in the aging brain and contribute to the exacerbation of other aging hallmarks.
Loss of basic proteins in the aging brain
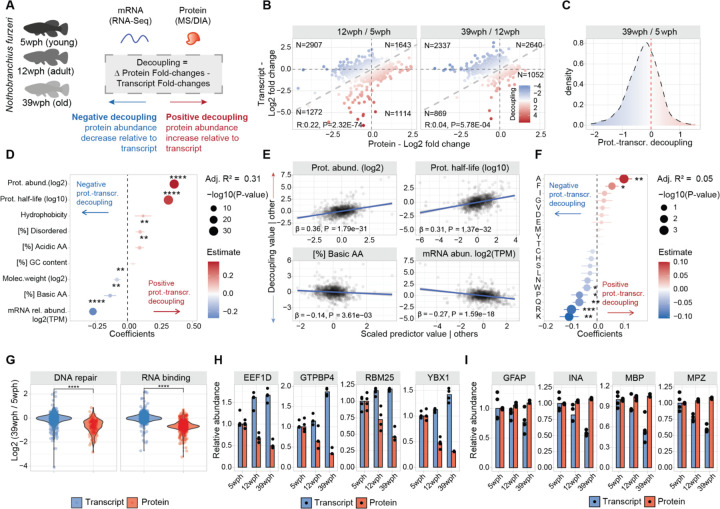
To investigate the disruption of protein homeostasis in aging, we focused on the loss of correlation between changes in gene transcripts (mRNA) and corresponding protein, an intra-species conserved phenomenon (9,10,11,12,13,14,6) here referred to as “decoupling”, for which a biological explanation is still lacking. To address the origin of decoupling, we combined the quantification of age-related changes in gene transcripts and protein levels obtained from RNA sequencing (RNAseq) and mass spectrometry-based proteomics data, respectively (Figure 1A–B, Figure S1A–H). We calculated a “decoupling score” by measuring the differences between protein and transcript changes (see methods). The decoupling score effectively describes discrepancies between transcript and protein changes by identifying subsets of proteins displaying “positive protein-transcript decoupling”, i.e., protein level higher than expected from changes of its corresponding transcript, or “negative protein-transcript decoupling”, i.e., protein level lower than expected from changes of its corresponding transcript (Figure 1B–C, Table S1).
Figure 1: Protein-transcript decoupling affects highly abundant and basic proteins in opposite manners.
A) Characterization of protein-transcript decoupling in the aging brain of killifish. Positive decoupling values indicate increased protein abundance relative to transcripts, while negative decoupling indicates decreased protein abundance compared to transcripts. B) Scatterplot comparing protein and transcript fold changes in aging brain. Color represents decoupling score, red – increased protein abundance relative to transcript, blue – decreased protein abundance compared to transcript. Grey dashed lines indicate equal changes. C) Density distribution of decoupling scores for 39 wph vs. 5 wph comparison. Red: positive decoupling, Blue: negative decoupling. D) Multiple linear regression analysis of decoupling scores based on transcript and protein features. asterisks represent the −log10 P-values of the F-test. E) Added variable plot between features and decoupling scores. F) Multiple linear regression analysis of decoupling scores based on protein amino acid composition. asterisks represent the −log10 P-values of the F-test. G) Transcript and protein fold changes for RNA binding and DNA repair proteins. Two-sample Wilcoxon test H and I) Examples of proteins with negative (H) and positive (I) decoupling (N=3–4). *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Related to Figure S1 and Table S1.
The obtained scores displayed a median shift towards negative values (Figure 1C) due to an overall skew towards negative fold changes at the proteome level (Figure S1D), which was independent of sample normalization (Figure S1C). To assess the reproducibility of this metric, we compared the decoupling scores from this study to the ones obtained using an independent brain aging dataset that we previously generated (6). Supporting our observations, there was a significant positive correlation between these datasets (Figure S1I), despite technical differences in the quantitative proteomics workflows: tandem-mass tags (TMT) based quantification (6) compared to label-free Data Independent Acquisition (DIA, this study).
We then applied a multiple linear regression model to interrogate the association between the measured decoupling scores (response variable, N=1188 complete observation) and distinct biophysical properties of transcripts and proteins (N=9 features). Our model explained 31% of the decoupling variance (Adjusted R2 = 0.31, Figure 1D). We detected estimated protein absolute abundance (see methods, β=0.36, P < 2.20E-16) and protein half-life (as described in (16), β=0.31, P < 2.20E-16) as the parameters with the highest correlation with higher protein levels than expected from transcript changes (Figure 1E). On the other hand, the parameters with the highest correlation with negative decoupling (i.e., lower protein levels than expected from transcript changes) were relative transcript abundance (expressed as log2 transcripts per million (TPM) β=−0.26, P < 2.20E-16) and proportion of basic amino acids (β=−0.13, P = 4.30E-03, Figure 1D–E). To understand the contribution of amino acid composition to the observed discrepancies, we employed a second regression model with protein amino acid composition as the sole predictor variable. Our analysis revealed significant correlations between negative decoupling and the content of lysine, proline, glutamine, and arginine (Figure 1F).
Intrigued by these findings, we investigated the behavior of proteins involved in DNA repair and RNA-binding, due to their high content of basic amino acids. Both these groups of proteins showed an age-dependent decrease of protein- but not transcript levels (Figure 1G and H). On the other hand, myelin components, e.g., myelin basic protein (MBP) and myelin protein P0 (MPZ), and intermediate filament proteins, e.g. glial fibrillary acidic protein (GFAP) and alpha-internecine (INA), showed decreased transcript- but not protein-levels with aging (Figure 1I), consistently with their long half-lives and low turnover rates. These results show that specific classes of proteins experience discrepancies between protein and transcript levels in the aging vertebrate brain. The distinct biophysical and biochemical characteristics promoting these discrepancies suggest the presence of shared molecular attributes that might drive these phenomena. In particular, our data highlight a potential mechanistic link between the content of basic amino acids and the age-related decrease in protein levels compared to the corresponding mRNAs.
Convergence of proteome alterations on ribosomes and respiratory chain complexes
We then explored how discrepancies between transcript and protein changes relate to other proteome alterations. To do so, we employed a comprehensive approach characterizing age-related changes in proteome insolubility by implementing a differential detergent extraction protocol (Figure 2A, Figure S2 and Table S2, see methods (17)) and organelle composition, using subcellular fractionation in combination with mass-spectrometry (18) (Figure S3 and Table S2, see methods). We additionally quantified changes in phosphorylation, ubiquitylation, and acetylation to detect how the landscape of post-translational modifications is affected in the aging brain (Figure 2A, Figure S4 and Table S3, see methods). The combined data showed changes in organelle proteome composition, proteasome solubility, alterations in the post-translational modification in the aging brain (Figure S5, see Supplementary text), and age-related proteome alterations in proteins encoded by genes genetically linked to neurodegeneration in humans (Table S4, see Supplementary text and Figure S6).
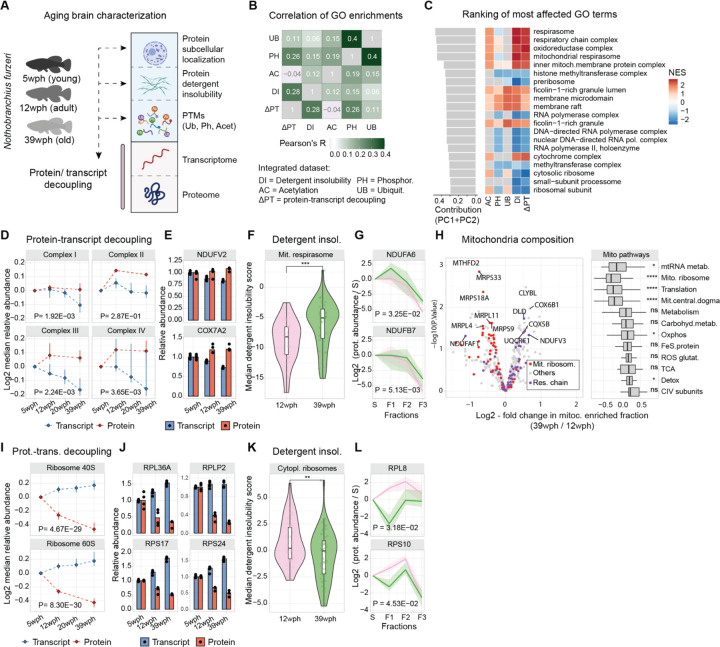
Figure 2: Proteome alterations converges on ribosomes and respiratory chain complexes.
A) Overview of the datasets generated at the beginning of this study (wph= weeks post-hatching). B) Heatmap shows correlations of normalized enrichment scores (NES) across datasets (DR=Detergent insolubility, ΔPT=protein-transcript decoupling, AC=Acetylation, PH=Phosphorylation, UB=Ubiquitylation). C) Top-ranking GO terms with strong contributions to PCA analysis. D) Line plots for respiratory chain proteins’ transcript (blue) and protein (red) median abundance across age groups (N=3–4, MANOVA). E) Examples of respiratory chain proteins with positive protein-transcript decoupling. F) Violin plot for detergent insolubility scores of mitochondrial respirasome proteins (N=4, two-sample Wilcoxon test). G) Examples of detergent insolubility profiles for respiratory chain proteins with increased detergent insolubility during aging (N=4, MANOVA). H) Volcano plot for changes in mitochondrial proteome due to aging. Box plot shows the effect of aging on different groups of mitochondrial pathways (N=4, two-sample Wilcoxon test). I) Ribosomal proteins’ transcript and protein abundance across age groups (N=3–4, MANOVA). J) Examples of ribosomal proteins displaying negative protein-transcript decoupling (N=3–4). K) Violin plot for detergent insolubility scores of cytoplasmic ribosomal subunits (N=4, two-sample Wilcoxon test). L) Examples of detergent insolubility profiles for ribosomal proteins with decreased detergent insolubility during aging (N=4, MANOVA). *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Related to Figure S2:S7 and Tables S2,S3.
To explore the relationship among different types of proteome alteration in the aging brain, we conducted a gene set enrichment analysis on the age-related proteome changes for each of the generated datasets (see methods). By calculating Pearson’s correlation coefficient between enrichment scores across datasets, we found a positive correlation between protein-transcript decoupling and increased detergent insolubility, a hallmark of protein aggregation (Pearson’s R = 0.28, P < 2.20E-16), as well as protein phosphorylation (Pearson’s R = 0.26, P = 6.67E-08), while other alterations, for instance, changes in protein ubiquitylation, showed a smaller correlation value (Pearson’s R = 0.11, P = 1.23E-02, Figure 2B).
To unbiasedly identify the most prominently affected cellular components in our analysis, we then used principal component analysis (PCA) to summarize the normalized enrichment scores (NES, see methods) and ranked GO terms by calculating the values of their projections on the first two principal component axes. We found that the highest-ranking terms were related to components of the mitochondrial respiratory chain and ribosomes (Figure 2C). These two sets of protein complexes were often affected by aging in opposite ways (Figure 2C). Components of the respiratory chain showed a progressive decrease in their transcripts together with a stable or modest increase of the corresponding protein levels (Figure 2D–E, Figure S7B). Respiratory chain proteins also showed an overall increase in detergent insolubility with aging (Figure 2F–G). Importantly, these alterations primarily affected respiratory chain components but not mitochondrial proteins in general (Figure S7C). To corroborate these findings, we interrogated our subcellular fractionation data (Figure S3). This analysis allowed us to identify two key aspects: (i) changes in the protein composition of aged mitochondria, notably a significant decrease in the relative abundance of mitochondrial ribosomes and an increase in the relative abundance of oxidative phosphorylation (Figure 2H and Figure S7D), and (ii) altered subcellular distribution of specific mitochondrial proteins (Figure S7E–F). These analyses provide support for a global remodeling of the mitochondria during aging.
In contrast, both cytosolic and mitochondrial ribosomal protein levels progressively decreased during aging (reaching, on average, a ~25% reduction in old brains), while their corresponding transcripts increased (Figure 2I–J, Figure S7G–H). The reduced level of ribosomal proteins was accompanied by a decreased detergent insolubility (Figure 2K–L, Figure S7H–I). This alteration might be related to the loss of ribosome stoichiometry and partial mis-/disassembly that we previously described in the old killifish brain (6). Interestingly, we noticed similar patterns for other large complexes rich in basic amino acids, like RNA polymerase II (Figure S7J–K), that might indicate common mechanisms altering the homeostasis of these key complexes. These combined analyses reveal that the mitochondrial respiratory chain, ribosomes, and other protein complexes enriched in basic amino acids are preferentially and differently affected in the aging brain. A web-based application is available for the exploration of these datasets (https://genome.leibniz-fli.de/shiny/orilab/notho-brain-atlas/ credentials username: reviewer password: nothobrain2023)
Impact of proteasome impairment on the brain proteome
Protein degradation by the ubiquitin-proteasome system regulates protein levels in organelles and complexes, including ribosomes and mitochondria. Previous studies (6,2,19) have linked aging with a decline in proteasome activity. To study the impact of proteasome activity on the aging brain, we simulated its impairment by chronically reducing its activity in adult killifish. We optimized in vivo dosage of bortezomib, a dipeptide that binds with high affinity and blocks the catalytic site of the proteasome, to maintain a ~50% inhibition in the brain of adult killifish over 4 weeks without inducing overt toxicity (Figure 3A, Table S5). GO enrichment analysis revealed brain proteome and transcriptome adaptive responses to proteasome inhibition, including over-representation of proteasome-related terms (Figure 3A) and specific proteostasis network alterations (Figure S8A). These include increased protein levels of proteasome activators (PSME1/PA28ɑ, PSME3/PA28γ, and PSME4/PA200) and increased mRNA levels of key autophagy genes such as ATG5 and ATG7 (Figure 3B). Some responses, like elevated HSPB1 and HSPA6, arose spontaneously in aged killifish brains (Figure 3B). Immunofluorescence analysis of lysosomes revealed a marked increase in their area, volume, and sphericity (Figure 3C), a phenotype typical of aging (Figure S8B), lysosomal storage disorders (20), and neurodegenerative diseases (21). Proteasome inhibition globally reduced mitochondrial protein levels independent of transcription (Figure 3D) and without altering master regulators of mitochondrial genes (Figure S8C). Similar to aging it also induced a reduction of mitochondrial content (estimated calculating ratio of mitochondrial DNA (mtDNA) to nuclear DNA, Figure 3D–S8D).
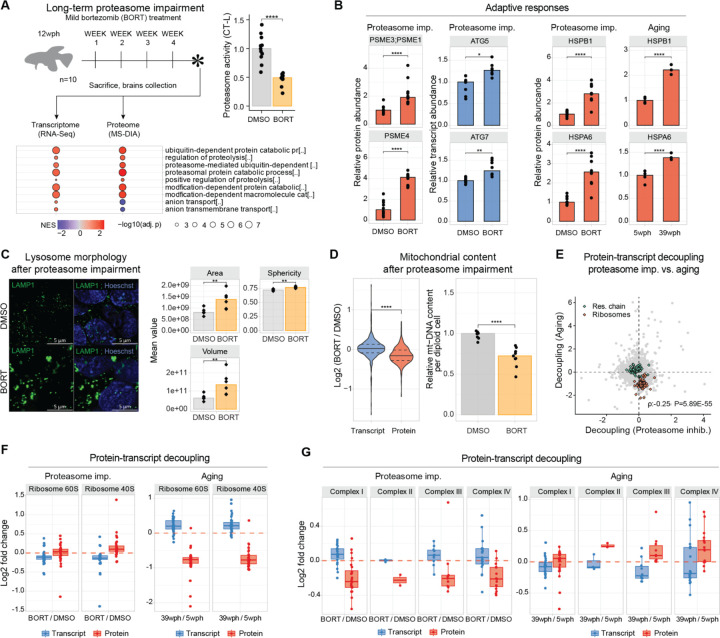
Figure 3: Effects of four weeks in vivo proteasome impairment on the adult killifish brain.
A) Adult killifish (12 wph, N=10) received weekly intraperitoneal injections of proteasome inhibitor bortezomib or DMSO control. Bottom panel: Gene Set Enrichment Analysis (GSEA) color-coded by normalized enrichment score (NES). Top-right: Chymotrypsin-like proteasome activity quantification (two-sample Wilcoxon test, N=10). B) Barplot: Protein (red) transcript (blue) quantity in proteostasis network proteins in aging and proteasome inhibition (two-sample T-test, N=10). Asterisks indicate Q.value (protein) and Adjusted P.Value (transcript). C) Left: Lysosome (LAMP1) immunofluorescence. Scale bars = 5μm; right: Lysosome morphology analysis (two-sample T-test N=6). D) Left: Proteasome effect on mitochondrial transcripts and proteins (two-sample Wilcoxon test); right: Relative mtDNA copy number was calculated using real-time quantitative PCR with primers for 16S rRNA mitochondrial gene and Cdkn2a/b nuclear gene for normalization (N=10, two-sample Wilcoxon tests). E) Decoupling scores comparison between aging and proteasome impairment for respiratory chain (green) and ribosomal (orange) proteins (Spearman correlation was selected due to the presence of outliers in the distribution). F) Ribosome decoupling comparison between aging and proteasome impairment. G) Oxidative phosphorylation protein decoupling comparison between aging and proteasome impairment. *P≤0.05; **P≤0.01, ***P≤0.001, ****P≤0.0001. Related to Figure S8, Table S5.
As expected, proteasome impairment led to an increased abundance of shorter-lived proteins, consistent with its role in regulating protein turnover (22)(Figure S8E). We then combined RNAseq and proteome data to understand the contribution of reduced proteasome activity to the age-related discrepancies between transcript and protein levels. We showed that proteasome impairment leads to decoupling between transcript and protein changes (Figure S8F, Table S5). However, when we applied the same linear regression models used for aging, we found distinct biophysical properties associated with decoupling by bortezomib compared to aging (Figure S8F). Specifically, proteasome inhibition caused an accumulation of basic proteins, independently of transcription (Figure S8F), and decoupling scores induced by proteasome impairment and aging were surprisingly negatively correlated (Spearman Rho = −0.25, P < 2.20E-16, Figure 3E). This included the cases of ribosomes (Figure 3F) and respiratory chain complexes (Figure 3G). Thus, our data reveal specific alterations induced in the adult killifish brain by partial proteasome impairment, some of which recapitulate aging brain phenotypes. Nonetheless, reduction in proteasome activity does not appear to be directly responsible for the loss of basic proteins observed in the old brains, hinting at other possible mechanisms.
Aberrant translation pausing correlates with decreased levels of basic proteins in old brains
Our findings show that imbalances in proteostasis during aging go beyond proteasome dysfunction. Other factors, such as differential mRNA translation at old age, could cause the observed discrepancies between transcript and protein levels. Therefore, we explored this hypothesis with a Ribo-Seq experiment in aging killifish brains (Figure 4A, Table S6). Tri-nucleotide periodicity and consistent replicates (Figure S9A–B) showed the overall quality of the data, while comparison between mRNA levels and ribosome occupancy showed the expected correlation between the two (R=0.25, P < 2.20E-16, Figure S9C). When we estimated translation efficiency (TE, see methods), we observed that this measure was better at explaining protein changes (R=0.32, P < 2.20E-16, Fig. 4B) in comparison to transcript changes (R=0.23, P < 2.20E-16), consistent with previous observations in mammals (23). For instance, shifts in TE led to consistent changes in protein levels for certain protein complexes, such as the Complex IV of the respiratory chain, and the 26S proteasome (Figure 4C–D and S9D). Interestingly though, ribosomes, RNA polymerase II, and other nucleic-acid binding proteins linked to DNA repair (Figure 4C–D, and S9D) didn’t exhibit the same behavior, excluding TE as the cause for their decreased abundance.
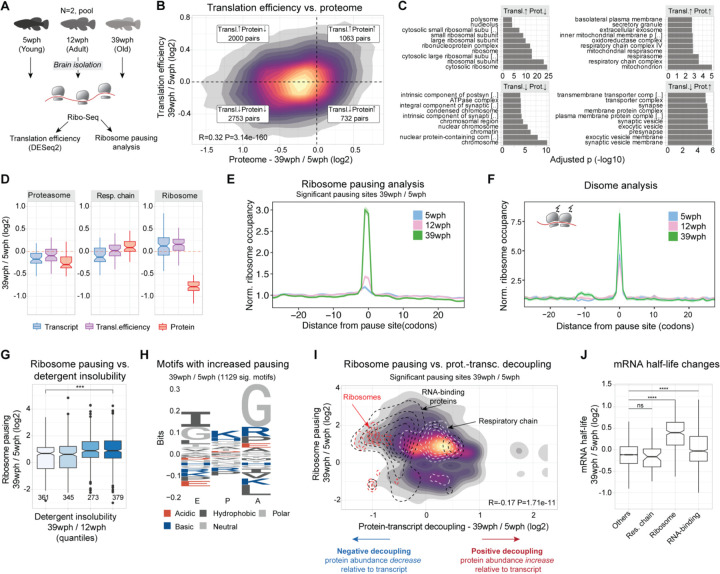
Figure 4: Increased translation pausing in the aging killifish brain.
A) Experimental Workflow: Ribosome profiling was conducted on brains of Nothobranchius furzeri at different ages—Young (5 weeks post-hatch, wph), Adult (12wph), and Old (39wph). Each age group had two replicates, each consisting of pooled samples from 10–15 animals. B) A 2-D density plot illustrates the connection between age-induced changes in protein abundance (x-axis) and alterations in translation efficiency (y-axis). Different quadrants highlight modes of translation regulation. C) GOEnrichment analysis (ORA) for each quadrant from B. x-axis: −log10(adjusted P-value) Fisher test, Holm correction. D) Differential regulations for key complexes, 26S Proteasome, oxidative phosphorylation, and cytoplasmic ribosomes – Transcriptome (blue), Translation efficiency (purple), and Proteome (red), in Old vs. Young. E) Lineplot showing the normalized ribosome distribution at pausing sites across different age groups. F) Lineplot depicts normalized disome ribosome distribution at disome pausing sites for various age groups. G) Boxplot showing solubility vs. ribosome pausing. x-axis: solubility quantiles (25% of total distribution each), y-axis: log2 fold changes in pausing for significant sites (Adj. P-value < 0.05). Numbers indicate observations. Two-sample Wilcoxon tests H) Peptide motif associated with age-dependent increased pausing (Pause score at 39wph > Pause score at 5wph and 12wph, and Pause score at 39wph > 6). y-axis: relative residue frequencies, x-axis: ribosome positions (E, P, A). I) 2-D density plot showing relation between significant pausing changes (Adj. P-value < 0.05) on y-axis and decoupling metrics (x-axis). Contours: cytoplasmic ribosomes (red), RNA-binding proteins (black), oxidative phosphorylation (white). J) Boxplot showing mRNA half-life estimate changes (methods) between 39 wph and 5 wph. x-axis: selected categories. Asterisks: two-sample Wilcoxon test, Holm correction. *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Related to Figure S9, Table S6.
To investigate further this intriguing aspect, we drew inspiration from studies on aged nematodes and yeasts, which exhibit age-related impaired translation elongation and ribosome pausing (24). We queried our Ribo-Seq data for signatures of translation pausing (see methods) and revealed an overall increase in site-specific pausing in the aging brain (Figure 4E, Table S6), with disome analysis confirming increased ribosome collisions (Figure 4F). Anisomycin-induced ribosome stalling in killifish cells (Figure S9E) showed a higher molecular weight ubiquitylated band in the immunoblot against the 40S subunit RPS3, commonly associated with ribosome collision (25–27). Aged brains showed similar changes (Figure S9F), even though most ribosomal protein ubiquitination decreased with age (Figure S9G). Similarly to aged yeast and nematodes, we also observed a decrease in a subset of proteins involved in ribosome quality control (RQC) (Figure S9H). This RQC reduction may worsen ribosome collisions and stalling as age progresses, potentially slowing stalled mRNA degradation and causing their accumulation in aging cells. When we investigated the impact of translation pausing on cellular proteostasis, we reported a clear association between translation pausing and increased detergent insolubility (Figure 4G). More interestingly, these alterations affected key proteostasis network components such as the proteasome (Figure S9I), hinting at a possible vicious cycle of proteostasis collapse. Stretches enriched in codons for basic residues (arginine and lysine), as well as glycine, were enriched at both pausing (Figure 5H) and disome sites (Figure S9J). Notably, our decoupling model linked the same residues (arginine and lysine) to reduced protein levels (Figure 1F). Furthermore, we found a significant correlation between pausing and protein-transcript decoupling (Figure 4I, R=−0.17, P < 2.20E-16), explaining cases for ribosomal and RNA-binding proteins, where protein decline does not follow transcript changes. On the contrary, components of the respiratory chain did not show any remarkable deviation from the overall pausing distribution (Figure 4I), although different complexes showed distinct pausing profiles (Figure S9K).
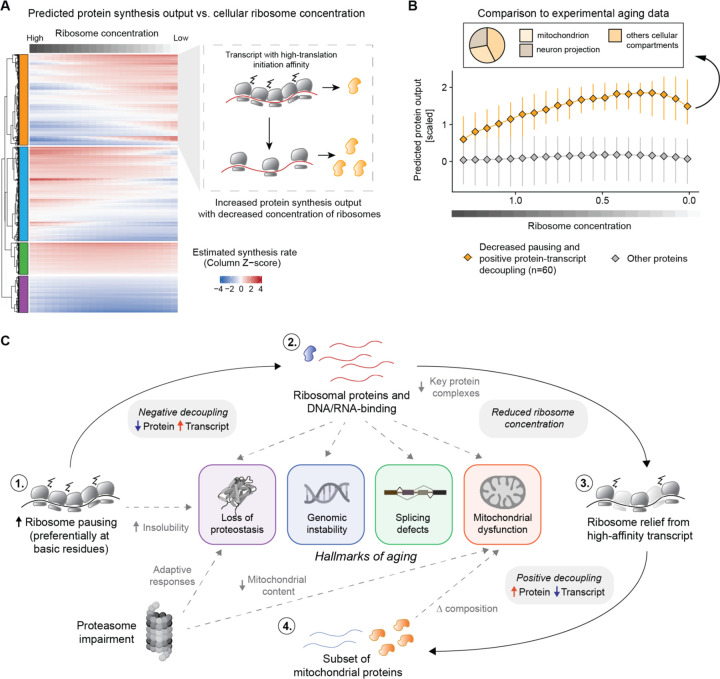
Figure 5: Reduced ribosome levels can lead to translation reprogramming in the aging brain.
A) Heatmap showing the estimated protein output, modeled as described in Mills and Green 2017 (32). Each column in the heatmap indicates the estimated protein output for a specific ribosome concentration. Transcripts are clustered with a hierarchical clustering using the “ward D2” algorithm on the dissimilarity (1 - Person’s correlation) measure. For display purposes, the heatmap represents 5000 rows randomly sampled from all datasets. In the right panel, an illustrative example of a cluster displaying increased estimated protein output as a function of reduced ribosome levels. For these transcripts, the general ribosome decrease is predicted to relieve trafficking and pausing, leading to overall improved protein production. B) Lineplot showing the estimated protein output for transcript displaying decreased ribosome pausing in the Ribo-Seq data (median per transcript log2 Pausing 39 wph / 5 wph < 0 and Adjusted P-value <=0.15) and increased protein levels relative to the transcript in the decoupling model (orange). The x-axis represents the simulated decreased ribosomal concentration, while the y-axis indicates the estimated protein output, as shown also in A. C) Schematic representation of the translation reprogramming model and its connection with the relevant hallmarks of aging. Aging is associated with increased ribosome collision and pausing on ribosomal proteins, leading to a ~25% reduction of ribosome levels. This generalized decrease of available ribosomes could drive the translation of other high-affinity mRNAs leading to increased protein levels in the aging brain. Related to Table S7.
Changes in translation and pausing affect mRNA half-life (28–30). We additionally investigated the association between translation pausing and mRNA half-life, by estimating it from RNA-Seq data (see methods, (31)). This led to the discovery that, in old brains, transcripts encoding for ribosomal proteins and RNA-binding proteins show an increased half-life compared to the rest of the transcriptome (Figure 4J). This highlights that alteration in translation might influence other general changes in the transcriptome’s structure. In summary, our results show that increased ribosome occupancy does not necessarily result in enhanced protein synthesis in the aging brain, of note we propose that translation dysfunction may represent the underlying cause for the decreased levels of ribosomal proteins and other nucleic-acid binding proteins (enriched in basic amino acids) in the aging brain. Such reduction might further exacerbate other aging hallmarks that depend on the activity of these proteins.
A possible model for protein biosynthesis in the aging brain
The results presented so far point to alterations in protein synthesis in old brains leading to a reduction of ribosomal proteins, among others. We hypothesized that the ensuing lower levels of ribosomes, particularly in light of increased load on the RQC machineries, may in turn lead to a vicious cycle of dysfunction. Altered ribosome concentration has been known to directly impact the translation of specific mRNAs, as observed in a group of inherited diseases collectively referred to as ‘ribosomopathies’ (32, 33). We thus attempted to extend a model proposed by (32, 33) to the aging scenario. The original model predicts that the protein output of specific mRNAs can be influenced by ribosome availability depending on transcript-specific translation initiation rate ki (where ki refers to the affinity of specific mRNAs sequences to bind ribosomes) (32, 33). Under these assumptions, a decrease in ribosome concentration can, for example, increase protein synthesis from transcripts that have a high translation initiation rate by lowering the total ribosome load on them and therefore relieving trafficking and pausing events (Figure 5A). To test this hypothesis in the context of aging brain, we estimated ki from killifish 5’-UTR sequences based on experimental data (34), and modeled the estimated synthesis rate as described in (32, 33) (see methods, Figure 5A, Table S7). In agreement with the model, a subset of killifish transcripts displayed an increase in predicted synthesis rate as a function of decreased ribosome concentration (orange cluster in Figure 5A and Table S7). To test these predictions on our experimental data, we selected a specific set of proteins showing decreased translation pausing and increased protein abundance in our decoupling model (60 proteins, bottom right quadrant in Figure 4I). We then estimated their predicted synthesis rates as a function of ribosome concentration. Consistent with the experimental data, the relative synthesis of this subset of proteins was predicted to increase following a reduction of ribosome concentration (Figure 5B). Approximately one-third of these proteins were mitochondrial (including 7 components of the respiratory chain), and another prominent fraction belonged to proteins related to neuron projections (Figure 5B). Intriguingly, the absence of ribosomal proteins in this subset, despite their high ki value, indicates distinct translation dynamics for these proteins resulting from their increased elongation pausing during aging. These results provide evidence that reduced ribosome concentration in aged brains, likely triggered by aberrant pausing events, might remodel a subset of the proteome independently of transcript levels and regulation (Figure 5C).
Discussion
Our study offers a comprehensive insight into how distinct mechanisms mediating proteostasis can influence the vertebrate brain proteome during aging. We show significant proteome changes in aging brains, encompassing protein synthesis, solubility, post-translational modifications, and organelle composition. Among these, we show that ribosomal and DNA/RNA binding protein levels decrease independently of mRNA abundance. We propose that translation alterations, including elongation pausing at basic residues, are central drivers of these changes leading to discrepancies between mRNA levels, ribosome occupancy, and protein synthesis.
At least two key implications emerge from our findings. Firstly, basic protein complexes like ribosomes, RNA-binding proteins involved in splicing, RNA and DNA polymerases, and the ones involved in DNA repair experience reduced availability with age. This phenomenon, caused by the presence of basic amino acids in their protein sequences, likely has a direct impact on each step of the gene expression process, and could mechanistically connect proteostasis impairment to other canonical aging hallmarks, including DNA damage, epigenetic alterations (35), aberrant splicing (36) and reduced RNA polymerase activity (37). Of note, individual manipulation of protein biosynthesis, as well as any of these pathways, ameliorates aging phenotypes (37–42,43), further highlighting their central role in the aging process.
A second implication is that aging leads to altered mitochondrial composition, partially driven by a reduced ribosomal content. This remodeling encompasses a decrease of mitochondrial ribosomes, while respiratory chain components remain stable or increase, as in the case of Complex IV. This is consistent with broader observations of aging-induced mitochondrial changes (44,45). These findings based on bulk tissue measurements were corroborated by more direct analysis of the composition of mitochondria from subcellular fractions and by other age-dependent alterations of mitochondrial proteins, e.g., in detergent insolubility. In addition, we show that a reduced mtDNA content is induced by decreased proteasome activity, showcasing the convergence of aging mechanisms affecting critical cellular structures.
The mechanisms leading to increased translation pausing remain unclear. Future analyses should clarify the mechanics of these events and their relationship with other age-related alterations of ribosomes, including loss of stoichiometry and aggregation (6). We speculate that one of the mechanisms contributing to increased translational pausing could reside in the decrease in ATP levels that is typically observed in old tissues (46–49). This reduction in energy levels might alter the decoding kinetics for specific non-optimal codons, such as those encoding basic amino acids (50, 51), leading to a decreased synthesis rate for these proteins. We also identified distinctive changes in protein ubiquitylation in ribosomal proteins, some of which have been previously associated with ribosome collision induced by different types of translation or proteotoxic stress (26, 27). However, it remains unclear whether these modifications are a cause or consequence of increased pausing. For ribosomes, decoupling in aging manifests as a decrease in protein levels together with a progressive increase in transcript levels. These findings are consistent with several observations. First, an age-dependent increase of transcripts encoding for ribosomal proteins has been observed by single-cell RNAseq in multiple cell types of the murine brain (52). Accordingly, increased levels of transcripts encoding for ribosomal proteins were one of the most consistent transcriptional signatures of longevity shared across multiple tissues and mammalian species (53). Interestingly, our results suggest that this increase might not result from increased transcription but rather from increased mRNA stability. Decreased abundance of ribosomal proteins with age has been described in multiple organs in mice (54), as well as in nematodes (55), and the protein half-life of ribosomes is affected by aging in the mouse brain (56). These data suggest that similar mechanisms might affect ribosomes in different cell types and organs during mammalian aging. Translation pausing may also represent a converging pathophysiological mechanism shared between aging and neurodegenerative diseases, as ribosome stalling has been linked to perturbation of proteostasis in different types of neurodegenerative diseases (57–60).
Other mechanisms that have not been investigated in this study can additionally contribute to alteration in proteome composition. For instance, age-dependent impairment of protein degradation by the autophagy-lysosome system can lead to the accumulation of specific proteins (61), as has been shown for myelin basic protein (MBP) in microglia (62). In addition, stalling of RNA polymerase II has been described to occur with aging, thereby skewing the output of transcription in a gene-length dependent manner (37), consistent with a systemic loss of long transcripts observed in multiple aging tissues and species (63). A reduction in the abundance of specific transcripts could increase transcriptional noise, lead to an imbalance in the stoichiometry of protein complexes, but also alter the relationship between mRNA and protein levels, especially for long-lived proteins.
Finally, our work might contribute to understanding the relationship between aging and the risk of neurodegenerative diseases. We provide an unprecedented resource (accessible at https://genome.leibniz-fli.de/shiny/orilab/notho-brain-atlas/ credentials username: reviewer password: nothobrain2023) of proteome alterations in the aging vertebrate brain and show that multiple proteins and signaling pathways associated with neurodegeneration in humans become perturbed in different ways during physiological aging in killifish (Supplementary text and Figure S5–S6). Such alterations might underlie convergent mechanisms between aging and mutations that increase the risk of neurodegeneration in old individuals.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from the FLI Core Facilities Proteomics, Sequencing, and the Fish Facility as well as the Stanford Genomics Facility. A.O. is supported by the German Research Council (Deutsche Forschungsgemeinschaft, DFG) via the Research Training Group ProMoAge (GRK 2155), the Else Kröner Fresenius Stiftung (award number: 2019_A79), the Fritz-Thyssen Foundation (award number: 10.20.1.022MN), the Chan Zuckerberg Initiative Neurodegeneration Challenge Network (award numbers: 2020-221617, 2021-230967 and 2022-250618), and the NCL Stiftung. J.H.L. was supported by the National Institute on Aging of the National Institutes of Health under Award Number T32 AG000266, and research by NIH grants GM05643319 and AG054407 to J.F. A.C. is supported by the German Research Council (Deutsche Forschungsgemeinschaft, DFG, award numbers CE 257/9-1 and CE 257/9-2), by Next Generation EU (PNRR), “Tuscany Health Ecosystem”, THE project code ECS 00000017 and the Italian Ministry of University and Research (MIUR) with the program “Joined research for Special Status School”, PRO3. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health. The FLI is a member of the Leibniz Association and is financially supported by the Federal Government of Germany and the State of Thuringia.
Footnotes
Declaration of interest
Authors declare no competing interests.
References
- 1.Labbadia J., Morimoto R. I., The biology of proteostasis in aging and disease. Annu. Rev. Biochem. (2015) (available at https://www.annualreviews.org/doi/abs/10.1146/annurev-biochem-060614-033955). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hipp M. S., Kasturi P., Hartl F. U., The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Valenzano D. R., Terzibasi E., Cattaneo A., Domenici L., Cellerino A., Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 5, 275–278 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Bagnoli S., Fronte B., Bibbiani C., Terzibasi T. E., Cellerino A., Quantification of noradrenergic-, dopaminergic-, and tectal-neurons during aging in the short-lived killifish Nothobranchius furzeri. Aging Cell. 21 (2022), doi: 10.1111/acel.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsui H., Kenmochi N., Namikawa K., Age- and α-Synuclein-Dependent Degeneration of Dopamine and Noradrenaline Neurons in the Annual Killifish Nothobranchius furzeri. Cell Rep. 26, 1727–1733.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Kelmer Sacramento E., Kirkpatrick J. M., Mazzetto M., Baumgart M., Bartolome A., Di Sanzo S., Caterino C., Sanguanini M., Papaevgeniou N., Lefaki M., Childs D., Bagnoli S., Terzibasi Tozzini E., Di Fraia D., Romanov N., Sudmant P. H., Huber W., Chondrogianni N., Vendruscolo M., Cellerino A., Ori A., Reduced proteasome activity in the aging brain results in ribosome stoichiometry loss and aggregation. Mol. Syst. Biol. 16, e9596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harel I., Chen Y. R., Ziv I., Singh P. P., Negredo P. N., Goshtchevsky U., Wang W., Astre G., Moses E., McKay A., Machado B. E., Hebestreit K., Yin S., Alvarado A. S., Jarosz D. F., Brunet A., Identification of protein aggregates in the aging vertebrate brain with prion-like and phase separation properties. bioRxiv (2022), p. 2022.02.26.482115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louka A., Bagnoli S., Rupert J., Esapa B., Tartaglia G. G., Cellerino A., Pastore A., Terzibasi T. E., New lessons on TDP-43 from old N. furzeri killifish. Aging Cell. 21 (2022), doi: 10.1111/acel.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens G. E., Meinema A. C., González J., Wolters J. C., Schmidt A., Guryev V., Bischoff R., Wit E. C., Veenhoff L. M., Heinemann M., Protein biogenesis machinery is a driver of replicative aging in yeast. Elife. 4, e08527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y.-N., Hu H.-Y., Xie G.-C., Fu N., Ning Z.-B., Zeng R., Khaitovich P., Transcript and protein expression decoupling reveals RNA binding proteins and miRNAs as potential modulators of human aging. Genome Biol. 16, 41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walther D. M., Kasturi P., Zheng M., Pinkert S., Vecchi G., Ciryam P., Morimoto R. I., Dobson C. M., Vendruscolo M., Mann M., Hartl F. U., Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell. 161 (2015), doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David D. C., Ollikainen N., Trinidad J. C., Cary M. P., Burlingame A. L., Kenyon C., Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLoS Biol. 8, e1000450 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemon Y., Chick J. M., Gerdes Gyuricza I., Skelly D. A., Devuyst O., Gygi S. P., Churchill G. A., Korstanje R., Proteomic and transcriptomic profiling reveal different aspects of aging in the kidney. Elife. 10 (2021), doi: 10.7554/eLife.62585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes Gyuricza I., Chick J. M., Keele G. R., Deighan A. G., Munger S. C., Korstanje R., Gygi S. P., Churchill G. A., Genome-wide transcript and protein analysis highlights the role of protein homeostasis in the aging mouse heart. Genome Res. 32, 838–852 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick F., Tysnes O. B., Alves G. W., Nido G. S., Tzoulis C., Altered transcriptome-proteome coupling indicates aberrant proteostasis in Parkinson’s disease. iScience. 26 (2023), doi: 10.1016/j.isci.2023.105925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornasiero E. F., Mandad S., Wildhagen H., Alevra M., Rammner B., Keihani S., Opazo F., Urban I., Ischebeck T., Sakib M. S., Fard M. K., Kirli K., Centeno T. P., Vidal R. O., Rahman R.-U., Benito E., Fischer A., Dennerlein S., Rehling P., Feussner I., Bonn S., Simons M., Urlaub H., Rizzoli S. O., Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 9, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebbenkamp A. T. N., Borchelt D. R., Protein Aggregate Characterization in Models of Neurodegenerative Disease. Neuroproteomics, 85–91 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Geladaki A., Britovšek N. K., Breckels L. M., Smith T. S., Vennard O. L., Mulvey C. M., Crook O. M., Gatto L., Lilley K. S., Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics. Nature Communications. 10 (2019), , doi: 10.1038/s41467-018-08191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray D. A., Tsirigotis M., Woulfe J., Ubiquitin, proteasomes, and the aging brain. Sci. Aging Knowledge Environ. 2003 (2003), doi: 10.1126/sageke.2003.34.re6. [DOI] [PubMed] [Google Scholar]
- 20.de Araujo M. E. G., Liebscher G., Hess M. W., Huber L. A., Lysosomal size matters. Traffic. 21, 60–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagi M., Klein Z. A., Gould T. J., Bewersdorf J., Strittmatter S. M., Lysosome size, motility and stress response regulated by fronto-temporal dementia modifier TMEM106B. Mol. Cell. Neurosci. 61, 226–240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickart C. M., Back to the future with ubiquitin. Cell. 116, 181–190 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Ori A., Toyama B. H., Harris M. S., Bock T., Iskar M., Bork P., Ingolia N. T., Hetzer M. W., Beck M., Integrated Transcriptome and Proteome Analyses Reveal Organ-Specific Proteome Deterioration in Old Rats. Cell Syst. 1, 224–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein K. C., Morales-Polanco F., van der Lienden J., Rainbolt T. K., Frydman J., Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis. Nature. 601, 637–642 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer C., Garzia A., Morozov P., Molina H., Tuschl T., The G3BP1-Family-USP10 Deubiquitinase Complex Rescues Ubiquitinated 40S Subunits of Ribosomes Stalled in Translation from Lysosomal Degradation. Mol. Cell. 77, 1193–1205.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Higgins R., Gendron J. M., Rising L., Mak R., Webb K., Kaiser S. E., Zuzow N., Riviere P., Yang B., Fenech E., Tang X., Lindsay S. A., Christianson J. C., Hampton R. Y., Wasserman S. A., Bennett E. J., The Unfolded Protein Response Triggers Site-Specific Regulatory Ubiquitylation of 40S Ribosomal Proteins. Mol. Cell. 59, 35–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan L. L., Simms C. L., McLoughlin F., Vierstra R. D., Zaher H. S., Oxidation and alkylation stresses activate ribosome-quality control. Nat. Commun. 10, 5611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A. K., Venezian J., Shiber A., Kramer G., Bukau B., O’Brien E. P., Combinations of slow-translating codon clusters can increase mRNA half-life in. Proc. Natl. Acad. Sci. U. S. A. 118 (2021), doi: 10.1073/pnas.2026362118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan L. Y., Mugler C. F., Heinrich S., Vallotton P., Weis K., Non-invasive measurement of mRNA decay reveals translation initiation as the major determinant of mRNA stability. Elife. 7 (2018), doi: 10.7554/eLife.32536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz D. C., Parker R., mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 20, 7933–7942 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaidatzis D., Burger L., Florescu M., Stadler M. B., Erratum: Analysis of intronic and exonic reads in RNA-seq data characterizes transcriptional and post-transcriptional regulation. Nat. Biotechnol. 34, 210 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Mills E. W., Green R., Ribosomopathies: There’s strength in numbers. Science. 358 (2017), doi: 10.1126/science.aan2755. [DOI] [PubMed] [Google Scholar]
- 33.Khajuria R. K., Munschauer M., Ulirsch J. C., Fiorini C., Ludwig L. S., McFarland S. K., Abdulhay N. J., Specht H., Keshishian H., Mani D. R., Jovanovic M., Ellis S. R., Fulco C. P., Engreitz J. M., Schütz S., Lian J., Gripp K. W., Weinberg O. K., Pinkus G. S., Gehrke L., Regev A., Lander E. S., Gazda H. T., Lee W. Y., Panse V. G., Carr S. A., Sankaran V. G., Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell. 173, 90–103.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noderer W. L., Flockhart R. J., Bhaduri A., Diaz de Arce A. J., Zhang J., Khavari P. A., Wang C. L., Quantitative analysis of mammalian translation initiation sites by FACS-seq. Mol. Syst. Biol. 10, 748 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G., Hallmarks of aging: An expanding universe. Cell. 186, 243–278 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Deschênes M., Chabot B., The emerging role of alternative splicing in senescence and aging. Aging Cell. 16, 918–933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyenis A., Chang J., Demmers J. J. P. G., Bruens S. T., Barnhoorn S., Brandt R. M. C., Baar M. P., Raseta M., Derks K. W. J., Hoeijmakers J. H. J., Pothof J., Genome-wide RNA polymerase stalling shapes the transcriptome during aging. Nat. Genet. 55, 268–279 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher B., Pothof J., Vijg J., Hoeijmakers J. H. J., The central role of DNA damage in the ageing process. Nature. 592, 695–703 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhadra M., Howell P., Dutta S., Heintz C., Mair W. B., Alternative splicing in aging and longevity. Hum. Genet. 139, 357–369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozukova M., Nikopoulou C., Kleinenkuhnen N., Grbavac D., Goetsch K., Tessarz P., Aging is associated with increased chromatin accessibility and reduced polymerase pausing in liver. Mol. Syst. Biol. 18, e11002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debès C., Papadakis A., Grönke S., Karalay Ö., Tain L. S., Mizi A., Nakamura S., Hahn O., Weigelt C., Josipovic N., Zirkel A., Brusius I., Sofiadis K., Lamprousi M., Lu Y.-X., Huang W., Esmaillie R., Kubacki T., Späth M. R., Schermer B., Benzing T., Müller R.-U., Antebi A., Partridge L., Papantonis A., Beyer A., Ageing-associated changes in transcriptional elongation influence longevity. Nature. 616, 814–821 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapahi P., Vijg J., Aging--lost in translation? N. Engl. J. Med. 361, 2669–2670 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Gonskikh Y., Polacek N., Alterations of the translation apparatus during aging and stress response. Mech. Ageing Dev. 168, 30–36 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Ingram T., Chakrabarti L., Proteomic profiling of mitochondria: what does it tell us about the ageing brain? Aging. 8, 3161–3179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heiby J. C., Ori A., Organelle dysfunction and its contribution to metabolic impairments in aging and age-related diseases. Current Opinion in Systems Biology. 30, 100416 (2022). [Google Scholar]
- 46.Miyoshi N., Oubrahim H., Chock P. B., Stadtman E. R., Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc. Natl. Acad. Sci. U. S. A. 103, 1727–1731 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braeckman B. P., Houthoofd K., Vanfleteren J. R., Aging Cell, in press.
- 48.Gkotsi D., Begum R., Salt T., Lascaratos G., Hogg C., Chau K.-Y., Schapira A. H. V., Jeffery G., Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp. Eye Res. 122, 50–53 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Espada L., Dakhovnik A., Chaudhari P., Martirosyan A., Miek L., Poliezhaieva T., Schaub Y., Nair A., Döring N., Rahnis N., Werz O., Koeberle A., Kirkpatrick J., Ori A., Ermolaeva M. A., Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat Metab. 2, 1316–1331 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Bazzini A. A., Del Viso F., Moreno-Mateos M. A., Johnstone T. G., Vejnar C. E., Qin Y., Yao J., Khokha M. K., Giraldez A. J., Codon identity regulates mRNA stability and translation efficiency during the maternal-to-zygotic transition. EMBO J. 35, 2087–2103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva P. T., Zhang Y., Theodorakis E., Martens L. D., Yépez V. A., Pelechano V., Gagneur J., Cellular energy regulates mRNA translation and degradation in a codon-specific manner. bioRxiv (2023), p. 2023.04.06.535836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ximerakis M., Lipnick S. L., Innes B. T., Simmons S. K., Adiconis X., Dionne D., Mayweather B. A., Nguyen L., Niziolek Z., Ozek C., Butty V. L., Isserlin R., Buchanan S. M., Levine S. S., Regev A., Bader G. D., Levin J. Z., Rubin L. L., Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22 (2019), doi: 10.1038/s41593-019-0491-3. [DOI] [PubMed] [Google Scholar]
- 53.Tyshkovskiy A., Ma S., Shindyapina A. V., Tikhonov S., Lee S.-G., Bozaykut P., Castro J. P., Seluanov A., Schork N. J., Gorbunova V., Dmitriev S. E., Miller R. A., Gladyshev V. N., Distinct longevity mechanisms across and within species and their association with aging. Cell. 186, 2929–2949.e20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Q., Xiao H., Jedrychowski M. P., Schweppe D. K., Navarrete-Perea J., Knott J., Rogers J., Chouchani E. T., Gygi S. P., Sample multiplexing for targeted pathway proteomics in aging mice. Proc. Natl. Acad. Sci. U. S. A. 117, 9723–9732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koyuncu S., Loureiro R., Lee H. J., Wagle P., Krueger M., Vilchez D., Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature. 596, 285–290 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kluever V., Russo B., Mandad S., Kumar N. H., Alevra M., Ori A., Rizzoli S. O., Urlaub H., Schneider A., Fornasiero E. F., Protein lifetimes in aged brains reveal a proteostatic adaptation linking physiological aging to neurodegeneration. Science Advances. 8, eabn4437 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Z., Tantray I., Lim J., Chen S., Li Y., Davis Z., Sitron C., Dong J., Gispert S., Auburger G., Brandman O., Bi X., Snyder M., Lu B., MISTERMINATE Mechanistically Links Mitochondrial Dysfunction with Proteostasis Failure. Mol. Cell. 75 (2019), doi: 10.1016/j.molcel.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rimal S., Li Y., Vartak R., Geng J., Tantray I., Li S., Huh S., Vogel H., Glabe C., Grinberg L. T., Spina S., Seeley W. W., Guo S., Lu B., Inefficient quality control of ribosome stalling during APP synthesis generates CAT-tailed species that precipitate hallmarks of Alzheimer’s disease. Acta neuropathologica communications. 9 (2021), doi: 10.1186/s40478-021-01268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S., Wu Z., Tantray I., Li Y., Chen S., Dong J., Glynn S., Vogel H., Snyder M., Lu B., Quality-control mechanisms targeting translationally stalled and C-terminally extended poly(GR) associated with ALS/FTD. Proc. Natl. Acad. Sci. U. S. A. 117 (2020), doi: 10.1073/pnas.2005506117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aviner R., Lee T.-T., Masto V. B., Gestaut D., Li K. H., Andino R., Frydman J., Ribotoxic collisions on CAG expansions disrupt proteostasis and stress responses in Huntington’s Disease. bioRxiv (2022), p. 2022.05.04.490528. [Google Scholar]
- 61.Aman Y., Schmauck-Medina T., Hansen M., Morimoto R. I., Simon A. K., Bjedov I., Palikaras K., Simonsen A., Johansen T., Tavernarakis N., Rubinsztein D. C., Partridge L., Kroemer G., Labbadia J., Fang E. F., Autophagy in healthy aging and disease. Nature Aging. 1, 634–650 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safaiyan S., Kannaiyan N., Snaidero N., Brioschi S., Biber K., Yona S., Edinger A. L., Jung S., Rossner M. J., Simons M., Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoeger T., Grant R. A., McQuattie-Pimentel A. C., Anekalla K. R., Liu S. S., Tejedor-Navarro H., Singer B. D., Abdala-Valencia H., Schwake M., Tetreault M.-P., Perlman H., Balch W. E., Chandel N. S., Ridge K. M., Sznajder J. I., Morimoto R. I., Misharin A. V., Budinger G. R. S., Nunes Amaral L. A., Aging is associated with a systemic length-associated transcriptome imbalance. Nature Aging. 2, 1191–1206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buczak K., Kirkpatrick J. M., Truckenmueller F., Santinha D., Ferreira L., Roessler S., Singer S., Beck M., Ori A., Spatially resolved analysis of FFPE tissue proteomes by quantitative mass spectrometry. Nat. Protoc. 15, 2956–2979 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Di Sanzo S., Spengler K., Leheis A., Kirkpatrick J. M., Rändler T. L., Baldensperger T., Dau T., Henning C., Parca L., Marx C., Wang Z.-Q., Glomb M. A., Ori A., Heller R., Mapping protein carboxymethylation sites provides insights into their role in proteostasis and cell proliferation. Nat. Commun. 12, 6743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bentley D. R., Balasubramanian S., Swerdlow H. P., Smith G. P., Milton J., Brown C. G., Hall K. P., Evers D. J., Barnes C. L., Bignell H. R., Boutell J. M., Bryant J., Carter R. J., Keira Cheetham R., Cox A. J., Ellis D. J., Flatbush M. R., Gormley N. A., Humphray S. J., Irving L. J., Karbelashvili M. S., Kirk S. M., Li H., Liu X., Maisinger K. S., Murray L. J., Obradovic B., Ost T., Parkinson M. L., Pratt M. R., Rasolonjatovo I. M. J., Reed M. T., Rigatti R., Rodighiero C., Ross M. T., Sabot A., Sankar S. V., Scally A., Schroth G. P., Smith M. E., Smith V. P., Spiridou A., Torrance P. E., Tzonev S. S., Vermaas E. H., Walter K., Wu X., Zhang L., Alam M. D., Anastasi C., Aniebo I. C., Bailey D. M. D., Bancarz I. R., Banerjee S., Barbour S. G., Baybayan P. A., Benoit V. A., Benson K. F., Bevis C., Black P. J., Boodhun A., Brennan J. S., Bridgham J. A., Brown R. C., Brown A. A., Buermann D. H., Bundu A. A., Burrows J. C., Carter N. P., Castillo N., Chiara M. Catenazzi E., Chang S., Neil Cooley R., Crake N. R., Dada O. O., Diakoumakos K. D., Dominguez-Fernandez B., Earnshaw D. J., Egbujor U. C., Elmore D. W., Etchin S. S., Ewan M. R., Fedurco M., Fraser L. J., Fuentes Fajardo K. V., Scott Furey W., George D., Gietzen K. J., Goddard C. P., Golda G. S., Granieri P. A., Green D. E., Gustafson D. L., Hansen N. F., Harnish K., Haudenschild C. D., Heyer N. I., Hims M. M., Ho J. T., Horgan A. M., Hoschler K., Hurwitz S., Ivanov D. V., Johnson M. Q., James T., Huw Jones T. A., Kang G.-D., Kerelska T. H., Kersey A. D., Khrebtukova I., Kindwall A. P., Kingsbury Z., Kokko-Gonzales P. I., Kumar A., Laurent M. A., Lawley C. T., Lee S. E., Lee X., Liao A. K., Loch J. A., Lok M., Luo S., Mammen R. M., Martin J. W., McCauley P. G., McNitt P., Mehta P., Moon K. W., Mullens J. W., Newington T., Ning Z., Ling Ng B., Novo S. M., O’Neill M. J., Osborne M. A., Osnowski A., Ostadan O., Paraschos L. L., Pickering L., Pike A. C., Pike A. C., Chris Pinkard D., Pliskin D. P., Podhasky J., Quijano V. J., Raczy C., Rae V. H., Rawlings S. R., Chiva Rodriguez A., Roe P. M., Rogers J., Rogert Bacigalupo M. C., Romanov N., Romieu A., Roth R. K., Rourke N. J., Ruediger S. T., Rusman E., Sanches-Kuiper R. M., Schenker M. R., Seoane J. M., Shaw R. J., Shiver M. K., Short S. W., Sizto N. L., Sluis J. P., Smith M. A., Ernest Sohna Sohna J., Spence E. J., Stevens K., Sutton N., Szajkowski L., Tregidgo C. L., Turcatti G., vandeVondele S., Verhovsky Y., Virk S. M., Wakelin S., Walcott G. C., Wang J., Worsley G. J., Yan J., Yau L., Zuerlein M., Rogers J., Mullikin J. C., Hurles M. E., McCooke N. J., West J. S., Oaks F. L., Lundberg P. L., Klenerman D., Durbin R., Smith A. J., Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 456, 53–59 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R., STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29, 15–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith T., Heger A., Sudbery I., UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao Y., Smyth G. K., Shi W., featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 30, 923–930 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGlincy N. J., Ingolia N. T., Transcriptome-wide measurement of translation by ribosome profiling. Methods. 126, 112–129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bagnoli S., Terzibasi Tozzini E., Cellerino A., Immunofluorescence and Aggresome Staining of Nothobranchius furzeri Cryosections. Cold Spring Harb. Protoc. (2023), doi: 10.1101/pdb.prot107791. [DOI] [PubMed] [Google Scholar]
- 73.Crook O. M., Breckels L. M., Lilley K. S., Kirk P. D. W., Gatto L., A Bioconductor workflow for the Bayesian analysis of spatial proteomics. F1000Res. 8, 446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breckels L. M., Mulvey C. M., Lilley K. S., Gatto L., A Bioconductor workflow for processing and analysing spatial proteomics data. F1000Res. 5, 2926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K., limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson J. L., Yaron T. M., Huntsman E. M., Kerelsky A., Song J., Regev A., Lin T. Y., Liberatore K., Cizin D. M., Cohen B. M., Vasan N., Ma Y., Krismer K., Robles J. T., van de Kooij B., van Vlimmeren A. E., Andrée-Busch N., Käufer N. F., Dorovkov M. V., Ryazanov A. G., Takagi Y., Kastenhuber E. R., Goncalves, Hopkins B. D., Elemento O., Taatjes D. J., Maucuer A., Yamashita A., Degterev A., Uduman M., Lu J., Landry S. D., Zhang B., Cossentino I., Linding R., Blenis J., Hornbeck P. V., Turk B. E., Yaffe M. B., Cantley L. C., An atlas of substrate specificities for the human serine/threonine kinome. Nature. 613 (2023), doi: 10.1038/s41586-022-05575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., Fu X., Liu S., Bo X., Yu G., clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2, 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 79.Strimmer K., fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 24, 1461–1462 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Cunningham F., Allen J. E., Allen J., Alvarez-Jarreta J., Amode M. R., Armean I. M., Austine-Orimoloye O., Azov A. G., Barnes I., Bennett R., Berry A., Bhai J., Bignell A., Billis K., Boddu S., Brooks L., Charkhchi M., Cummins C., Da Rin Fioretto L., Davidson C., Dodiya K., Donaldson S., El Houdaigui B., El Naboulsi T., Fatima R., Giron C. G., Genez T., Martinez J. G., Guijarro-Clarke C., Gymer A., Hardy M., Hollis Z., Hourlier T., Hunt T., Juettemann T., Kaikala V., Kay M., Lavidas I., Le T., Lemos D., Marugán J. C., Mohanan S., Mushtaq A., Naven M., Ogeh D. N., Parker A., Parton A., Perry M., Piližota I., Prosovetskaia I., Sakthivel M. P., Salam A. I. A., Schmitt B. M., Schuilenburg H., Sheppard D., Pérez-Silva J. G., Stark W., Steed E., Sutinen K., Sukumaran R., Sumathipala D., Suner M.-M., Szpak M., Thormann A., Tricomi F. F., Urbina-Gómez D., Veidenberg A., Walsh T. A., Walts B., Willhoft N., Winterbottom A., Wass E., Chakiachvili M., Flint B., Frankish A., Giorgetti S., Haggerty L., Hunt S. E., IIsley G. R., Loveland J. E., Martin F. J., Moore B., Mudge J. M., Muffato M., Perry E., Ruffier M., Tate J., Thybert D., Trevanion S. J., Dyer S., Harrison P. W., Howe K. L., Yates A. D., Zerbino D. R., Flicek P., Ensembl 2022. Nucleic Acids Res. 50, D988–D995 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quinlan A. R., Hall I. M., BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S.-E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., Mootha V. K., A mitochondrial protein compendium elucidates complex I disease biology. Cell. 134, 112–123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin M., Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10 (2011). [Google Scholar]
- 84.Langmead B., Trapnell C., Pop M., Salzberg S. L., Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauria F., Tebaldi T., Bernabò P., Groen E. J. N., Gillingwater T. H., Viero G., riboWaltz: Optimization of ribosome P-site positioning in ribosome profiling data. PLoS Comput. Biol. 14, e1006169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomsen M. C. F., Nielsen M., Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res. 40, W281–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Putri G. H., Anders S., Pyl P. T., Pimanda J. E., Zanini F., Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics. 38, 2943–2945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gatto L., Breckels L. M., Lilley K. S., Assessing sub-cellular resolution in spatial proteomics experiments. Curr. Opin. Chem. Biol. 48, 123–149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vecchi G., Sormanni P., Mannini B., Vandelli A., Tartaglia G. G., Dobson C. M., Hartl F. U., Vendruscolo M., Proteome-wide observation of the phenomenon of life on the edge of solubility. Proc. Natl. Acad. Sci. U. S. A. 117, 1015–1020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alfonso S. I., Callender J. A., Hooli B., Antal C. E., Mullin K., Sherman M. A., Lesné S. E., Leitges M., Newton A. C., Tanzi R. E., Malinow R., Gain-of-function mutations in protein kinase Cα (PKCα) may promote synaptic defects in Alzheimer’s disease. Sci. Signal. 9 (2016), doi: 10.1126/scisignal.aaf6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morshed N., Lee M. J., Rodriguez F. H., Lauffenburger D. A., Mastroeni D., White F. M., Quantitative phosphoproteomics uncovers dysregulated kinase networks in Alzheimer’s disease. Nature Aging. 1, 550–565 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Bai B., Wang X., Li Y., Chen P.-C., Yu K., Dey K. K., Yarbro J. M., Han X., Lutz B. M., Rao S., Jiao Y., Sifford J. M., Han J., Wang M., Tan H., Shaw T. I., Cho J.-H., Zhou S., Wang H., Niu M., Mancieri A., Messler K. A., Sun X., Wu Z., Pagala V., High A. A., Bi W., Zhang H., Chi H., Haroutunian V., Zhang B., Beach T. G., Yu G., Peng J., Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer’s Disease Progression. Neuron. 106, 700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guillozet A. L., Weintraub S., Mash D. C., Mesulam M. M., Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch. Neurol. 60, 729–736 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Chatterjee S., Sealey M., Ruiz E., Pegasiou C. M., Brookes K., Green S., Crisford A., Duque-Vasquez M., Luckett E., Robertson R., Richardson P., Vajramani G., Grundy P., Bulters D., Proud C., Vargas-Caballero M., Mudher A., Age-related changes in tau and autophagy in human brain in the absence of neurodegeneration. PLoS One. 18, e0262792 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Mandelkow E., Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 22–35 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Li L., Jiang Y., Wang J.-Z., Liu R., Wang X., Tau Ubiquitination in Alzheimer’s Disease. Front. Neurol. 12 (2022), doi: 10.3389/fneur.2021.786353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Datta D., Leslie S. N., Wang M., Morozov Y. M., Yang S., Mentone S., Zeiss C., Duque A., Rakic P., Horvath T. L., van Dyck C. H., Nairn A. C., Arnsten A. F. T., Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers. Dement. 17, 920–932 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baumgart M., Priebe S., Groth M., Hartmann N., Menzel U., Pandolfini L., Koch P., Felder M., Ristow M., Englert C., Guthke R., Platzer M., Cellerino A., Longitudinal RNA-Seq Analysis of Vertebrate Aging Identifies Mitochondrial Complex I as a Small-Molecule-Sensitive Modifier of Lifespan. Cell Syst. 2, 122–132 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Iordanov M. S., Pribnow D., Magun J. L., Dinh T. H., Pearson J. A., Chen S. L., Magun B. E., Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17, 3373–3381 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.