Abstract
To investigate the role of chemokines during the initial local response to Mycobacterium tuberculosis in the human lung, we studied chemokine production by the human alveolar epithelial cell line A549 after infection with M. tuberculosis. M. tuberculosis-infected A549 cells produced mRNAs and protein for monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8) but not mRNAs for macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES. Chemokine production in response to M. tuberculosis was not dependent on production of tumor necrosis factor alpha, IL-1β, or IL-6. Two virulent clinical M. tuberculosis isolates, the virulent laboratory strain H37Rv, and the avirulent strain H37Ra elicited production of comparable concentrations of MCP-1 and IL-8, whereas killed M. tuberculosis and three Mycobacterium avium strains did not. The three virulent M. tuberculosis strains grew more rapidly than the avirulent M. tuberculosis strain in the alveolar epithelial cell line, and the three M. avium strains did not grow intracellularly. These findings suggest that intracellular growth is necessary for mycobacteria to elicit production of MCP-1 and IL-8 by alveolar epithelial cells but that virulence and the rate of intracellular growth do not correlate with chemokine production. Alveolar epithelial cells may contribute to the local inflammatory response in human tuberculosis by producing chemokines which attract monocytes, lymphocytes, and polymorphonuclear cells.
During human infection with Mycobacterium tuberculosis, the first cells to encounter the organisms are alveolar macrophages and alveolar epithelial cells. M. tuberculosis can invade and grow in human alveolar epithelial cells (3), and virulent M. tuberculosis strains replicate more rapidly and are more cytotoxic for human alveolar epithelial cells (3, 15). During the course of the immune response to M. tuberculosis infection, T cells and macrophages are recruited to the site of infection, resulting in tissue inflammation and granuloma formation. The mechanism for recruitment of these cells is unknown but is likely to involve chemokines, which are a large family of proteins that include chemoattractants for neutrophils, lymphocytes, and macrophages. To investigate the potential role of chemokines during the initial host response to M. tuberculosis, we studied chemokine production by the human alveolar epithelial cell line A549 in response to virulent and avirulent strains of M. tuberculosis.
MATERIALS AND METHODS
Alveolar epithelial cell line.
The human alveolar epithelial cell line A549 (American Type Culture Collection, Rockville, Md.) was cultured in RPMI 1640 (GIBCO, Grand Island, N.Y.)–5% fetal calf serum (FCS) (Hyclone, Logan, Utah)–100 U of penicillin (Sigma, St. Louis, Mo.) per ml.
Mycobacteria.
M. tuberculosis H37Ra and H37Rv were kindly provided by John Belisle, Colorado State University, Fort Collins, and M. tuberculosis isolates S29 and S51 were obtained from two tuberculosis patients. Mycobacterium avium 100 and 101 were kindly provided by Luiz Bermudez, Kuzell Institute, San Francisco, Calif., and M. avium 109 was generously provided by Clark Inderlied, Children’s Hospital of Los Angeles, Los Angeles, Calif. Each strain was initially cultured in Middlebrook 7H9 medium (Difco, Detroit, Mich.) with 0.5% glycerol, aliquoted, and frozen at −70°C. Single-cell suspensions of mycobacteria were obtained by a modification of standard methods. Briefly, aliquots of frozen M. tuberculosis were cultured in 7H9 broth with 0.5% glycerol at 37°C and 5% CO2 for 7 to 10 days to mid-exponential growth phase. Bacterial cultures were pelleted at 3,000 × g for 10 min and resuspended in 7H9. Clumps of mycobacteria were dispersed with an ultrasonic cell disrupter (Virtis Co., Gardiner, N.Y.) for 15 to 20 s. The sample was centrifuged at 200 × g for 10 min to pellet clumps of bacilli, and the upper bacterial suspension was used in all experiments. Bacteria were counted in a Petroff-Hauser chamber. By this technique, 90 to 95% of the organisms were single bacilli, with the remaining 5 to 10% in small aggregates of up to five organisms. Mycobacterial viability, as assessed by the number of CFU, was 50 to 60% for all mycobacterial strains.
Mycobacterial growth in A549 cells.
A549 cells were seeded in 1 ml of medium in 2-ml wells (Falcon, Lincoln Park, N.J.) at 105 cells per well and grown for 3 days to confluence, at which point there were approximately 5 × 105 cells/well. In experiments to measure chemokine production, A549 cells were infected in triplicate with 104 to 107 M. tuberculosis organisms per well. Based on bacterial viability of 50% and an estimated 5 × 105 A549 cells/well, the multiplicity of infection ranged from approximately 0.01 to 10 live M. tuberculosis organisms per A549 cell. After 2 h, cells were washed twice with warm RPMI to remove extracellular bacteria. A549 cells were then cultured in fresh RPMI with 5% FCS at 37°C and 5% CO2 for 1 to 6 days, and cells and supernatants were collected as outlined below.
In experiments to measure mycobacterial growth in A549 cells, only 104 bacilli per well (approximately 1 organism per 100 A549 cells) were used. This low ratio was used to ensure that the A549 cells remained viable during the culture period and that mycobacterial growth was intracellular. After 20 h, the cells were washed twice with warm RPMI to remove extracellular bacteria. This time point was considered to be day 0. A549 cells were then cultured in fresh RPMI 1640 with 5% FCS for up to 10 days. We chose to evaluate intracellular proliferation of M. tuberculosis in A549 cells for up to 10 days because this provided sufficient time for the bacilli to grow by 2 log units or more and because viability of the A549 cells was not reduced during this period. A549 cells were collected at days 0, 3, 5, 7, and 10. The supernatants was aspirated, and the A549 cells were lysed by incubation with 0.5 ml of 0.1% Triton X-100 (Sigma) in sterile water for 20 min. Bacterial suspensions in cell lysates and supernatants were ultrasonically dispersed, serially diluted, and plated in triplicate on 7H10 agar plates, and the number of CFU was counted after 3 weeks.
In some experiments, viability of A549 cells after the culture period was assessed by using trypsin-EDTA (GIBCO) to detach the cells and by counting the numbers of viable and nonviable cells by trypan blue exclusion, using a hemocytometer.
Measurement of MCP-1 and IL-8 concentrations.
A549 cells were cultured for up to 6 days in the presence or absence of mycobacteria. In some experiments, neutralizing antibodies to interleukin-1β (IL-1β) (10 μg/ml; R & D Systems, Minneapolis, Minn.) and IL-6 (1 μg/ml; R & D Systems) were added at 0 h and daily until day 6. Supernatants were collected at 0 h and after 2, 4, and 6 days, passed through a 0.8-μm/0.2-μm Acrodisc PF filter (Gelman Sciences, Ann Arbor, Mich.) to remove bacteria, and frozen at −70°C. Cytokine concentrations in supernatants were measured by enzyme-linked immunosorbent assay (for monocyte chemotactic protein-1 [MCP-1] [R & D Systems], the sensitivity was 5 pg/ml; for IL-6 [R & D Systems], the sensitivity was 1 pg/ml; and for IL-8 [Biosource International, Camarillo, Calif.], the sensitivity was 10 pg/ml). Optical density readings were obtained with a Microplate Reader model 450 and analyzed with Microplate Manager/PC Data Analysis software (both from Bio-Rad Laboratories, Hercules, Calif.).
Measurement of cytokine mRNA in M. tuberculosis-infected A549 cells by RT-PCR.
Replicate wells of A549 cells, either infected with mycobacteria or uninfected, were harvested after 1 to 6 days, lysed with 4 M guanidinium isothiocyanate, and stored at −20°C. Total cellular RNA was extracted and cDNA was synthesized, as described previously (20). To determine if A549 cells expressed mRNAs for certain chemokines and cytokines in response to M. tuberculosis infection, reverse transcriptase PCR (RT-PCR) was performed with primers specific for the chemokines MCP-1, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, IL-8, and RANTES and the cytokines tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, IL-10, and the p40 chain of IL-12. The primer sequences and reaction conditions for IL-10 and IL-12 p40 have been published (16, 20). For the chemokines and other cytokines, the 5′ and 3′ primer sequences, respectively, were as follows: MCP-1, CAAACTGAAGCTCGCACTCTCGCC and ATTCTTGGGTTGTGGAGTGAGTGTTCA; MIP-1α, TGCATCACTTGCTGCTGACACG and CAACCAGTCCATAGAAGAGG; MIP-1β, CCAAACCAAAAGAAGCAAGC and AGAAACAGTGACAGTGGACC; IL-8, ATGACTTCCAAGCTGGCCGTG and TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC; RANTES, TCATTGCTACTGCCCTCTGC and CGTCGTGGTCAGAATCTGGG; TNF-α, TCTCGAACCCCGAGTGACAA and TATCTCTCAGCTCCACGCCA; IL-1β, GACACATGGGATAACGAGGC and ACGCAGGACAGGTACAGATT; and IL-6, ATGTAGCCGCCCCACACAGA and CATCCATCTTTTTCAGCCAT. ForMCP-1, MIP-1α, MIP-1β, RANTES, and IL-1β, a DNA thermocycler 480 (Perkin-Elmer Cetus, Norwalk, Conn.) ran 35 cycles of denaturation at 94°C for 1 min and annealing-extension at 65°C for 2 min. For IL-8, the same reaction conditions were used with 26 cycles. For IL-6 and TNF-α, 35 cycles of denaturation at 94°C for 1 min were used, with annealing-extension at 55°C for 2 min. The PCR product (10 μl) was subjected to electrophoresis on 1.5% agarose gels and visualized by staining with ethidium bromide.
For quantitation of mRNAs for MCP-1 and IL-8, we used competitive RT-PCR, as previously described (13, 21). Briefly, we first normalized all samples for β-actin cDNA content by competitive PCR, in which one set of primers amplifies the target cDNA and a competitor cDNA (MIMIC; Clontech Laboratory, Inc., Palo Alto, Calif.), which generate PCR products of different sizes. Aliquots containing equivalent amounts of β-actin cDNA were used as substrates and amplified by PCR with primers specific for MCP-1 and IL-8, using the reaction conditions outlined above. For quantifying PCR products, gels were photographed with a SPEEDLIGHT gel documentation system (B/T Scientific Technologies, Carlsbad, Calif.) and analyzed with QGEL software (Kendrick Laboratories, Madison, Wis.), an imaging and analysis system that permits accurate comparison of the integrated densities of the PCR product bands for target and MIMIC cDNAs. By plotting the ratio of integrated density of sample to MIMIC PCR product against the known amount of MIMIC substrate cDNA, the amount of substrate CD3 cDNA in each sample was calculated. This method detects twofold differences in sample cDNA concentrations. Positive controls containing cytokine cDNA (from phytohemagglutinin-stimulated peripheral blood mononuclear cells) and negative controls containing no cDNA were employed in each set of reactions.
Statistical analysis.
Data for different groups were expressed as the mean ± standard error (SE) and were compared by the paired or unpaired Student t test, as appropriate.
RESULTS
Chemokine mRNAs expressed by A549 cells infected with M. tuberculosis H37Ra.
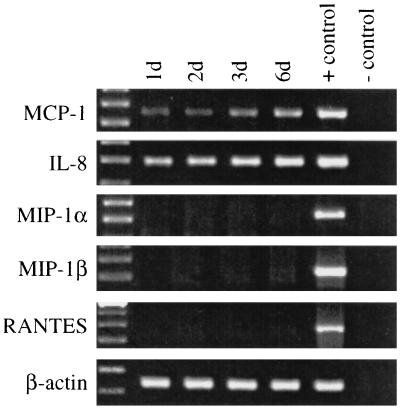
Using RT-PCR, we evaluated expression of mRNAs for five chemokines by A549 cells 24 h after infection with 107 M. tuberculosis H37Ra organisms (approximately 10 bacilli per cell). In infected A549 cells, PCR products for MCP-1 and IL-8 increased over 6 days of culture compared to the case for A549 cells cultured in the presence of medium alone (Fig. 1). In contrast, mRNAs for MIP-1α, MIP-1β, and RANTES were not detected (Fig. 1).
FIG. 1.
Chemokine mRNA expression of A549 cells infected with M. tuberculosis H37Ra. A549 cells were infected with H37Ra, and mRNA expression for five chemokines at various time points was determined by RT-PCR. The far-left lane shows molecular weight markers, the positive control was cDNA prepared from phytohemagglutinin-stimulated cells, and the negative control contained no cDNA.
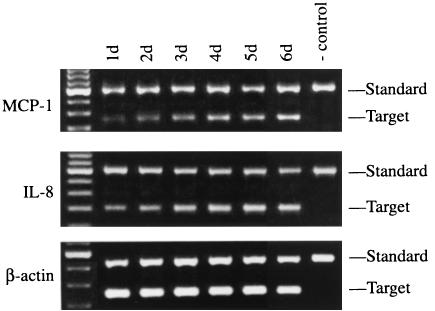
To confirm the results of the qualitative RT-PCR, competitive PCR was performed to quantitate cDNAs for MCP-1 and IL-8, which increased steadily during 6 days of culture (Fig. 2).
FIG. 2.
Competitive RT-PCR to quantitate mRNAs for MCP-1 and IL-8 in A549 cells infected with M. tuberculosis H37Ra. A549 cells were infected with H37Ra, and mRNA expression for MCP-1 and IL-8 at various time points was determined by competitive RT-PCR. The far-left lane shows molecular weight markers; the negative control contains no cDNA.
Production of MCP-1 and IL-8 by A549 cells infected with M. tuberculosis H37Ra.
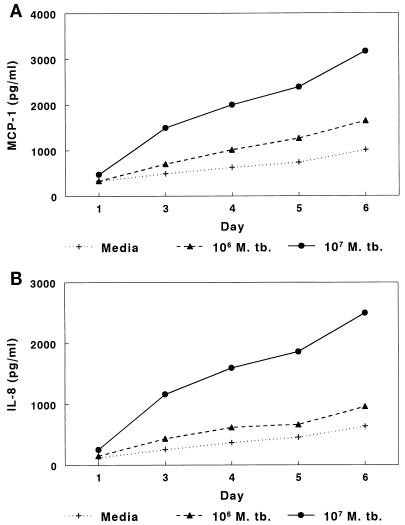
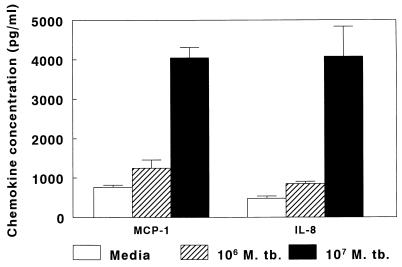
Because mRNAs for MCP-1 and IL-8 were upregulated in A549 cells after infection with M. tuberculosis, we measured the concentrations of these chemokines in supernatants of A549 cells. When A549 cells were cultured in medium alone, MCP-1 concentrations in supernatants rose to approximately 800 pg/ml after 6 days of culture, indicating spontaneous production of MCP-1. Addition of 104 or 105 M. tuberculosis H37Ra organisms per well (equivalent to 0.01 or 0.1 organism per cell) did not affect MCP-1 concentrations (data not shown). However, when 106 organisms (1 organism per cell) were added, MCP-1 concentrations increased slightly, and 107 organisms (10 organisms per cell) elicited production of 3,400 pg of MCP-1 per ml after 6 days of culture (Fig. 3A). In seven experiments, the mean MCP-1 concentrations (± SE) after 6 days of culture were 764 ± 55 pg/ml with medium alone, 1,242 ± 210 pg/ml with 106 bacilli, and 4,044 ± 265 pg/ml with 107 bacilli (Fig. 4). When A549 cells were cocultured with 107 heat-killed M. tuberculosis organisms, MCP-1 concentrations were similar to those found in cells cultured with medium alone (data not shown).
FIG. 3.
Concentrations of MCP-1 (A) and IL-8 (B) in supernatants of A549 cells infected with M. tuberculosis (M. tb.) H37Ra or uninfected. A quantity of 106 organisms represents approximately one bacillus per A549 cell.
FIG. 4.
Mean concentrations of MCP-1 and IL-8 in supernatants of A549 cells infected with M. tuberculosis (M. tb.) H37Ra or uninfected. A quantity of 106 organisms represents approximately one bacillus per A549 cell.
Findings for IL-8 were similar to those for MCP-1. A549 cells produced IL-8 spontaneously, but IL-8 concentrations were four- to sixfold higher after addition of 107 bacilli (Fig. 3B). In seven experiments, the mean IL-8 concentrations (± SE) after 6 days of culture were 479 ± 56 pg/ml with medium alone, 844 ± 62 pg/ml with 106 M. tuberculosis organisms, and 4,075 ± 765 pg/ml with 107 M. tuberculosis organisms (Fig. 4). When A549 cells were cocultured with 107 heat-killed M. tuberculosis organisms, IL-8 concentrations were similar to those found in cells cultured with medium alone (data not shown).
Factors that elicit production of MCP-1 and IL-8.
Human mononuclear phagocytes produce IL-1β, IL-6, TNF-α, IL-10, and IL-12 in response to M. tuberculosis (2, 6, 20, 22). In human renal tubercular epithelial cells and osteoblasts, IL-1, IL-6, and TNF-α enhance production of MCP-1 (17, 23), and in human alveolar macrophages, IL-1 and TNF-α elicit production of IL-8 (22). We therefore evaluated the potential contribution of these cytokines to the capacity of A549 cells to produce MCP-1 and IL-8. In A549 cells infected with H37Ra, RT-PCR revealed that mRNA for IL-6 was significantly greater than that in uninfected A549 cells. In contrast, no mRNA for TNF-α, IL-10, or IL-12 p40 was detected, and mRNA for IL-1β was present but not upregulated by infection with H37Ra (data not shown).
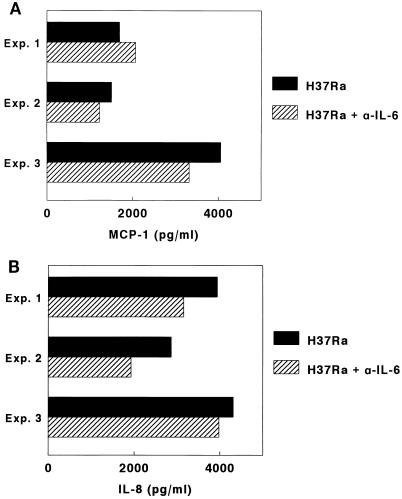
Because IL-6 mRNA was increased in A549 cells after infection with M. tuberculosis, we measured IL-6 concentrations in supernatants of A549 cells infected with 107 H37Ra organisms (10 bacilli per cell). In five experiments, mean IL-6 concentrations (± SE) were 257 ± 44 pg/ml, compared to 2 ± 0.4 pg/ml in supernatants of uninfected A549 cells. To determine if IL-6 contributed to production of MCP-1 and IL-8, neutralizing antibodies to IL-6 were used. In three experiments, anti-IL-6 antibodies had no significant effect on MCP-1 or IL-8 concentrations (Fig. 5). Anti-IL-1β antibodies also had no effect on MCP-1 or IL-8 production (data not shown).
FIG. 5.
Effect of anti-IL-6 (α-IL-6) on MCP-1 (A) and IL-8 (B) concentrations in supernatants of A549 cells infected with M. tuberculosis H37Ra. Exp., experiment.
Production of MCP-1 and IL-8 by A549 cells infected with different mycobacterial strains.
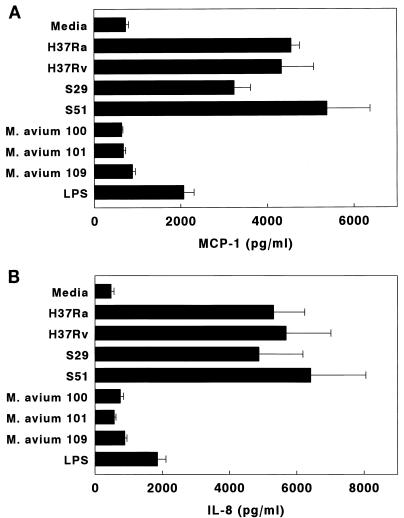
In order to determine if MCP-1 production varied in response to different mycobacterial strains, A549 cells were infected with four M. tuberculosis strains (avirulent H37Ra, virulent H37Rv, and two isolates from patients, S29 and S51) or M. avium 100, 101, or 109. Some A549 cells were cultured in the presence of lipopolysaccharide (10 μg/ml, derived from Escherichia coli serotype O127:B8 [Sigma]). In four experiments, all M. tuberculosis strains induced similar MCP-1 concentrations (Fig. 6A). In contrast, the three M. avium strains did not elicit MCP-1 production over baseline levels. Lipopolysaccharide elicited less MCP-1 production than M. tuberculosis. M. tuberculosis strains induced significantly higher MCP-1 concentrations than lipopolysaccharide (P < 0.04 for each comparison) and the M. avium strains (P < 0.001 for each comparison).
FIG. 6.
Mean concentrations of MCP-1 (A) and IL-8 (B) in supernatants of A549 cells cultured for 6 days with M. tuberculosis strains, M. avium, or lipopolysaccharide (LPS). The M. tuberculosis strains were H37Ra, H37Rv, and the clinical isolates S29 and S51.
Parallel experiments were performed to measure IL-8 production by A549 cells infected with different mycobacteria or cultured with lipopolysaccharide. Findings were similar to those for MCP-1 (Fig. 6B). M. tuberculosis strains induced significantly higher MCP-1 concentrations than lipopolysaccharide (P < 0.05 for each comparison) and the M. avium strains (P < 0.02 for each comparison).
Intracellular growth of mycobacteria in A549 cells.
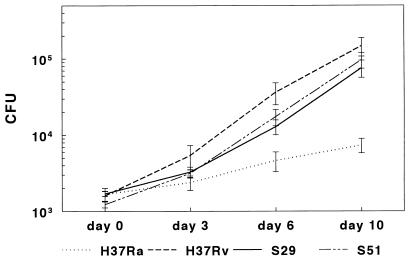
Our results show that all M. tuberculosis strains elicited similar MCP-1 and IL-8 production by A549 cells, whereas M. avium strains did not. To determine if intracellular mycobacterial growth rates were related to production of IL-8 and MCP-1, we measured intracellular growth of the mycobacterial strains in A549 cells. The numbers of CFU obtained from A549 lysates were comparable at day 0 for all strains of M. tuberculosis, but H37Rv and the patient isolates S29 and S51 grew much more rapidly than H37Ra. In four experiments, after 10 days, the mean CFU were significantly higher for H37Rv than for H37Ra (P = 0.03) (Fig. 7). Mean CFU were similar for the clinical isolates S29 and S51 and were significantly higher than those for H37Ra (P < 0.04 for each comparison) (Fig. 7). In contrast to results for the M. tuberculosis strains, M. avium 100, 101, and 109 showed no significant growth after 10 days of culture in A549 cells (data not shown).
FIG. 7.
Growth of M. tuberculosis strains in A549 cells. A549 cells were infected with H37Ra, H37Rv, or clinical isolate S29 or S51 at a bacterium/cell ratio of 1:100, and CFU were measured at different time points. The values shown are the means and SEs for four separate experiments.
The differences in CFU observed between H37Ra and virulent M. tuberculosis strains may have resulted from differential extracellular growth in the culture medium. However, none of the M. tuberculosis strains grew in medium alone (data not shown). Because our experimental system did not support extracellular growth of M. tuberculosis, reduced viability of A549 cells could decrease the number of cells available for intracellular growth and artifactually lower the number of CFU isolated. Therefore, if H37Ra reduced viability of A549 cells more than the other M. tuberculosis strains, this could selectively reduce the CFU measured for H37Ra. However, after 10 days of culture, trypan blue exclusion revealed no significant differences in total numbers of A549 cells per well (H37Ra, 1.3 × 106; H37Rv, 1.4 × 106; S29, 1.6 × 106; S51, 1.6 × 106). The percentage of viable cells ranged from 94 to 98%. Therefore, the reduced growth of H37Ra did not result from excessive destruction of infected A549 cells. To determine if the reduced rate of intracellular growth of H37Ra resulted from an intrinsic difference in the growth characteristics of this strain, we cultured H37Ra, H37Rv, and the clinical isolates S29 and S51 in 7H9 medium. In four experiments, the mean CFU for all strains were similar at 6 and 10 days (data not shown).
DISCUSSION
An increasing body of evidence suggests that epithelial cells play an important role in initiation of the acute inflammatory response to microbial pathogens by producing chemokines. Colonic epithelial cells secrete TNF-α, IL-8, and MCP-1 in response to invasive bacterial pathogens but not upon exposure to noninvasive organisms (9), and Pseudomonas aeruginosa elicits IL-8 production by airway epithelial cells (5). The results of this study demonstrate that M. tuberculosis infection of a human alveolar epithelial cell line elicits production of the chemokines MCP-1 and IL-8 through upregulation of expression of their respective mRNAs. Chemokine production in response to M. tuberculosis was not dependent on production of TNF-α, IL-1β, or IL-6. Virulent clinical and laboratory M. tuberculosis isolates and the avirulent H37Ra strain elicited comparable production of MCP-1 and IL-8, whereas killed M. tuberculosis and live M. avium strains did not. The virulent M. tuberculosis strains grew more rapidly than the avirulent M. tuberculosis strain in alveolar epithelial cells, and the M. avium strains did not grow intracellularly. These findings suggest that intracellular growth is necessary for mycobacteria to elicit production of MCP-1 and IL-8 by alveolar epithelial cells but that virulence and the rate of intracellular growth do not correlate with production of these chemokines. If these findings can be extrapolated to the situation in vivo, our results suggest that M. tuberculosis induces normal alveolar epithelial cells to produce chemokines which can contribute to the local inflammatory response by attracting monocytes, lymphocytes, and polymorphonuclear cells.
Although most studies of the interactions between M. tuberculosis and pulmonary cells have focused on alveolar macrophages, interactions between M. tuberculosis and alveolar epithelial cells may contribute significantly to the pathogenesis of tuberculosis. Bermudez and Goodman showed that the virulent M. tuberculosis strain H37Rv grew more rapidly than the avirulent H37Ra strain in A549 cells, suggesting that alveolar epithelial cells serve as a reservoir for M. tuberculosis in the lung (3). We confirmed and extended these results, showing that M. tuberculosis clinical isolates that are virulent in vivo behave similarly to H37Rv and differently from H37Ra. In contrast, M. avium, which is much less virulent than M. tuberculosis in humans, did not replicate in A549 cells.
MCP-1 is a chemoattractant for CD4+ T cells and monocytes (19), and depletion of MCP-1 markedly reduces mononuclear cell recruitment and clearance of Cryptococcus neoformans in a murine model of fungal pneumonia (8). CD4+ T cells and monocytes are central components of the granulomatous response to M. tuberculosis, and MCP-1 may play a critical role in initiating the local immune response. Peripheral blood monocytes exposed to killed M. tuberculosis or purified protein derivative produce high concentrations of MCP-1 (10). In addition, MCP-1 is produced in vivo at the site of disease in human tuberculosis. MCP-1 concentrations are elevated in pleural fluid of patients with tuberculous pleuritis, and high MCP-1 concentrations correlate strongly with the number of monocytes in pleural fluid (1). Furthermore, in bronchoalveolar lavage fluid of patients with pulmonary tuberculosis, MCP-1 concentrations are elevated, and they decrease during convalescence (11). Our findings with a human alveolar epithelial cell line suggest that normal alveolar epithelial cells may contribute to development of the local inflammatory response by production of MCP-1.
Human monocytes and alveolar macrophages produce IL-8 upon exposure to live M. tuberculosis or the M. tuberculosis heteropolysaccharide lipoarabinomannan, a putative mycobacterial virulence factor (4). IL-8 predominately attracts neutrophils, which are a prominent component of the inflammatory infiltrate in the lungs of some tuberculosis patients (7, 12, 22). In patients with pulmonary tuberculosis, IL-8 concentrations are elevated in bronchoalveolar lavage fluid and correlate linearly with neutrophil concentration (11, 22). Alveolar macrophages from tuberculosis patients spontaneously produce increased concentrations of IL-8 (22). In contrast, IL-8 concentrations are not elevated in pleural fluid of patients with tuberculous pleuritis (1), suggesting that production of this chemokine varies at different anatomic sites. Our findings suggest that alveolar epithelial cells can produce IL-8 in response to M. tuberculosis and can contribute to the initial neutrophilic inflammatory response in human tuberculosis.
M. tuberculosis is more virulent than M. avium because it commonly causes disease in normal persons, whereas M. avium is generally a pathogen only in those with compromised local or systemic immune defenses. M. tuberculosis strains elicited production of MCP-1 and IL-8 by A549 cells, whereas M. avium strains did not. This difference in chemokine production is unlikely to simply reflect differences in virulence between M. tuberculosis and M. avium, as the avirulent M. tuberculosis strain H37Ra and virulent M. tuberculosis strains elicited comparable degrees of MCP-1 and IL-8 production. This conclusion is supported by studies showing similar patterns of MCP-1 production in mice infected with M. tuberculosis strains with different degrees of virulence (18). We believe that the inability of M. avium to induce production of these chemokines is more likely to reflect failure to grow intracellularly in A549 cells, and this conclusion is supported by the failure of killed M. tuberculosis to elicit chemokine production. However, because virulent M. tuberculosis strains grew more rapidly intracellularly than H37Ra but elicited comparable production of MCP-1 and IL-8, the rate of intracellular growth does not appear to correlate with the level of production of these chemokines.
In human alveolar macrophages infected with M. tuberculosis, IL-8 production is dependent on production of TNF-α and IL-1 (22). The role of other cytokines in eliciting MCP-1 upon exposure of cells to mycobacteria has previously been unexplored. However, in human osteoblasts and renal proximal epithelial cells, TNF-α, IL-1, and IL-6 elicit MCP-1 production (17, 23), whereas TNF-α and IL-1 do not affect MCP-1 production by human monocyte-endothelial cell interactions (14). Our findings demonstrate that in a human alveolar epithelial cell line infected with M. tuberculosis, production of IL-8 and MCP-1 is independent of TNF-α, IL-1, and IL-6, suggesting that these chemokines are part of the initial inflammatory response rather than a component of the secondary cytokine cascade.
In summary, we demonstrated that a human alveolar epithelial cell line infected in vitro with M. tuberculosis but not M. avium produces the chemokines MCP-1 and IL-8 through upregulation of their respective mRNAs. Production of these cytokines depended on intracellular mycobacterial growth but was not related to virulence. These findings suggest that alveolar epithelial cells can contribute to immune defenses by attracting monocytes, lymphocytes, and neutrophils. Further studies of the human alveolar epithelium in tuberculosis patients are needed to characterize the significance of these observations in vivo.
ACKNOWLEDGMENTS
This work was supported by the UNDP/World Bank/World Health Organization Special Programme for Vaccine Development (IMMMYC) and the National Institutes of Health (AI27285 and AI36069). P. F. Barnes holds the Margaret E. Byers Cain Chair for Tuberculosis Research. Molecular core laboratory facilities were provided by the National Institutes of Health National Center for Research Resources of the General Clinical Research Centers grant M01 RR-43. Mycobacterial products were provided through contract AI05074 from the National Institute of Allergy and Infectious Diseases.
We thank Luiz Bermudez and Clark Inderlied for providing the M. avium strains.
REFERENCES
- 1.Antony V B, Godbey S W, Kunkel S L, Hott J W, Hartman D L, Burdick M D, Strieter R M. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151:7216–7223. [PubMed] [Google Scholar]
- 2.Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 3.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J, Fan X, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoheisel G B, Tabak L, Teschler H, Erkan F, Kroegel C, Costabel U. Bronchoalveolar lavage cytology and immunocytology in pulmonary tuberculosis. Am J Respir Crit Care Med. 1994;149:460–463. doi: 10.1164/ajrccm.149.2.8306046. [DOI] [PubMed] [Google Scholar]
- 8.Huffnagle G B, Strieter R M, Standiford T J, McDonald R A, Burdick M D, Kunkel S L, Toews G B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 9.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasahara K, Tobe T, Tomita M, Mukaida N, Shao-Bo S, Matsushima K, Yoshida T, Sugihara S, Kobayashi K. Selective expression of monocyte chemotactic and activating factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–1247. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T, Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–1477. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 12.Law K F, Jagirdar J, Weiden M D, Bodkin M, Rom W N. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153:1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Zhang M, Hofman F M, Gong J, Barnes P F. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukacs N W, Strieter R M, Ellner V, Evanoff H L, Burdick M D, Kunkel S L. Production of chemokines, interleukin-8 and monocyte chemoattractant protein-1, during monocyte:endothelial cell interactions. Blood. 1995;86:2767–2773. [PubMed] [Google Scholar]
- 15.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceiçao-Silva F, Modlin R L. Cytokine patterns in pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prodjosudjadi W, Gerritsma S J, Klar-Mohamad N, Gerritsen A F, Bruijn J A, Daha M R, van Es L A. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 18.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub D D, Proost P, Murphy W J, Anver M, Longo D L, Damme J V, Oppenheim J J. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95:1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Gately M K, Wang E, Gong J, Wolf S F, Lu S, Modlin R L, Barnes P F. Interleukin-12 at the site of disease in tuberculosis. J Clin Invest. 1994;93:1733–1739. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom W N. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Valente A J, Lorenzo J A, Carnes D, Graves D T. Expression of monocyte chemoattractant protein 1 in human osteoblastic cells stimulated by proinflammatory mediators. J Bone Miner Res. 1994;9:1123–1130. doi: 10.1002/jbmr.5650090721. [DOI] [PubMed] [Google Scholar]