Abstract
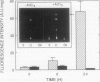
The major effort in developing pathogenic fungi into potential mycoherbicides is aimed at increasing fungal virulence to weeds without affecting crop selectivity. Specific suppression of biosynthesis of a phytoalexin derived from the shikimate pathway in Cassia obtusifolia L. by a sublethal dose (50 micromolar) of glyphosate increased susceptibility to the mycoherbicide Alternaria cassiae Jurair & Khan. Glyphosate applied with conidia suppressed phytoalexin synthesis beginning at 12 hours, but not an earlier period 8 to 10 hours after inoculation. The phytoalexin synthesis elicited by fungal inoculation was also suppressed by darkness. The magnitudes of virulence of the mycoherbicide in the dark or with glyphosate in the light were both higher than after inoculation in the light with the same concentration of conidia in the absence of glyphosate. Five times less inoculum was needed to cause disease symptoms when applied with glyphosate than without. Glyphosate did not render A. cassiae virulent on soybean (Glycine max), a crop related to the host. These results suggest that a specific inhibition of a weed's elicited defense response can be a safe way to enhance virulence and improve the efficacy of the mycoherbicide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Straney D., Ciuffetti L., VAN Etten H. D., Yoder O. C. One enzyme makes a fungal pathogen, but not a saprophyte, virulent on a new host plant. Science. 1989 Oct 13;246(4927):247–249. doi: 10.1126/science.246.4927.247. [DOI] [PubMed] [Google Scholar]
- Sharon A., Ghirlando R., Gressel J. Isolation, Purification, and Identification of 2-(p-Hydroxyphenoxy)-5, 7-Dihydroxychromone: A Fungal-Induced Phytoalexin from Cassia obtusifolia. Plant Physiol. 1992 Jan;98(1):303–308. doi: 10.1104/pp.98.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal B., Robeson D. J. The metabolism of sunflower phytoalexins ayapin and scopoletin: plant-fungus interactions. Plant Physiol. 1986 Sep;82(1):167–172. doi: 10.1104/pp.82.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. W., Cahill D. M., Bhattacharyya M. K. Abscisic Acid Suppression of Phenylalanine Ammonia-Lyase Activity and mRNA, and Resistance of Soybeans to Phytophthora megasperma f.sp. glycinea. Plant Physiol. 1989 Sep;91(1):23–27. doi: 10.1104/pp.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender R., Röhrig H., Höricke C., Schell J. cis-regulatory elements involved in ultraviolet light regulation and plant defense. Plant Cell. 1990 Oct;2(10):1019–1026. doi: 10.1105/tpc.2.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]