Abstract
We have employed both 31P nuclear magnetic resonance spectroscopy and two intracellular fluorescent pH indicator dyes to monitor the pH of the vacuole and cytoplasm of suspension-cultured soybean cells (Glycine max Merr cv Kent). For the 31P nuclear magnetic resonance studies, a flow cell was constructed that allowed perfusion of the cells in oxygenated growth medium throughout the experiment. When the perfusion medium was transiently adjusted to a pH higher than that of the ambient growth medium, a rapid elevation of vacuolar pH was observed followed by a slow (approximately 30 minute) return to near resting pH. In contrast, the concurrent pH changes in the cytoplasm were usually fourfold smaller. These data indicate that extracellular pH changes are rapidly communicated to the vacuole in soybean cells without significantly perturbing cytoplasmic pH. When elicitors were dissolved in a medium of altered pH and introduced into the cell suspension, the pH of the vacuole, as above, quickly reflected the pH of the added elicitor solution. In contrast, when the pH of either a polygalacturonic acid or Verticillium dahliae elicitor preparation was adjusted to the same pH as the ambient medium, no significant change in either vacuolar or cytoplasmic pH was observed during the 35 minute experiment. These results were confirmed in experiments with pH-sensitive fluorescent dyes. We conclude that suspension-cultured soybean cells do not respond to elicitation by significantly changing the pH of their vacuolar or cytoplasmic compartments.
Full text
PDF

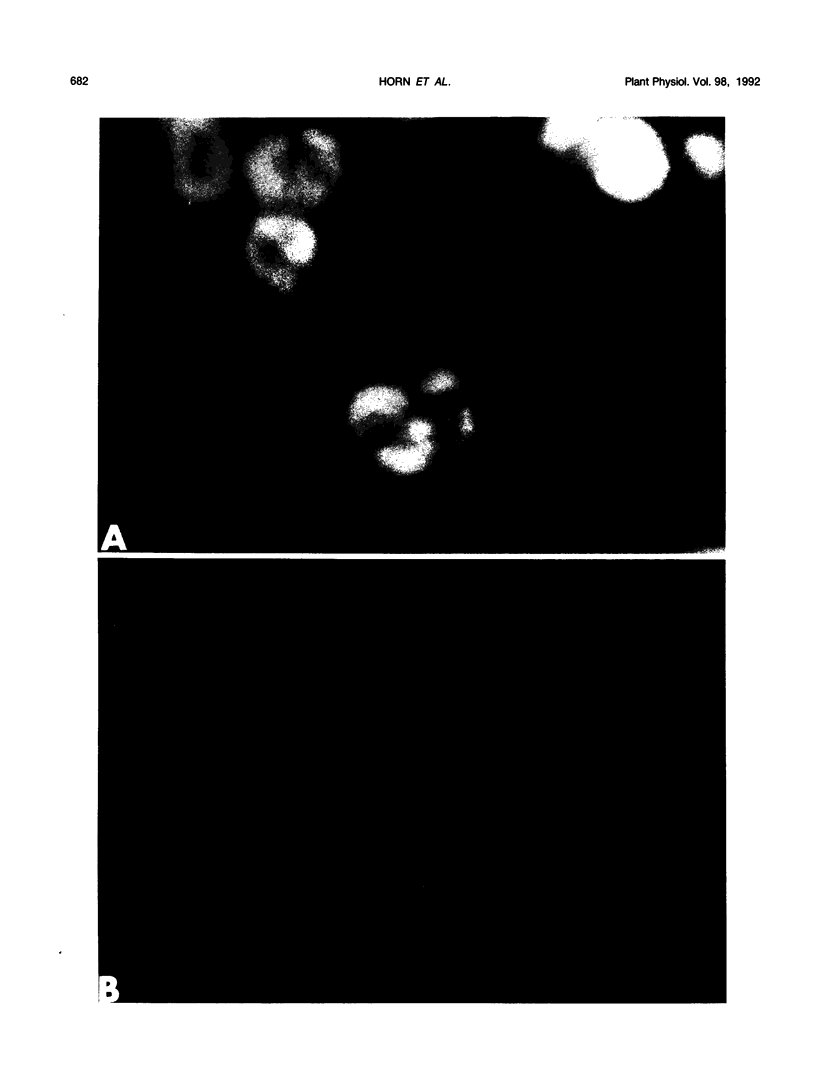
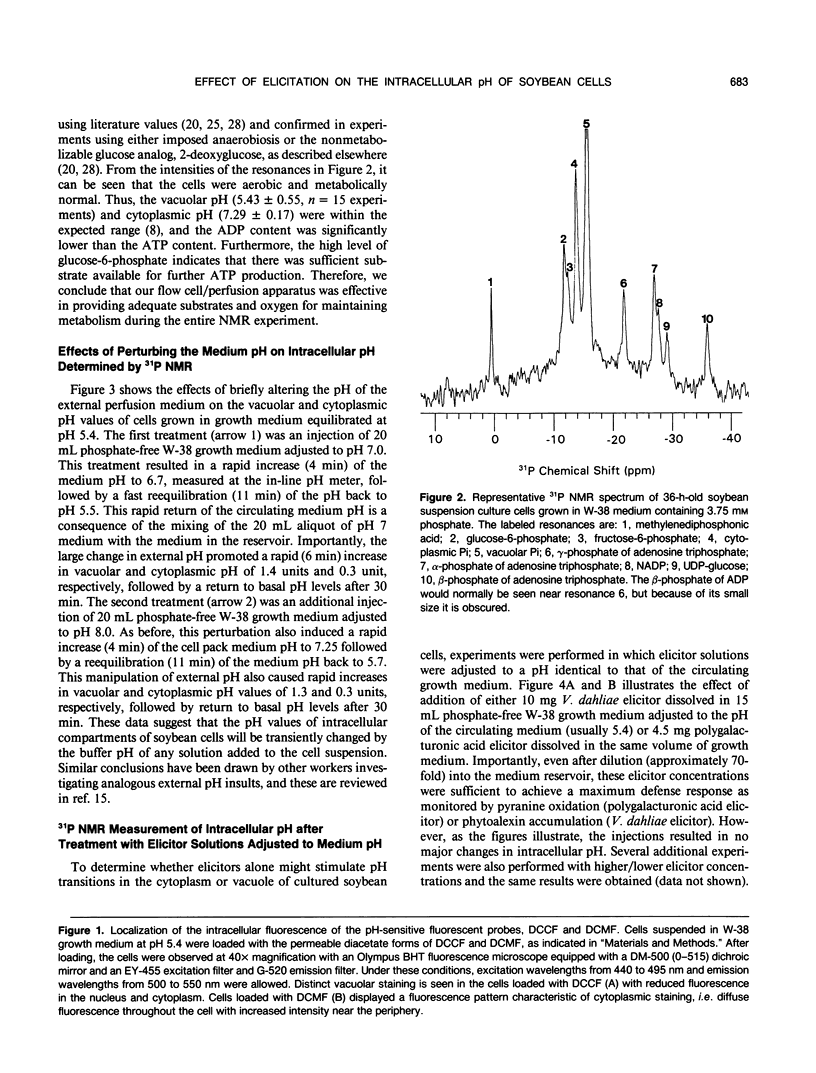
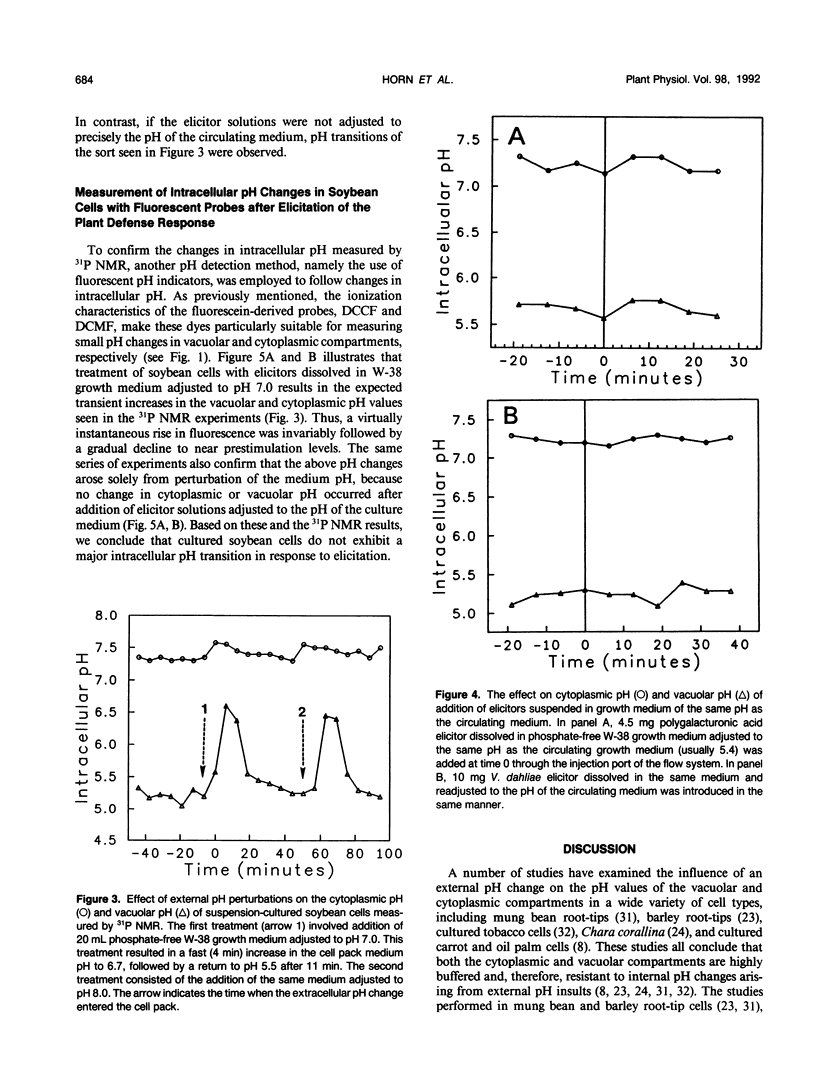
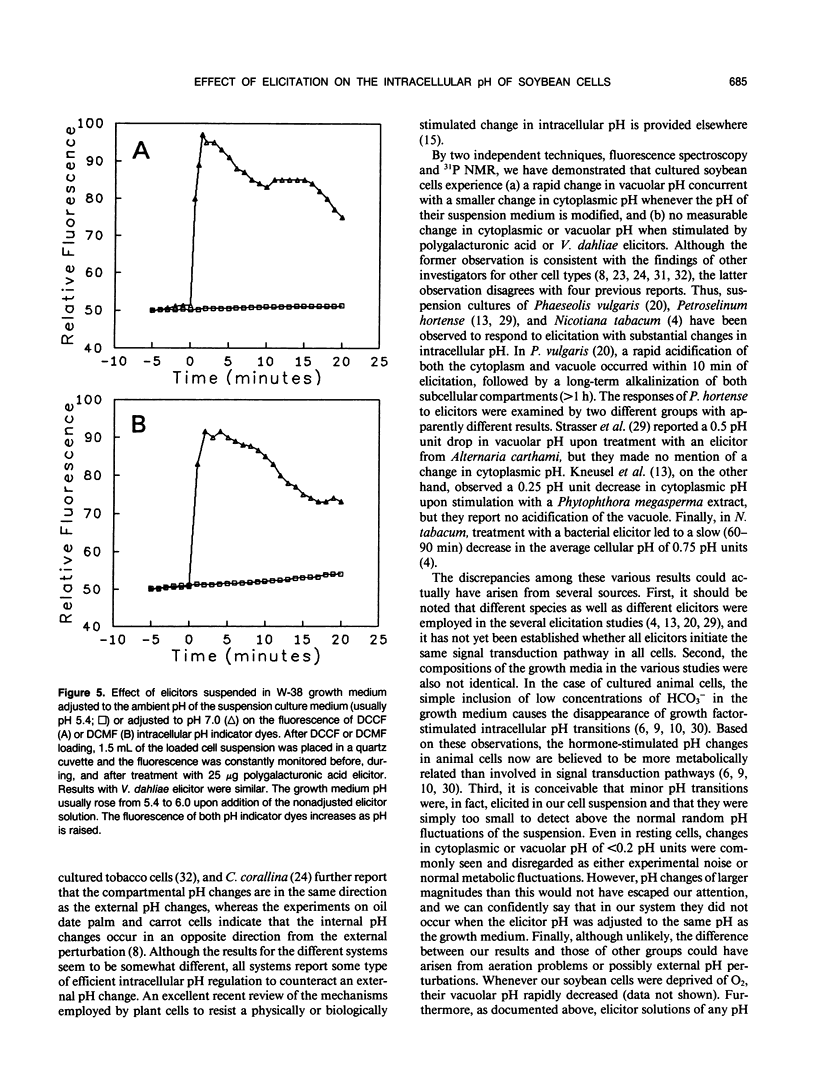

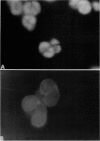
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I., Low P. S., Heinstein P., Stipanovic R. D., Altman D. W. Inhibition of elicitor-induced phytoalexin formation in cotton and soybean cells by citrate. Plant Physiol. 1987 Aug;84(4):1276–1280. doi: 10.1104/pp.84.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Huang J. S., Knopp J. A. The Hypersensitive Reaction of Tobacco to Pseudomonas syringae pv. pisi: Activation of a Plasmalemma K/H Exchange Mechanism. Plant Physiol. 1985 Nov;79(3):843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Cassel D., Whiteley B., Zhuang Y. X., Glaser L. Mitogen-independent activation of Na+/H+ exchange in human epidermoid carcinoma A431 cells: regulation by medium osmolarity. J Cell Physiol. 1985 Feb;122(2):178–186. doi: 10.1002/jcp.1041220203. [DOI] [PubMed] [Google Scholar]
- Fox G. G., Ratcliffe G. P NMR Observations on the Effect of the External pH on the Intracellular pH Values in Plant Cell Suspension Cultures. Plant Physiol. 1990 Jun;93(2):512–521. doi: 10.1104/pp.93.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Boron W. F., Sterzel R. B. Effects of angiotensin II and vasopressin on intracellular pH of glomerular mesangial cells. Am J Physiol. 1988 Jun;254(6 Pt 2):F787–F794. doi: 10.1152/ajprenal.1988.254.6.F787. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Horn M. A., Heinstein P. F., Low P. S. Receptor-Mediated Endocytosis in Plant Cells. Plant Cell. 1989 Oct;1(10):1003–1009. doi: 10.1105/tpc.1.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneusel R. E., Matern U., Nicolay K. Formation of trans-caffeoyl-CoA from trans-4-coumaroyl-CoA by Zn2+-dependent enzymes in cultured plant cells and its activation by an elicitor-induced pH shift. Arch Biochem Biophys. 1989 Mar;269(2):455–462. doi: 10.1016/0003-9861(89)90129-x. [DOI] [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Bligny R., Rebeille F., Douce R., Leguay J. J., Mathieu Y., Guern J. A P Nuclear Magnetic Resonance Study of Intracellular pH of Plant Cells Cultivated in Liquid Medium. Plant Physiol. 1982 Oct;70(4):1156–1161. doi: 10.1104/pp.70.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo I., Rokem J. S., Navon G., Goldberg I. P NMR Study of Elicitor Treated Phaseolus vulgaris Cell Suspension Cultures. Plant Physiol. 1987 Nov;85(3):716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Cell growth factors. Bicarbonate and pHi response. Nature. 1989 Feb 16;337(6208):601–601. doi: 10.1038/337601a0. [DOI] [PubMed] [Google Scholar]
- Wray V., Schiel O., Berlin J., Witte L. Phosphorus-31 nuclear magnetic resonance investigation of the in vivo regulation of intracellular pH in cell suspension cultures of Nicotiana tabacum: the effects of oxygen supply, nitrogen, and external pH change. Arch Biochem Biophys. 1985 Feb 1;236(2):731–740. doi: 10.1016/0003-9861(85)90679-4. [DOI] [PubMed] [Google Scholar]