Abstract
Little is known about the role of peripheral blood mononuclear cells (PBMCs) in lipopolysaccharide (LPS) elimination. We studied the endotoxin elimination capacities (EEC) of PBMCs of 15 healthy volunteers, 13 patients with sepsis, and 1 patient suffering from paroxysmal nocturnal hemoglobinuria (PNH). Although expression of CD14, the best-characterized receptor for LPS to date, was reduced from 93.6% ± 0.8% in healthy subjects to 50.5% ± 6.5% in patients with sepsis and was 0.3% in a patient with septic PNH, EEC were found to be unchanged. There was no difference in the amount of tumor necrosis factor alpha (TNF-α) released by PBMCs of healthy donors and patients with sepsis. Anti-CD14 antibodies (MEM-18) completely suppressed EEC, binding of fluorescein isothiocyanate-labeled LPS to monocytes as determined by FACScan analysis, and TNF-α release in all three groups studied. The concentrations of soluble CD14 (sCD14) secreted by endotoxin-stimulated PBMCs from healthy donors and patients with sepsis amounted to 4.5 ± 0.4 and 20.1 ± 1.8 ng/ml, respectively. Based on our results, we suggest that PBMCs eliminate LPS by at least two different mechanisms; in healthy subjects, the membrane CD14 (mCD14) receptor is the most important factor for LPS elimination, while in patients with sepsis (including the septic state of PNH), increased sCD14 participates in LPS elimination. Secretion of sCD14 is strongly enhanced under conditions of low expression of mCD14 in order to counteract the reduction of mCD14 and maintain the function of monocytes. This sCD14 may substitute the role of mCD14 in LPS elimination during sepsis. The elimination of LPS by PBMCs correlates with the binding reaction and the secretion of TNF-α.
Monocytes and macrophages play a central role in the inflammatory response of the human organism to bacterial lipopolysaccharides (LPS) in terms of the release of pro- and anti-inflammatory mediators. The binding of LPS to monocytes is the prerequisite for signal transduction followed by the activation of the cells. Several putative LPS membrane receptors have been recognized in humans, such as membrane-bound CD14 (mCD14) (54), the CD11b-CD18 complex (53), an 80-kDa membrane protein (51) occurring in a broad variety of cell systems, and scavenger receptor ligands such as acetylated low-density lipoprotein (27).
CD14 is a 55-kDa glycoprotein existing in a membrane-bound form and a soluble form. mCD14 has been characterized as the most important LPS receptor of monocytes that is involved in the process of signal transduction. mCD14 is attached to the membrane by a glycosylphosphatidylinositol anchor which excludes direct signal transduction without the involvement of other membrane constituents (54). Studies relying on the use of blocking antibodies against mCD14 were confirmed by experiments with fluorescence-labeled (20, 22, 31, 36, 49) or radioactively (21, 35, 44) labeled LPS which supported the relevance of the LPS receptor system concerning binding and signal transduction. The lack of transmembrane anchorage of mCD14 and the necessity of other membrane constituents such as an 80-kDa glycoprotein (51) for signaling processes explain recent findings of the dissociation between the binding reaction and cell activation (21, 44).
The soluble form of CD14 (sCD14) is present in plasma at concentrations of 2 to 6 μg/ml (4). sCD14 has recently been revealed to be responsible for the LPS-mediated activation of cell systems lacking mCD14 (11, 23, 26). Endothelial cells (19, 50), smooth muscle cells (43), and monocytes of patients suffering from paroxysmal nocturnal hemoglobinuria (PNH) (11, 23, 26) do not express mCD14 but can be activated by LPS in the presence of sCD14. Even the response of mCD14-bearing cells on LPS stimuli is known to be enhanced in the presence of sCD14 (23, 26). The interaction between LPS and mCD14 and sCD14 is catalyzed by the LPS-binding protein, an acute-phase protein synthesized by the liver which forms complexes with LPS and CD14 (26, 56).
During sepsis, expression of mCD14 was shown to be reduced (3, 7, 13, 14, 41, 42). In addition, reduction in mCD14 (30) and increase in sCD14 (39) are both poor prognostic signs of mortality. The stimulatory effect of LPS on monocytes derived from septic patients is reduced. This could in part be due to reduced mCD14 expression (42).
Considerable work has been performed to elucidate the mechanisms of LPS binding and internalization and also of signal transduction in monocytes (20–22, 31, 36, 44, 49, 54). However, little is known about whether the binding is associated with a significant elimination of circulating endotoxin. Although the cellular mechanisms of LPS detoxification such as dephosphorylation (48), deacylation (47), and other LPS degradation processes (16, 18) which in part occur in macrophages have been identified, the neutralizing activity of circulating endotoxin is believed to be based mainly on humoral factors such as high-density lipoprotein (15, 40), bactericidal and permeability-increasing protein (24, 32), antiendotoxin antibody (2), and transferrin (6). Therefore, the aim of our study was to characterize and quantify the cellular elimination capacities of human mononuclear cells from healthy volunteers, patients with sepsis, and a patient with PNH in healthy, septic, and recovery statuses, respectively, in an ex vivo setting. Elimination capacities were correlated with binding capacities and cytokine secretion by studying the binding of fluorescein isothiocyanate (FITC)-labeled LPS and the stimulatory effect of exogenously added LPS on monocytes in terms of the release of proinflammatory cytokines. Furthermore, the influence of spontaneous and LPS-stimulated secretion of sCD14 on cellular LPS elimination was studied.
MATERIALS AND METHODS
Patients.
Thirteen patients (8 male and 5 female, ranging in age from 35 to 87 years) who fulfilled the criteria of severe sepsis (1) were enrolled in this study. The simplified APACHE II score of all patients was 17.3 ± 1 (mean ± standard errors of the means [SEM]). The diagnoses were pancreatitis (n = 7 [6 patients were surgically treated and 5 patients died]), peritonitis (n = 4 [all patients were surgically treated and survived]), or pneumonia (n = 2 [2 patients died]). We excluded patients with early sepsis (within 4 days after onset of sepsis) and patients within 7 days after major surgical procedures. All patients were antibiotically treated. Fifteen healthy volunteers and a patient suffering from PNH with healthy, septic, and recovery statuses who lacked a hemolytic crisis and who had not required blood transfusion during the preceding 6 months were studied. The protocol was approved by the local review board.
Isolation of PBMCs.
Venous blood (15 ml) anticoagulated with 10 IU of endotoxin-free sodium heparinate (B/Braun, Melsungen, Germany) per ml was separated in a 50-ml polystyrene tube with a porous filter disk (LuecoSep; Greiner, Frickenhausen, Germany) by centrifugation at 630 × g for 20 min over a Ficoll-Paque gradient (Seromed, Berlin, Germany). Peripheral blood mononuclear cells (PBMCs) were washed three times with phosphate-buffered saline (PBS) and were finally suspended in a concentration of 2 × 106 cells per ml in RPMI 1640 (GIBCO, Eggenstein, Germany) supplemented with 10% fetal calf serum (FCS) (GMN, Frankfurt, Germany) containing 2 mmol of l-glutamine and 25 mmol of HEPES buffer per liter. The endotoxin contamination of the incubation mixture was routinely examined and proved to be less than 0.05 EU per ml.
(i) Determination of the EEC of PBMCs.
PBMCs (2 × 106) were plated onto 24-well polystyrene plates (Greiner) and the plates were incubated with 1 ng of Escherichia coli O55:B5 (Bio-Whittaker, Walkersville, Md.) at 37°C in a 5% CO2 atmosphere for 30 min, 2, 4, and 6 h. Supernatants from non-LPS-stimulated cells and cell-free 10% FCS medium served as controls. Furthermore, cell lines of a pancreatic cancer (ATCC SW1116), a colonic cancer (ATCC HT 29), and a hepatoblastoma (ATCC Hep G2) were used as negative controls demonstrating the specificity of LPS elimination and its binding reaction. The LPS content of the supernatant was measured, and total cellular endotoxin elimination capacity (total EEC) was expressed as a percentage of the amount of exogenously added endotoxin which was no longer recovered [EEC (total)]. In each assay, the EEC of the cell-free 10% FCS medium was subtracted from total EEC to obtain the cellular EEC by the equation EEC (total) − EEC (10% FCS medium) = EEC (cellular). In experiments testing the role of CD14, the cells were preincubated with 15-μg/ml of the anti-CD14 monoclonal antibody (MAb) MEM-18 (immunoglobulin G1 [IgG1], kindly provided by V. Horejsi, Institute of Molecular Genetics, Prague, Czechoslovakia) (3) for 30 min at 4°C before addition of LPS. Aliquots of the supernatants were immediately frozen and stored for as many as 4 weeks at −80°C after centrifugation at 630 × g for 10 min.
(ii) Determination of supernatant LPS contents.
Determination of LPS contents was performed by a chromogenic modification of the Limulus amoebocyte lysate (LAL) test (a two-step and end point micromethod) as previously described (5), with only minor modifications. The lysate (Endosafe) was obtained from Charles River Endosafe, Sulzfeld, Germany. The supernatant samples were pretreated by dilution (1:20) with pyrogen-free water and subsequent heat inactivation for 15 min at 75°C. The endotoxin contents of unknown samples were determined according to a simultaneously established standard curve with 10% FCS medium with the endotoxin of E. coli O55:B5 (Bio-Whittaker).
(iii) Cytokine assays.
Supernatant samples were assayed for human interleukin 1β (Il-1β), Il-6, and tumor necrosis factor alpha (TNF-α) by commercially available enzyme-linked immunosorbent assay (ELISA) kits obtained from Immunotech Co., Hamburg, Germany.
(iv) sCD14 assays.
Supernatant concentrations of CD14 were tested by a recently available CD14 capture ELISA (IBL, Hamburg, Germany). The lower detection limit was <1 ng/ml.
Analysis of the binding of FITC-labeled LPS to monocytes by flow cytometry.
Freshly prepared PBMCs (2 × 106 per ml of the above-described medium) were incubated with 1-μg/ml FITC-labeled LPS of E. coli O55:B5, which was trichloroacetic acid extracted (Sigma, Delsenhofen, Germany) for 30 min and for 2 and 4 h at 37°C in a 5% CO2 atmosphere. Binding at an LPS concentration of 10 ng/ml was detected; however, the most efficient binding was observed at 1 μg/ml. Blocking experiments with anti-CD14 MAb were performed after preincubation of the cells with 15 μg of MEM-18 per ml for 30 min at 4°C as it was done in the elimination experiments. Then, the cells were subjected to two washes with PBS (supplemented with 1% bovine serum albumin and 0.1% acetic acid), resuspended in PBS, and assayed with a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany). The analysis was performed with a forward light scatter and a side scatter to obtain data for monocytes. At least 30,000 cells were analyzed for each determination. The binding of FITC-labeled LPS was measured as the mean percentage of positive gated monocytes. To estimate binding specificities, cell lines of pancreatic cancer, colonic cancer, and hepatoblastoma were used as controls.
Evaluation of the monocytic expression of HLA-DR and CD14.
PBMCs (2 × 106 per ml) were centrifuged at 630 × g for 5 min. The sedimented cells were simultaneously incubated with 10 μl of rhodamine-phycoerythrin-conjugated anti-CD14 MAb (DACO, Hamburg, Germany) and 10 μl of FITC-conjugated anti-HLA-DR MAb (DACO) after resuspension in 100 μl of PBS supplemented with 1% bovine serum albumin and 0.1% acetic acid for 30 min at 4°C in the dark. Cells were also incubated with IgG1-FITC and IgG2-RPE isotype-matched control antibodies (DACO) as controls. After the staining procedure, the cells were washed twice and fixed in supplemented PBS. For analysis, monocytes were gated by using forward- and sight-scatter properties. Specific cell fluorescence was studied by side-scatter properties to estimate the levels of mCD14 and HLA-DR expression in gated monocytes.
Statistical analysis.
Statistical evaluation was performed with by Student’s t test for independent data. A P value of <0.05 was considered significant unless otherwise stated. The data are given as means ± SEM.
RESULTS
Specificity and time scale of the EECs of PBMCs.
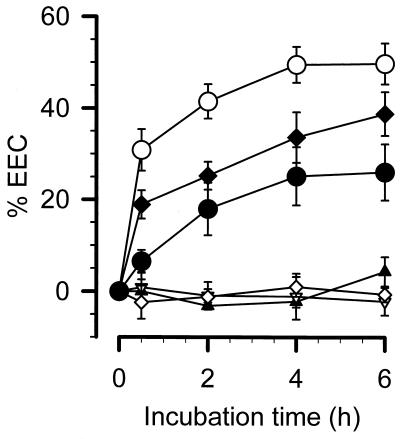
PBMCs (2 × 104, 2 × 105, and 2 × 106 per ml) isolated from 15 healthy volunteers were incubated with 1 ng of LPS per ml. The LPS concentrations of the supernatant were determined after different incubation periods as shown in Fig. 1. Pancreatic cancer, colonic cancer, and hepatoblastoma cell lines (2 × 106 cells per ml) served as controls. EECs were expressed as percentages of the originally added endotoxin which had disappeared from the supernatant. The EEC of the cell-free incubation buffer in each assay was tested (maximal EEC, 19.2% ± 4%) and was subtracted from total EEC to obtain cellular EEC. In order to study the EEC in a 10% FCS containing medium, we tested the EECs for 0.1, 0.3, 0.5, and 1 ng of LPS per ml in the FCS medium; the concentration of 1 ng of LPS per ml was chosen because lower concentrations (≦0.1 ng of LPS per ml) were completely eliminated by 10% FCS medium (data not shown). On the other hand, more than 1 ng of endotoxin per ml is rarely found in patients with sepsis. Maximal endotoxin clearance values obtained with 2 × 106, 2 × 105, and 2 × 104 cells per ml amounted to 49.7% ± 4.5%, 38.8% ± 4.7%, and 26.0% ± 6.1%, respectively. In contrast, almost no cellular EEC could be detected in any of the control cell lines tested.
FIG. 1.
Specificity and time scale of the EECs of PBMCs from healthy volunteers. Different numbers of PBMCs were incubated with 1 ng of smooth LPS per ml, and changes in EECs determined by the LAL test are expressed. ○, 2 × 106 PBMCs from 15 healthy volunteers; ⧫, 2 × 105 PBMCs from 10 healthy volunteers; •, 2 × 104 PBMCs from 7 healthy volunteers; ▿, 2 × 106 cells of the pancreatic cancer cell line SW1116 (n = 3), ▴, 2 × 106 cells of the colorectal cancer cell line HT29 (n = 3); ◊, 2 × 106 cells of the hepatoblastoma cell line HepG2 (n = 3).
Comparison of cellular EECs of PBMCs derived from healthy and septic subjects.
PBMCs (2 × 106 per ml) were prepared from 13 patients suffering from severe sepsis and were assayed for EEC. Table 1 demonstrates that PBMCs of subjects with sepsis and healthy subjects were equally effective in eliminating LPS. The maximal cellular EEC during sepsis was achieved after 4 and 6 h and reached 45.2% ± 5.4%. There was no difference between patients and healthy volunteers. The preincubation of PBMCs with MEM-18 almost completely abolished EEC in healthy subjects and patients with sepsis.
TABLE 1.
Comparison of EECs of PBMCs and levels of FITC-labeled LPS binding by human monocytes for healthy and septic states
| Group (n) | Value for the following
timesa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h
|
30
min
|
2 h
|
4 h
|
6 h
|
|||||
| EEC (%) | FITC-labeled LPS binding (%) | EEC (%) | FITC-labeled LPS binding (%) | EEC (%) | FITC-labeled LPS binding (%) | EEC (%) | FITC-labeled LPS binding (%) | EEC (%) | |
| Healthy volunteers (15) | 0.0 | 0.0 | 30.9 ± 4.5 | 60.3 ± 9.0 | 41.5 ± 3.8 | 86.4 ± 4.3 | 49.5 ± 4.5 | 85.1 ± 3.5 | 49.7 ± 4.5 |
| Patients with sepsis (13) | 0.0 | 0.0 | 30.8 ± 4.3 | 48.2 ± 11.1 | 37.3 ± 3.4 | 82.0 ± 2.9 | 41.0 ± 3.4 | 85.4 ± 3.1 | 45.2 ± 5.4 |
| Healthy volunteers + MEM-18 (15) | 0.0 | 0.0 | 0.8 ± 0.3b | 3.2 ± 1.3b | 0.2 ± 0.3b | 4.4 ± 2.3b | 6.5 ± 1.0b | 10.8 ± 4.6b | 8.3 ± 6.0b |
| Patients with sepsis + MEM-18 (13) | 0.0 | 0.0 | 0.8 ± 0.4c | 1.9 ± 0.3c | 0.2 ± 0.3c | 6.8 ± 1.0c | 0.9 ± 0.1c | 13.2 ± 1.0c | 6.5 ± 2.0c |
| Control (8) | 0.0 | 0.0 | 0.7 ± 0.4 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.3 | 1.2 ± 0.1 | 1.5 ± 0.4 | 6.8 ± 1.0 |
Values are means ± SEM.
Significantly different from the values for healthy volunteer (P < 0.05).
Significantly different from the values for patients with sepsis (P < 0.05; unpaired t test).
EEC of a patient suffering from PNH.
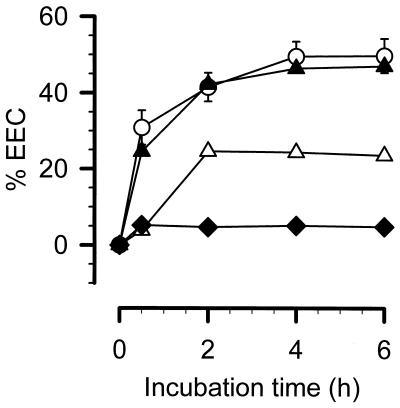
Recently, a patient known to have PNH was admitted to our department because of an ileus with sepsis due to an unknown origin. We took blood samples from him pre- and postoperatively, which we used to test the elimination capacity, just as we did 3 months later, when the patient was readmitted for the take down of an ileostomy (representing healthy status). Four weeks later, the patient had to have a third operation because of acute cholecystitis without any clinical signs of severe sepsis. Four days postoperatively, we took the fourth sample (corresponding to a recovery status, with slight signs of a postoperative acute-phase reaction). As seen in Fig. 2, maximal EEC during sepsis was 47%, which is indistinguishable from values for healthy volunteers or other patients with sepsis (Table 1). During health status, only 5.1% of the exogenously added LPS was removed by PBMCs. During recovery from cholecystitis, the EEC reached 24.6%. It should be pointed out that the EEC of the PNH patient varied as the clinical status changed, indicating an inducible underlying mechanism responsible for the EEC.
FIG. 2.
EEC of a patient suffering from PNH under different clinical states. PBMCs (2 × 106) from a patient suffering from PNH with different clinical statuses were incubated with 1 ng of smooth LPS (E. coli O55:B5) per ml. Changes in EEC determined by the LAL test are expressed. ○, 2 × 106 PBMCs from 15 healthy volunteers; ▴, ▵, and ⧫, 2 × 106 PBMCs from the PNH patient in a septic state, in a state of recovery from acute cholecystitis and in a healthy state, respectively.
Binding of endotoxin to monocytes.
We tested different concentrations of FITC-conjugated LPS to determine the capacity of binding to monocytes (1, 10, and 100 ng and 1 μg/ml). We found a concentration-dependent binding that reached maximal binding at 100 ng/ml. There is almost no difference in the binding of FITC-labeled LPS between the concentrations of 100 ng and 1 μg of LPS per ml. However, the variability at 1 μg of LPS per ml is lower than that at 100 ng/ml. Therefore, we chose 1 μg of FITC-labeled LPS per ml as the suitable concentration for the binding study. The levels of binding of FITC-conjugated LPS of E. coli O55:B5 (1 μg/ml) to monocytes are indicated in Table 1. As is true for the EECs, the binding capacities of monocytes from healthy subjects and patients with sepsis did not differ significantly. Maximal binding amounted to 86.4% ± 4.3% in healthy donors and 85.4% ± 3.4% in donors with sepsis, with no differences in the time scale. Preincubation of the cells with anti-CD14 MAbs reduced the binding capacity to 10.8% ± 4.6% in healthy subjects and 13.2% ± 1.0% in patients with sepsis. When values of EEC and FITC-labeled LPS binding were compared, the elimination capacities of PBMCs seemed to agree with the binding capacities of monocytes. The binding capacities of the cell lines were very low, reaching 1.8% ± 0.2% (pancreatic cells), 1.7% ± 0.1% (colonic cells) and 3.4% ± 0.3% (hepatoblastoma).
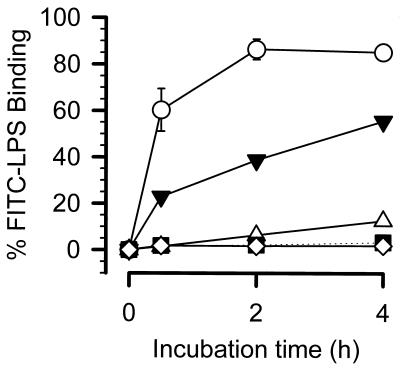
The monocytes of the patient suffering from PNH were also tested for their binding capacities (Fig. 3). Unfortunately, we did not perform fluorescence-activated cell sorter (FACS) analysis during the initial septic state but only during recovery from sepsis. The binding capacities varied from 3.3% (during health) and 12.6% (recovery from cholecystitis) to 55.5% (recovery from sepsis), which was largely consistent with the EEC data (Fig. 2).
FIG. 3.
Comparison of levels of binding of FITC-labeled LPS by monocytes from a PNH patient in different clinical states. ○, 2 × 106 PBMCs from eight healthy volunteers; ▾, ▵, and ▪, 2 × 106 PBMCs from the PNH patient in a state of recovery from sepsis, in a state of recovery from cholecystitis, in a healthy state, respectively; ◊, control without any FITC-labeled LPS.
Expression of mCD14 and HLA-DR.
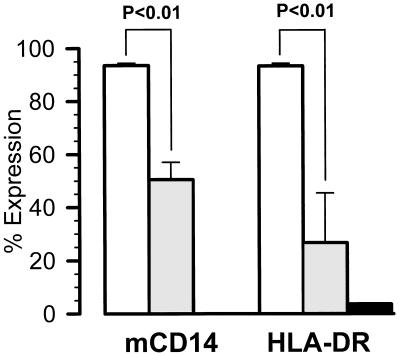
Expression of HLA-DR on monocytes was used to characterize the severity of septic disease (Fig. 4). A total of 93.3% ± 1.0% of monocytes from healthy volunteers were HLA-DR positive, compared to only 26.6% ± 5.0% in patients with sepsis (P < 0.01). The expression of CD14 was also significantly reduced, from 93.6% ± 0.8% to 50.5% ± 6.5% (P < 0.01). The CD14 and HLA-DR expression levels of monocytes from PNH patients during sepsis were only 0.3 and 3.7%, respectively.
FIG. 4.
Expression of mCD14 and HLA-DR on gated monocytes; 106 PBMCs harvested from healthy volunteers (n = 15) (□), patients with sepsis (n = 13) (░⃞), and a PNH patient in a state of sepsis (▪) were stained with RPE-labeled anti-CD14 (Leu-M3) or FITC-labeled anti-HLA-DR. Values are means of percent positive monocytes (P < 0.01 compared to healthy subjects).
Endotoxin-induced TNF-α release from PBMCs.
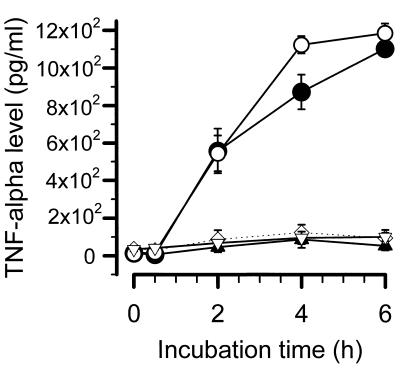
Stimulation of PBMCs with LPS (1 ng/ml) led to a time-dependent release of TNF-α (Fig. 5). No statistically significant difference between PBMCs from healthy subjects and patients with sepsis could be calculated, except for a slight decrease in TNF-α release after 4 h. Preincubation with anti-CD14 MAbs reduced the LPS-induced release of TNF-α to nearly basal values in both groups. Similar results were obtained for IL-1β and IL-6 levels (data not shown).
FIG. 5.
Endotoxin-induced TNF-α release from PBMCs. The levels of TNF-α after stimulation with 1 ng of LPS (E. coli O55:B5) per ml are given. ○ and ▿, 2 × 106 PBMCs from 15 healthy volunteers with and without MEM-18, respectively; • and ▴, 2 × 106 PBMCs from 13 patients with sepsis with and without MEM-18, respectively; ◊, control with no LPS stimulation.
Spontaneous and endotoxin-induced release of sCD14 (Fig. 6).
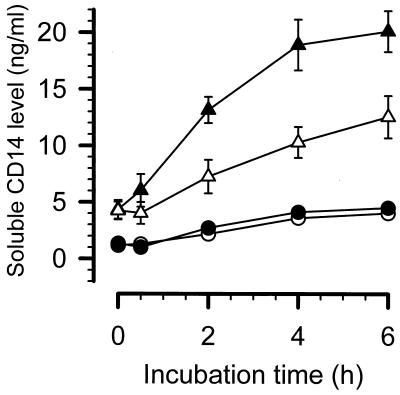
FIG. 6.
Spontaneous and endotoxin-induced release of sCD14. The levels of sCD14 after stimulation with 1 ng of LPS (E. coli O55:B5) per ml are indicated. Values are for • and ○, 2 × 106 PBMCs from six healthy volunteers, stimulated with LPS and unstimulated, respectively; ▴ and ▵, 2 × 106 PBMCs from six patients with sepsis, stimulated with LPS and unstimulated, respectively.
PBMCs from healthy volunteers secrete very low amounts of sCD14 spontaneously (4.0 ± 0.4 ng/ml), which did not increase after endotoxic stimulation (4.5 ± 0.4 ng/ml). In contrast, PBMCs from patients with sepsis spontaneously released significantly higher quantities of sCD14 (12.5 ± 1.9 ng/ml; P < 0.01 versus healthy volunteers), which further increased after addition of LPS (20.1 ± 1.8 ng/ml; P < 0.02 versus spontaneous secretion during sepsis). The maximal stimulated values of sCD14 were more than five times higher during sepsis than during health. In addition, these data were substantiated by increased sCD14 levels in plasma from patients with sepsis compared to healthy subjects (6.8 ± 0.7 versus 3.5 ± 0.3 μg/ml). The concentrations of sCD14 in plasma from the patient with PNH showed the same tendency: healthy state, 3.5 μg/ml; septic state, 8.6 μg/ml; and recovery from cholecystitis, 6.5 μg/ml.
DISCUSSION
Circulating endotoxin is believed to be cleared mainly by liver cell systems (9, 18, 46). It has been shown that LPS is internalized by hepatocytes and released into the bloodstream after chemical modification (16–18, 46). However, the modified LPS retains specific activities such as bioactivity in the LAL test and induces lethality in animal experiments (12). Therefore, specific and unspecific LPS-neutralizing mechanisms of the blood itself should play a pathophysiologically important role. Some humoral factors have been shown to be involved in these neutralizing processes (2, 6, 15, 24, 32, 40). The microscopic observation of the uptake of LPS in monocytes involving specific receptor pathways (33, 34), as well as the characterization of intracellular detoxification processes (16, 18, 48), suggests that PBMCs play an important role in the clearance of circulating endotoxin.
We applied different test systems to the question of endotoxin-monocyte interaction. Determination of the disappearance of LPS from the supernatant of PBMCs represents its elimination from the medium and is therefore called elimination capacity, although we do not clearly know that endotoxin is definitely eliminated in the cells. The binding assay with FITC-labeled LPS is termed binding to facilitate the differentiation between both assays.
Our results with PBMCs derived from healthy volunteers strongly support this assumption. After addition of 1 ng of endotoxin per ml to PBMCs (2 × 106 cells), more than 50% was eliminated by the cells. The elimination capacity was demonstrated to depend on the time of incubation and the number of cells. Cell lines such as pancreatic cancer, colonic cancer, and hepatoblastoma cells did not remove any significant amounts of endotoxin from the supernatant, underlining the specificity of the elimination process. By testing the binding capacities of monocytes with FITC-labeled LPS, the time scale resembled the elimination capacities of PBMCs. No binding of LPS to the cell lines was found. The concordance between EEC and the binding reaction may be a hint that the elimination capacities of PBMCs are based mainly on monocytes. In addition, the kinetics of the cellular neutralizing and binding reactions may require the presence of LBP in FCS, which is known to accelerate the interaction between LPS and mCD14 and sCD14 (2, 26, 45, 52, 56). Actually, we have not directly confirmed whether EEC and LPS binding contributes to LPS internalization or to further detoxification. However, numerous attempts that used confocal microscopy (20), immunocytochemistry (33, 34), observation of human monocytes, and fractionating neutrophil lysates on Percol gradients (44) have clearly demonstrated that plasma membrane-bound LPS can be internalized in phagocytes after 5 to 10 min. Therefore, our EEC assay may reflect not only binding of LPS to the cell surface resulting in elimination of LPS from the supernatant but also a further internalization and accompanying detoxification.
Different LPS receptor mechanisms were shown by several studies to exist (10, 36) that used nonphysiological conditions such as high amounts of LPS in the absence of serum. However, under physiological conditions, mCD14 represents the most important monocytic receptor system for LPS binding and signal transduction. This has been demonstrated by using blocking anti-CD14 antibodies and radioactive- or FITC-labeled LPS (8, 21, 22, 31, 35, 36, 49). Further support of the relevance of mCD14 under low-level endotoxin conditions in the presence of serum is provided by studies of monocytes derived from patients suffering from PNH (10, 11, 23). These monocytes express almost no mCD14. They cannot be stimulated by low concentrations of endotoxin (11). Yet in the presence of sCD14, they regain their capacity to respond to low endotoxin concentrations (11, 23, 26). The sCD14-dependent cellular response of monocytes from the patient with PNH is completely abolished by anti-CD14 MAb (23). During septic diseases, the expression of mCD14 is also significantly reduced (3, 7, 13, 14, 41, 42).
To study the involvement of mCD14 in elimination capacity, we tested PBMCs from patients with severe sepsis. The severity of the underlying septic disease is demonstrated by the low level of expression of HLA-DR (26.6% ± 5% in patients with sepsis compared with 93.3% ± 2% in healthy volunteers). The expression of mCD14 was also reduced to 53.5% ± 7% (P < 0.01 compared with healthy volunteers). No difference in EECs or levels of binding of FITC-labeled LPS could be seen in patients with sepsis compared to healthy donors. Similarly, the endotoxin-induced release of TNF-α and other proinflammatory cytokines from PBMCs was not changed. Even PBMCs from the patient suffering from PNH during sepsis eliminated exogenously added endotoxin in a manner identical to that of PBMCs from healthy donors and donors with sepsis. The expression of mCD14 was reduced in the former patient to 0.3%, as was expected for PNH, and did not change during recovery from cholecystitis or during health. However, PBMCs derived from the same patient during health could only eliminate 5.1% of the exogenously added endotoxin. During recovery from cholecystectomy, the EEC amounted to 24.6%. The binding of FITC-labeled LPS to monocytes was found to be closely correlated to the EEC. During health, only a very low binding capacity could be established, whereas during recovery from cholecystitis, the binding capacity increased. During recovery from the septic episode induced by an ileus, the binding capacity was found to be the highest.
The fact that the EECs and binding capacities did not change between subjects with sepsis and healthy volunteers despite a decrease in mCD14 expression suggests that mCD14-independent pathways by which LPS is eliminated and signal transduction is mediated do exist. Moreover, the changing EECs and binding capacities of PBMCs and monocytes obtained from a patient suffering from PNH during sepsis, recovery, and health have not been described until now and lead to the conclusion that a mechanism different from mCD14 is induced by sepsis and postoperative acute-phase reaction.
To further elucidate the role of CD14, we studied EECs, binding capacities, and release of cytokines after the addition of MEM-18, a blocking anti-CD14 MAb (4). During health and sepsis, EECs, binding capacities, and endotoxin-induced release of cytokines were almost completely abolished by blocking CD14. No difference in the effects of MEM-18 on PBMCs or monocytes derived from healthy subjects or patients with sepsis was observed. Therefore, it may be concluded that very low amounts of mCD14 are sufficient for mediating the binding or neutralizing processes. However, the results of experiments with cells derived from the PNH patient, which clearly indicate that an inducible mechanism is involved in cellular endotoxin neutralizing and binding capacities, cannot be explained in that way. A further possibility may be the participation of sCD14 in these processes, because MEM-18 has been clearly demonstrated to block the function not only of mCD14 but also that of sCD14. Determination of the sCD14 concentration of the supernatant of PBMCs strongly supports the importance of sCD14. PBMCs of healthy subjects secrete only low amounts of sCD14 spontaneously and after endotoxic stimulation. The spontaneous and stimulated secretion of sCD14 by PBMCs from patients with sepsis was at least five times higher.
Complexes of LPS and sCD14 are considered to play a role as intermediates in the neutralization of LPS (55) with reconstituted high-density lipoprotein. The efficacy of high doses (>2 μg/ml) of recombinant sCD14 was confirmed to improve mice survival in vivo (29) and the inhibition of TNF-α secretion in vitro (28). Grunwald et al. (25) showed that binding of FITC-labeled LPS to bovine monocytes and endotoxin-induced cell activation were abrogated by an exogenously added high dose of sCD14. On the other hand, low amounts of sCD14 (between 10 ng and 1 μg/ml) play a harmful role by transmitting LPS effects in endothelial cells (19) and PNH monocytes (11, 23) and also by sensitizing normal human phagocytes to low endotoxin concentrations (23, 26).
Our study revealed a much higher secretion of sCD14 from PBMCs obtained from patients with sepsis compared with healthy subjects. Supernatant levels of sCD14 were higher than 10 ng/ml after 2 h of incubation and peaked at 20.1 ± 1.8 ng/ml. These concentrations are consistent with previously described optimal levels of LPS-dependent cell activation (23, 26). We also found high levels of sCD14 in plasma from patients with sepsis and the patient with PNH during sepsis, supporting the physiological relevance of an elevated secretion of sCD14. Recent clinical studies demonstrate an elevated concentration of sCD14 in plasma during gram-negative sepsis (38, 39) or during a late stage of a severe burn with existing septicemia (37).
We conclude from our results that mononuclear cells and especially monocytes significantly participate in the elimination process of circulating endotoxin. The elimination process definitely depends on the binding reaction. mCD14 plays a major role in LPS binding, cell activation, and elimination capacity during health. Reduced expression of mCD14 during sepsis can be completely substituted by the enhanced secretion of sCD14, which represents a compensatory mechanism. Under physiological conditions, in the presence of serum and with a small amount of endotoxin, mCD14 or sCD14 represents the main receptor system, mediating not only signal transduction but also the LPS elimination processes of monocytes. With the exclusion of the effect of preexisting anti-inflammatory humoral factors from using mononuclear cells in our experiments, we could find no reduction in the responsiveness of the cells from patients with sepsis compared to normal controls in terms of endotoxin-induced cytokine release. This observation supports the hypothesis that the secretion of sCD14 may be a physiologically important mechanism counteracting cellular events that lead to reduction in responsiveness. The secretion of sCD14 also restores the EEC of monocytes with reduced or even without mCD14 expression under circumstances in which endotoxin may reach the circulation, such as sepsis or during the postoperative period.
ACKNOWLEDGMENTS
We thank V. Horejsi for providing anti-CD14 MAb MEM-18 and A. K. Nussler for providing the hepatoblastoma cell line and reading the manuscript. We thank Mark Racz for checking the English. Furthermore, we are deeply indebted to S. Hauschildt for fruitful discussions during preparation of the manuscript.
REFERENCES
- 1.American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 2.Baumgartner J D, O’Brien T X, Kirkland T N, Glauser M P, Ziegler E J. Demonstration of cross-reactive antibodies to smooth gram-negative bacteria in antiserum to Escherichia coli J5. J Infect Dis. 1987;156:136–143. doi: 10.1093/infdis/156.1.136. . (Erratum, 156:1043.) [DOI] [PubMed] [Google Scholar]
- 3.Bazil V, Horejsi V. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992;149:747–753. [PubMed] [Google Scholar]
- 4.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 5.Berger D, Bolke E, Huegel H, Seidelmann M, Hannekum A, Beger H G. New aspects concerning the regulation of the post-operative acute phase reaction during cardiac surgery. Clin Chim Acta. 1995;239:121–130. doi: 10.1016/0009-8981(95)06105-m. [DOI] [PubMed] [Google Scholar]
- 6.Berger D, Schleich S, Seidelmann M, Beger H G. Demonstration of an interaction between transferrin and lipopolysaccharide—an in vitro study. Eur Surg Res. 1991;23:309–316. doi: 10.1159/000129169. [DOI] [PubMed] [Google Scholar]
- 7.Birkenmaier C, Hong Y S, Horn J K. Modulation of the endotoxin receptor (CD14) in septic patients. J Trauma. 1992;32:473–478. [PubMed] [Google Scholar]
- 8.Burd R S, Battafarano R J, Cody C S, Farber M S, Ratz C A, Dunn D L. Anti-endotoxin monoclonal antibodies inhibit secretion of tumor necrosis factor-alpha by two distinct mechanisms. Ann Surg. 1993;218:250–259. doi: 10.1097/00000658-199309000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey F J, Braude A I, Zalesky M. Studies with radioactive endotoxin. III. The effect of tolerance on the distribution of radioactivity after intravenous injection of Escherichia coli endotoxin labeled with CR51 1. J Clin Invest. 1958;37:441–457. doi: 10.1172/JCI103624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrales I, Weersink A J, Verhoef J, van Kessel K P. Serum-independent binding of lipopolysaccharide to human monocytes is trypsin sensitive and does not involve CD14. Immunology. 1993;80:84–89. [PMC free article] [PubMed] [Google Scholar]
- 11.Duchow J, Marchant A, Crusiaux A, Husson C, Alonso Vega C, De Groote D, Neve P, Goldman M. Impaired phagocyte responses to lipopolysaccharide in paroxysmal nocturnal hemoglobinuria. Infect Immun. 1993;61:4280–4285. doi: 10.1128/iai.61.10.4280-4285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan R L, Jr, Morrison D C. The fate of E. coli lipopolysaccharide after the uptake of E. coli by murine macrophages in vitro. J Immunol. 1984;132:1416–1424. [PubMed] [Google Scholar]
- 13.Ertel W, Krombach F, Kremer J P, Jarrar D, Thiele V, Eymann J, Muenzing S, Faist E, Messmer K, Schildberg F W. Mechanisms of cytokine cascade activation in patients with sepsis: normal cytokine transcription despite reduced CD14 receptor expression. Surgery. 1993;114:243–250. [PubMed] [Google Scholar]
- 14.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler Heitbrock H W. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 15.Flegel W A, Baumstark M W, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low-and high-density lipoproteins and by apolipoprotein A-I. Infect Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox E S, Thomas P, Broitman S A. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96:456–461. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- 17.Freudenberg M A, Freudenberg N, Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982;63:56–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg M A, Kleine B, Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984;6:483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- 19.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallay P, Jongeneel C V, Barras C, Burnier M, Baumgartner J D, Glauser M P, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–5093. [PubMed] [Google Scholar]
- 21.Gegner J A, Ulevitch R J, Tobias P S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995;270:5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- 22.Gessani S, Testa U, Varano B, Di Marzio P, Borghi P, Conti L, Barberi T, Tritarelli E, Martucci R, Seripa D, Peschle C, Belardelli F. Enhanced production of LPS-induced cytokines during differentiation of human monocytes to macrophages. Role of LPS receptors. J Immunol. 1993;151:3758–3766. [PubMed] [Google Scholar]
- 23.Golenbock D T, Bach R R, Lichenstein H, Juan T S, Tadavarthy A, Moldow C F. Soluble CD14 promotes LPS activation of CD14-deficient PNH monocytes and endothelial cells. J Lab Clin Med. 1995;125:662–671. [PubMed] [Google Scholar]
- 24.Gray P W, Flaggs G, Leong S R, Gumina R J, Weiss J, Ooi C E, Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989;264:9505–9509. [PubMed] [Google Scholar]
- 25.Grunwald U, Kruger C, Schutt C. Endotoxin-neutralizing capacity of soluble CD14 is a highly conserved specific function. Circ Shock. 1993;39:220–225. [PubMed] [Google Scholar]
- 26.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 27.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 28.Haziot A, Rong G W, Bazil V, Silver J, Goyert S M. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-alpha production by cells in whole blood. J Immunol. 1994;152:5868–5876. [PubMed] [Google Scholar]
- 29.Haziot A, Rong G W, Lin X Y, Silver J, Goyert S M. Recombinant soluble CD14 prevents mortality in mice treated with endotoxin (lipopolysaccharide) J Immunol. 1995;154:6529–6532. [PubMed] [Google Scholar]
- 30.Heinzelmann M, Mercer Jones M, Cheadle W G, Polk H C. CD14 expression in injured patients correlates with outcome. Ann Surg. 1996;224:91–96. doi: 10.1097/00000658-199607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch R J, Tobias P S, Glauser M P, Baumgartner J D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 32.Heumann D, Gallay P, Betz Corradin S, Barras C, Baumgartner J D, Glauser M P. Competition between bactericidal/permeability-increasing protein and lipopolysaccharide-binding protein for lipopolysaccharide binding to monocytes. J Infect Dis. 1993;167:1351–1357. doi: 10.1093/infdis/167.6.1351. [DOI] [PubMed] [Google Scholar]
- 33.Kang Y H, Dwivedi R S, Lee C H. Ultrastructural and immunocytochemical study of the uptake and distribution of bacterial lipopolysaccharide in human monocytes. J Leukoc Biol. 1990;48:316–332. doi: 10.1002/jlb.48.4.316. [DOI] [PubMed] [Google Scholar]
- 34.Kang Y H, Lee C H, Monroy R L, Dwivedi R S, Odeyale C, Newball H H. Uptake, distribution and fate of bacterial lipopolysaccharides in monocytes and macrophages: an ultrastructural and functional correlation. Electron Microsc Rev. 1992;5:381–419. doi: 10.1016/0892-0354(92)90016-j. [DOI] [PubMed] [Google Scholar]
- 35.Kirkland T N, Finley F, Leturcq D, Moriarty A, Lee J D, Ulevitch R J, Tobias P S. Analysis of lipopolysaccharide binding by CD14. J Biol Chem. 1993;268:24818–24823. [PubMed] [Google Scholar]
- 36.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruger C, Schutt C, Obertacke U, Joka T, Muller F E, Knoller J, Koller M, Konig W, Schonfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landmann R, Reber A M, Sansano S, Zimmerli W. Function of soluble CD14 in serum from patients with septic shock. J Infect Dis. 1996;173:661–668. doi: 10.1093/infdis/173.3.661. [DOI] [PubMed] [Google Scholar]
- 39.Landmann R, Zimmerli W, Sansano S, Link S, Hahn A, Glauser M P, Calandra T. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171:639–644. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 40.Levine D M, Parker T S, Donnelly T M, Walsh A, Rubin A L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin R Y, Astiz M E, Saxon J C, Rackow E C. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expression. Chest. 1993;104:847–853. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- 42.Lin R Y, Astiz M E, Saxon J C, Saha D C, Rackow E C. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit Care Med. 1994;22:1595–1602. . (Erratum, 23:1162, 1995). [PubMed] [Google Scholar]
- 43.Loppnow H, Stelter F, Schonbeck U, Schluter C, Ernst M, Schutt C, Flad H D. Endotoxin activates human vascular smooth muscle cells despite lack of expression of CD14 mRNA or endogenous membrane CD14. Infect Immun. 1995;63:1020–1026. doi: 10.1128/iai.63.3.1020-1026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luchi M, Munford R S. Binding, internalization, and deacylation of bacterial lipopolysaccharide by human neutrophils. J Immunol. 1993;151:959–969. [PubMed] [Google Scholar]
- 45.McCall C E, Grosso Wilmoth L M, LaRue K, Guzman R N, Cousart S L. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest. 1993;91:853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 47.Munford R S, Hall C L. Purification of acyloxyacyl hydrolase, a leukocyte enzyme that removes secondary acyl chains from bacterial lipopolysaccharides. J Biol Chem. 1989;264:15613–15619. [PubMed] [Google Scholar]
- 48.Peterson A A, Munford R S. Dephosphorylation of the lipid A moiety of Escherichia colilipopolysaccharide by mouse macrophages. Infect Immun. 1987;55:974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollack M, Espinoza A M, Guelde G, Koles N L, Wahl L M, Ohl C A. Lipopolysaccharide (LPS)-specific monoclonal antibodies regulate LPS uptake and LPS-induced tumor necrosis factor-alpha responses by human monocytes. J Infect Dis. 1995;172:794–804. doi: 10.1093/infdis/172.3.794. [DOI] [PubMed] [Google Scholar]
- 50.Pugin J, Ulevitch R J, Tobias P S. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993;178:2193–2200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schletter J, Brade H, Brade L, Kruger C, Loppnow H, Kusumoto S, Rietschel E T, Flad H D, Ulmer A J. Binding of lipopolysaccharide (LPS) to an 80-kilodalton membrane protein of human cells is mediated by soluble CD14 and LPS-binding protein. Infect Immun. 1995;63:2576–2580. doi: 10.1128/iai.63.7.2576-2580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobias P S, Soldau K, Gegner J A, Mintz D, Ulevitch R J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 53.Wright S D, Levin S M, Jong M T, Chad Z, Kabbash L G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. . (See comments.) [DOI] [PubMed] [Google Scholar]
- 55.Wurfel M M, Hailman E, Wright S D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu B, Wright S D. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]