Abstract
Climate change poses significant threats to public health, with dengue representing a growing concern due to its high existing burden and sensitivity to climatic conditions. Yet, the quantitative impacts of temperature warming on dengue, both in the past and in the future, remain poorly understood. In this study, we quantify how dengue responds to climatic fluctuations, and use this inferred temperature response to estimate the impacts of historical warming and forecast trends under future climate change scenarios. To estimate the causal impact of temperature on the spread of dengue in the Americas and Asia, we assembled a dataset encompassing nearly 1.5 million dengue incidence records from 21 countries. Our analysis revealed a nonlinear relationship between temperature and dengue incidence with the largest marginal effects at lower temperatures (around 15°C), peak incidence at 27.8°C (95% CI: 27.3 – 28.2°C), and subsequent declines at higher temperatures. Our findings indicate that historical climate change has already increased dengue incidence 18% (12 – 25%) in the study region, and projections suggest a potential increase of 40% (17 – 76) to 57% (33 – 107%) by mid-century depending on the climate scenario, with some areas seeing up to 200% increases. Notably, our models suggest that lower emissions scenarios would substantially reduce the warming-driven increase in dengue burden. Together, these insights contribute to the broader understanding of how long-term climate patterns influence dengue, providing a valuable foundation for public health planning and the development of strategies to mitigate future risks due to climate change.
Introduction
Anthropogenic climate change is a major health threat that is already causing significant morbidity, mortality, and economic loss through its effects on biological processes and ecological systems1,2. Describing the causal relationship between climate and biological processes is necessary to anticipate and respond to health hazards and attribute harms to fossil fuel emissions as part of climate accountability and justice efforts3,4,5. Changing temperatures and increasingly frequent extreme weather events are generally expected to drive changes in the global burden and distribution of infectious diseases6. However, the implications of warming for vector-borne diseases remain particularly unclear and are often difficult to estimate compared to other climate-associated risks due to data sparsity and confounding by climate-independent factors7,8,9.
By building from a biological understanding of the temperature sensitivity of ectothermic mosquito vectors, we can better understand the relationship between temperature and cases of vector-borne diseases that pose particularly significant health threats10. Prior experimental work has measured transmission-relevant mosquito traits (e.g., biting rate, survival, development time) across a temperature gradient and used these values to estimate relative R0, a measure of transmission intensity, as a function of temperature11,12. Across mosquito-borne diseases, we expect a nonlinear and unimodal relationship between temperature and transmission11,13. Dengue, vectored by Aedes aegypti and Aedes albopictus, is expected to present a growing risk with warming temperatures relative to other vector-borne diseases due to its particularly warm thermal optimum (29 and 26°C for the two mosquito vectors, respectively)14,15,16. However, relative transmission risk metrics cannot be translated directly into cases, morbidity, and mortality, the outcomes most relevant to quantifying health costs of climate change, because of complexities and nonlinearities of infectious disease dynamics and the potential for additional covariates to modulate the relationship17,18. We therefore aim to directly estimate the quantitative relationship between cases and temperature in the field over space and time, while controlling for potentially confounding variation in other factors.
In addition to considering temperature, studies to estimate drivers of disease burden may incorporate several additional covariates including El Niño Southern Oscillation (ENSO), urban infrastructure, age structure, human mobility, and immunity (based on past exposure)19,20,21,22,23,24. Several other covariates have been identified as important and potential confounders in existing analyses (e.g., vector control, serotype changes, and global trade and migration), but many of these variables are difficult to measure and report in a standardized manner19,25,26. The field of applied econometrics has developed a set of causal inference tools for quantifying causal effects of hypothesized drivers in complex systems where randomized-controlled trials are impractical or unethical (such as for estimating large-scale impacts of climate change). These tools, called panel regression, allow us to account for spatiotemporal variation in unmeasured covariates and focus on the relationship between anomalies in both temperature and dengue cases6. Further, because deviations in temperature from the average conditions in a given space and time are plausibly random, we can interpret our result as a causal estimate27.
Previous work has addressed impacts of temperature on dengue transmission, but has not been able to conclusively quantify the relationship at a large scale. Statistical models have generally considered dengue across smaller geographic areas, either within a single geographic region (e.g., a district, island, or city)28,23,29 or across multiple administrative subunits (e.g., municipalities or districts) within a single country or province30,31,19. The relationship between weather and disease may not be consistent globally. For example, a meta-analysis found that population density, precipitation, and prior disease burden (and resulting immunity) may modulate the reported correlation between dengue and temperature32, and urban infrastructure (particularly water supply) may alter the effects of precipitation and drought24. We therefore examine whether covariates like average dengue incidence, continent, health expenditure, and population density significantly moderate the relationship between dengue and temperature.
After deriving a causal estimate of the relationship between dengue and temperature and determining that it is robust across regions with endemic transmission in East Asia and Central and South America, we apply our model to attribute historical dengue burden to existing climate change–one of the first vector-borne disease climate attribution studies ever done–and to forecast how dengue burden may change by mid-century under different emissions scenarios5,33. The distribution of dengue is generally expected to expand while the burden increases under climate change, but prior studies have focused on more limited geographic regions26,34,35. By building from a nonlinear dengue-temperature relationship that is well-supported by previous mechanistic experimental work and leveraging a global dataset encompassing a large temperature gradient, we expect to find both increases in dengue in regions where temperature warms toward the thermal optimum and declines in warmer tropical regions where the thermal optimum for transmission is exceeded36,37.
Here, we assess the impact of temperature on dengue in the Americas and Asia. Specifically, we (i) capture the causal nonlinear relationship between temperature and dengue incidence, (ii) assess the impact of socioeconomic and environmental covariates on this relationship, and (iii) estimate the change in dengue incidence due to historical and future warming. To do so, we assembled a dataset of dengue incidence, climate, and covariates (Fig. 1). We then use future climate scenarios and our temperature-dengue response function to project future changes in incidence.
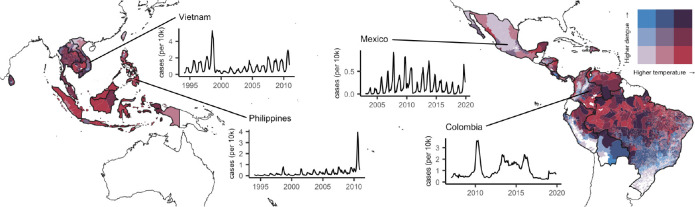
Figure 1: Subnational data on dengue and temperature from 21 countries.
Darker red indicates warmer temperatures and darker blue indicates higher incidence of dengue. Insets show four examples of epidemic dynamics in different countries.
Results
Thermal sensitivity of dengue
We find that dengue incidence responds nonlinearly to temperature, increasing up to a peak at 27.8°C(95% CI: 27.3 – 28.2) and declining at higher temperatures (Fig. 2). This dengue - temperature response corresponds to a marginal effect of temperature that is highest at low temperatures (15–20°C), declines to zero at 27.8 °C, and becomes negative at higher temperatures. The general shape and nonlinearity of this relationship is consistent across a range of alternative model specifications, including higher degree polynomials, varied lags of temperature, alternative fixed effects and weightings, and removing Brazil, which makes up 74% of the spatial units in our dataset (Fig. 2, Fig. S4). These results are consistent with previous mechanistic models based on laboratory-derived mosquito thermal performance curves, which predicted maximum transmission at 29°C for Ae. aegypti and 26°C for Ae. albopictus 14.
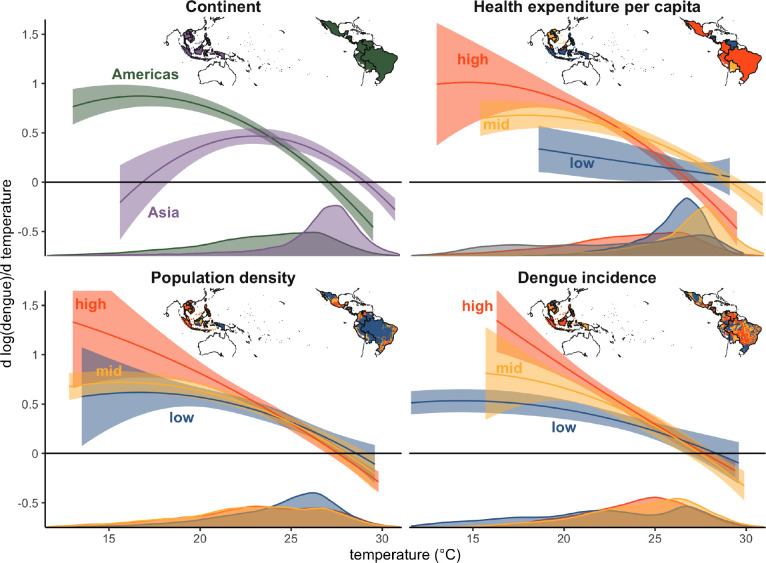
Figure 2: Effect of temperature on dengue.
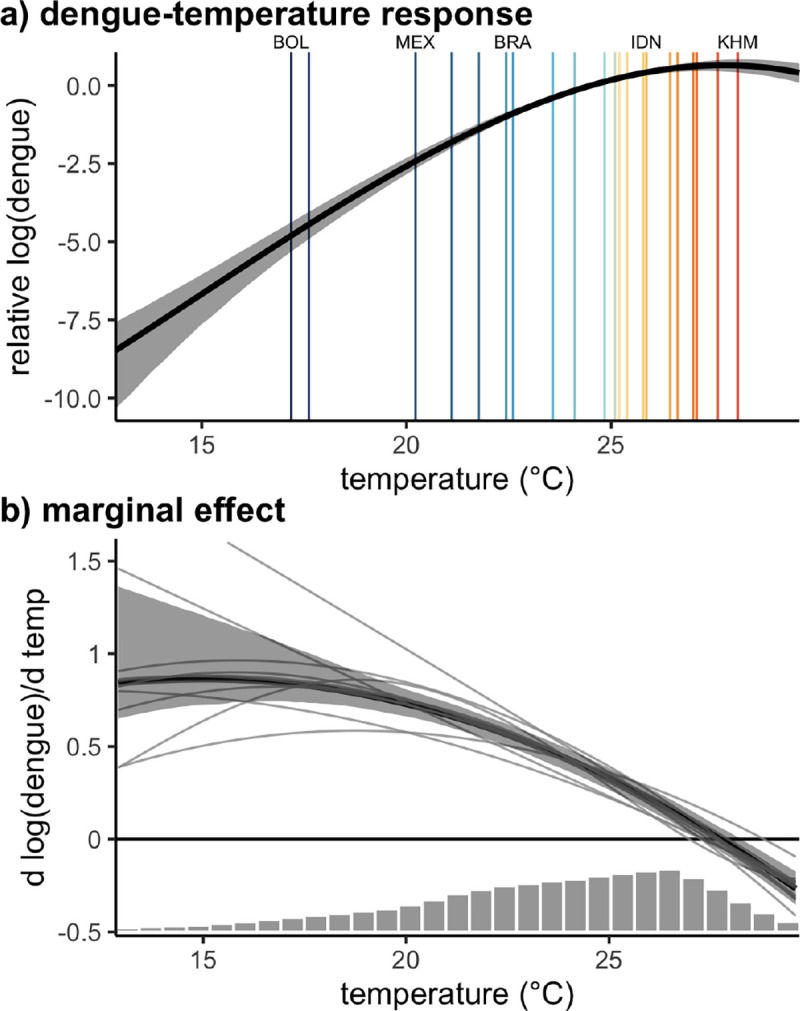
(a) Global nonlinear relationship between dengue cases and temperature and (b) the slope of that relationship indicating the marginal effect of temperature on dengue incidence. Main panel regression model fit in black with 95% confidence interval in gray shading. Vertical lines in (a) indicate country mean temperatures, with labels highlighting the coldest and warmest countries as well as the three highest population countries in the sample: Bolivia (BOL), Mexico (MEX), Brazil (BRA), Indonesia (IDN), and Cambodia (KHM). Thin gray lines in (b) represent variations on the main model using alternative specifications. Histogram in (b) shows the distribution of observed monthly temperatures. Model estimates are restricted to the 1st to 99th percentiles of the observed temperature distribution.
The impacts of temperature changes on dengue incidence are likely to be heterogeneous based on existing conditions, including serotype dynamics in the location, population immunity levels from previous exposure, availability of vector breeding habitat, the dominant vector species with Ae. aegypti having a slightly warmer temperature optimum, living conditions and exposure to vectors, and public health response. We focused on investigating whether the thermal sensitivity of dengue incidence varied spatially over large continental regions, with country health expenditure, with population density, and with average dengue incidence. We found the largest variation in thermal responses between continental regions, and smaller variations by health expenditure, population density, and existing dengue incidence (Fig. 3). For all responses, we see larger positive marginal effects at low temperatures, a decline to zero around 27–30 °C, and negative marginal effects at higher temperatures. The temperature responses differed slightly in the exact optimum temperature and the magnitude of the marginal impacts at low temperatures. The temperature response for Asia displayed a slightly higher optimum temperature than the Americas, and smaller marginal impacts at low temperatures, although support in the lower temperature range was limited for Asia given the observed temperature distribution. For health expenditure, we originally hypothesized that higher expenditure might enable countries to better limit transmission resulting in lower marginal effects of temperature, but instead found slightly larger impacts in higher health expenditure countries, although confidence intervals overlapped. For population density, we similarly see overlapping estimates among all terciles, although slightly higher median estimates for high population density follow by intermediate and low population density, consistent with higher transmission rates in high density areas. Finally, for dengue incidence, one possibility was that higher incidence locations might have more immunity and more health infrastructure for dealing with dengue transmission resulting in lower marginal impacts, while a competing hypothesis was that places with higher incidence might have more interactions between serotypes and thus more severe cases of dengue likely to be reported as well as the other environmental and socioeconomic condition conducive to transmission so more suitable temperature would lead to larger marginal effects. Again we see overlapping confidence intervals between different terciles of dengue incidence, but some evidence supporting the second set of hypotheses.
Figure 3: Spatial heterogeneity in the dengue-temperature relationship.
Marginal effect of temperature on dengue incidence by (a) continental region, (b) health expenditure tercile, (c) population density tercile, and (d) dengue incidence tercile. Dengue incidence and population density are subnational covariates, and continent and health expenditure are country-level covariates. Lines are the mean estimates and shaded areas are 95% confidence intervals, with estimated marginal effects trimmed to the 1st to 99th percentiles of the observed temperature distribution for each tercile or continental region. Where relevant, confidence intervals were truncated for visibility. Density plots show the distribution of monthly temperatures for each tercile or continental region and inset maps depict the spatial units included in each tercile for the different covariates, with colors matching those of the estimated marginal effects in each panel.
Historical and future impacts of climate change
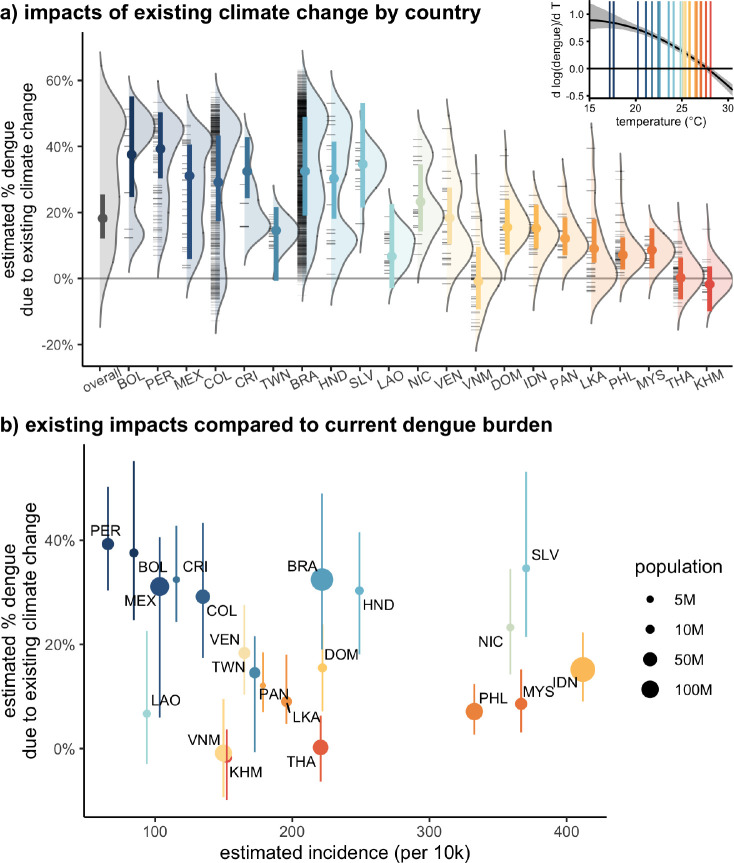
To quantify the impact of existing climate change, we estimate the change in dengue incidence under observed temperatures for 1995–2014 compared to a counterfactual of global circulation models (GCMs) without anthropogenic forcing. We estimate that on average over the locations included in the study, 18% (95% CI: 12 – 25%) of existing dengue incidence is due to anthropogenic climate change already (Fig. 4, Table S3). The estimates vary both within and between countries, with cooler areas in Bolivia, Peru, Mexico, Brazil, and Colombia seeing 30–40% of existing dengue from climate change and warmer countries like Thailand and Cambodia having little impact from existing warming. These impacts equate to a large number of dengue cases and affected populations: while some of these cooler countries where a larger portion of existing dengue is due to climate change have relatively low current dengue burdens, other countries like Brazil, El Salvador, and Indonesia with intermediate average temperatures (22–26°C) have high current burdens of dengue and 10–30% of that existing burden is estimated to be due to climate change (Fig. 4).
Figure 4: Climate change has already contributed to increases in dengue.
(a) Distributions show estimated median percent of the dengue burden that is attributable to anthropogenic climate warming across administrative units within each country, estimated as the average % change between observed and counterfactual climate from 1995–2014. Black tick marks indicate individual unit median values, and colored points and bars show the median and 95% CI of the population-weighted average estimate by country. Countries are ordered by current average temperatures with warmer countries to the right. Only administrative units with reported dengue are included in the distributions and country averages. Inset: marginal effect of temperature on dengue from the main model specification, with country-average temperatures indicated with vertical lines matching the colors in the main panel. (b) Climate change to date has already increased dengue transmission. These impacts are largest in cooler countries where current incidence is low, but impacts are also substantial in moderate temperature countries with high current dengue burdens (e.g., Brazil, Honduras, El Salvador, and Nicaragua). Point colors (indicating temperature) are the same in (a) and (b) and point sizes in (b) indicate population size. Line ranges are 95% CIs as in (a).
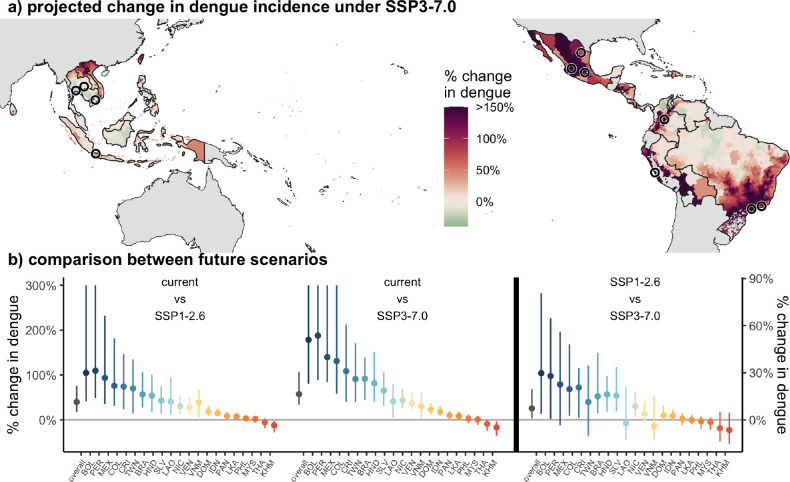
Future warming will also play a role in determining dengue incidence. On average in the study region, we predict a 57% (95% CI: 33 – 107%) increase in dengue incidence under SSP3–7.0. While some low elevation equatorial areas will warm beyond the peak temperature and are projected to see small declines in dengue incidence due to climate change, the majority of locations are projected to see increases in dengue incidence under all climate scenarios (Fig. 5, Fig. S5). Some cooler regions of Mexico, Peru, Bolivia, and Brazil are predicted to see over 150% increases in dengue incidence due to climate change under all climate scenarios. Many of the largest cities in the Americas are located in these cooler regions where large increases in dengue are projected. Among the 21 countries included in the study, only one country (Cambodia) is projected to see declines in dengue incidence under all three climate scenarios while 17 countries are projected to see increases under all scenarios. Despite the estimated increases in dengue in the majority of locations even under the most optimistic (low emissions) scenario (SSP1–2.6, Fig. S5), we find that these increases would be on average 7% (95%CI: 1 – 20%) larger under the high emissions scenario (SSP3–7.0), with some countries seeing up to 30% greater increases in dengue incidence in SSP3–7.0 compared to SSP1–2.6 (Fig. 5b, Table S3). These estimates are largely similar when using continent-specific temperature responses, which showed the greatest difference between temperature responses, with the largest differences in projected impacts in Asia where no countries are predicted to have see significant declines in dengue under future climate scenarios due to the slightly higher estimated temperature where dengue incidence peaks (Fig. S6).
Figure 5: Estimated impacts from climate change for 2040–2059 are widespread and largest in more temperate regions, but few places will become too warm.
(a) Most locations are expected to see an increase in dengue under climate change, with a small fraction of locations seeing slight declines due to temperatures exceeding the current predicted optimal temperature. Many of the areas where large increases are predicted (shown in dark red), especially in the Americas, are areas with large cities and high population density. Black circles show cities over 5M in population. Administrative units are colored by the median percent change in dengue incidence under the high emissions scenario at mid-century (SSP3–7.0 in 2040–2059) compared to current temperatures (1995–2014). (b) Across all future climate scenarios, dengue incidence is predicted to increase for a majority of countries, with the largest increases in cold countries (left panel), and these impacts are estimated to be up to 30% larger under the highest emissions scenarios compared to the lowest (right panel). Countries retain ordering by average temperature, and country colors are consistent across all figures. Points show median estimates and lines show 95% CIs. Confidence intervals are truncated to 300% for visibility.
Discussion
A wide range of existing literature documents that temperature affects dengue transmission32,14,18,38, but quantifying the exact nature of this relationship has been challenging due to the confounding effects of multiple other drivers19,24,26. Yet, understanding this relationship precisely is critical for projecting impacts under climate change to better anticipate future changes and design adaptations to meet them. Here, we aim to comprehensively quantify the temperature-dependence of dengue transmission using human case data. We find clear nonlinear effects, consistent with the thermal biology of the vectors11. Increases in temperature have the largest marginal impact at low temperatures (15–20°C) and become negative at very high temperatures (¿27.8°C), allowing us to identify geographic regions where temperature change is likely to have the largest impact.
Based on these marginal effects of temperature, we estimate that 18% of the current dengue burden in the study region is due to increases in temperature from existing climate change, and up to 40% of dengue incidence due to climate change in some cooler countries. Projecting forward, we expect the impacts of climate change to be even larger by the mid-century, with 40 – 57% increases in dengue incidence overall in the study region depending on the scenario. While dengue is expected to increase under all climate scenarios, climate mitigation still has large benefits, with estimated increases in incidence 7% smaller overall under the lowest emissions scenario, and some countries seeing 30% smaller increases.
We project that most areas will see increases in dengue transmission due to climate change in contrast to malaria, which is projected to decline in sub-Saharan Africa by mid-century due to climate change-driven temperature increases39. These divergent impacts are consistent with the differences in thermal biology between malaria and dengue, with malaria predicted to have a cooler optimum temperature around 25°C compared to the 27.8°C optimum inferred here for dengue11. Relative to other health impacts of climate change, especially direct heat-related mortality, which is predicted to increase the most in already warm regions40, the impacts on dengue are projected to be largest in relatively cool regions while the hottest regions in our study are projected to see declines in dengue incidence due to climate change (Fig. 5). In fact, our study likely underestimates these future warming-driven dengue increases in cool areas as many such regions do not yet have consistent dengue transmission and/or reporting, and thus are not included in our dataset.
While our results highlight the benefit of climate mitigation in reducing the projected increases in dengue incidence, the projected increases under all scenarios suggest that climate adaptation will also be necessary even in the best case (Fig. 5). Moreover, the consistency of the estimated dengue-temperature relationship across places with both high health expenditures and high existing dengue incidence (Fig. 3) suggests that even the locations most likely to have existing public health systems able to withstand the impacts of temperature are still strongly affected.
Although this work presents the most globally comprehensive (spanning 21 countries), quantitative estimate of temperature impacts on dengue to date, our estimates are constrained by the set of locations with available sub-annual, sub-national dengue data, which are primarily located in tropical areas of the Americas and southeast Asia. The study omits both other tropical areas with endemic dengue (notably south Asia and sub-Saharan Africa, Fig. S1) and cooler temperate regions that do not have consistent dengue transmission. As a result of the latter, our estimates focus on the impact of temperature changes in intensification of dengue incidence rather than invasion of dengue into new regions. Taken together, this suggests that our estimated change in dengue case from climate change will be conservative due to the omitted dengue-endemic regions and the temperate regions where dengue transmission occurs sporadically and could expand (including recently in California, Texas, and Florida, USA). Some of the excluded areas may be at or above the optimum temperature for dengue transmission and see declines in dengue incidence due to climate change, but the majority will see increases with future warming. In addition to focusing on endemic regions, our projections center on the effect of changes in monthly average temperature rather than any of the myriad of other impacts expected with climate change, including altered precipitation patterns, changes in temperature variability, increased extreme weather events, and behavioral adaptation to climate change. Further, our temperature response is estimated from real dengue dynamics in the field, and in doing so, implicitly incorporates complexities like serotype dynamics, vector control, policy, and population growth and mobility. Our projections assume that these other factors will not change in a way that alters the estimated dengue-temperature response (i.e., in a way that is interactive with temperature). Finally, our projections focus solely on temperature’s impacts on dengue incidence, and the landscape of dengue transmission in the future will also be impacted by a range of other factors including urbanization, migration, the emergence of new serotypes, and/or potential medical advances in treating or preventing dengue in a way that could either reduce or exacerbate the future temperature effects.
Our study contributes in several important ways to the growing body of literature attributing climate impacts on health5. First, it makes quantitative predictions for how dengue burden will change under climate warming at the local, sub-national scale across a major swath of its endemic range, which can be used to develop targeted public health planning and responses and compare the consequences of different emissions scenarios. Second, it is among the first studies to attribute changes in infectious disease to climate change—expanding on the attribution literature centered on more direct effects such as heat waves, storms, and fires—providing a road map for future studies on other ecological and health impacts33,41,39. Third, this work provides a rare demonstration that theoretical models based on laboratory experiments can capture the thermal biology of complex infectious disease systems, reinforcing the idea that such models can be used to predict climate responses in places with limited existing data. Finally, attribution studies like this one are increasingly used in litigation aimed at holding governments and fossil fuel companies financially accountable for negative societal effects of carbon emissions due to climate change.
Methods
Dengue Case Data
To obtain a comprehensive global dataset of dengue cases at the sub-annual and sub-national scale, we searched the Pan-American Health Organization (PAHO) and Project Tycho databases, Ministry of Health websites, and published literature. For Project Tycho, we selected “Dengue” for condition, “Month” as the interval type, and downloaded data for any country with >2 years of data at admin level 1 (state or province) or below. For all countries with endemic cases listed on the WHO website, we found their Ministry of Health (or equivalent) website and navigated to any relevant tabs or databases reporting health data. If we still could not locate relevant data, we searched the following on Google: “[country name] immunological bulletin OR dengue”. Additionally, we searched the literature using the same query for references to data sources; however, these were typically not publicly available. We found 25 datasets from 21 countries that span an average of 11 years (Table S1). We excluded datasets that spanned less than 2 years and trimmed our data to the end of 2019 to avoid confounding effects of COVID-19. We aggregated weekly data to monthly as follows: if a week spanned two months we split the cases into months based on the number of days within that week that fell into each month. To obtain incidence from the raw case counts, population size was obtained by summing population count within each administrative boundary (see “Subnational boundaries”) in the midyear of the time series, using population counts from WorldPop42 on available on Google Earth Engine43. In total, our dataset consisted of roughly 1.5 million monthly observations of dengue incidence at the first or second administrative level.
Subnational boundaries
We matched the names of the subnational administrative units with those in shapefiles downloaded from the Humanitarian Data Exchange44 except for Taiwan, which was downloaded separately45. Because case data in Costa Rica is reported for socioeconomic regions, which does not fall cleanly in administrative level 1 or 2, a shapefile of administrative boundaries within Costa Rica was manually modified to correspond to socioeconomic regions. We used the district (administrative level 3) shapefile and aggregated to six socioeconomic regions based on a mapping from canton (administrative level 2) to socioeconomic region46. Four districts (admin level 3) in socioeconomic regions different from the rest of their cantons are switched to the correct socioeconomic region.
Historical temperature data
To obtain downscaled daily temperature values with consistent performance across regions, we compared ERA5 daily climate reanalysis product47 to Global Historical Climatology Network (GHCN) station observations in the countries included in this study. We found that ERA5 temperatures were on average downward biased relative to GHCN station observations, with large (up to 10°C) negative biases at high elevations (Fig. S7). To avoid this differential bias in the ERA5 product, we debias using WorldClim, a high resolution climatology product with monthly average temperature from 1970 – 200048:
| (1) |
where is the debiased daily temperature, is observed ERA5 temperature in location on day in month and year is the month- and location- specific ERA5 climatology over 1970 – 2000 and is the WorldClim climatology over the same time period. We calculate from monthly average temperature in ERA5 monthly products, and use from the daily average temperature product available on Google Earth Engine43. We find that this correction reduces the mean error between satellite-based temperature estimates and ground-based measurements from the GHCN, especially at higher elevation sites (Fig. S7). We calculate higher powers of debiased temperature data for each day and grid cell before calculating administrative unit - month population-weighted averages using the subnational boundaries described above and WorldPop population estimates42. We use the same population-weighted average approach to calculate monthly average precipitation from ERA5.
Climate scenario temperature projections
To estimate the change in dengue transmission under future climate change, we use projected temperature from the Coupled Model Intercomparison Project Phase 6 (CMIP6)49 under different scenarios. In keeping with recommendations from the latest IPCC report50 and Hausfather et al.51, we use SSP3–7.0 as a high baseline emissions scenario, and SSP2–4.5 and SSP1–2.6 as medium and low emissions scenarios, respectively. We also consider the historical-natural projections as our counterfactual for historical temperature absent anthropogenic forcing to understand how climate change has already impacted dengue transmission. Of the 39 global climate models (GCMs) available from the CMIP6, we follow recent guidance on excluding “hot models” and limit to models with transient climate response (TCR) in the likely range (1.4 – 2.2°C)51 using existing TCR calculations for the GCMs52. We further limit to models that have monthly temperature available for projections for all three future climate scenarios specified above, resulting in 22 GCMs included in this analysis. A full list of included models and scenarios can be found in Supplementary Table S2.
Given known biases in average temperatures and unreliable daily temperature anomalies in GCMs53,54 we use the delta change method55 to calculate temperature under future climate scenarios as follows. We determine the estimated change in temperature from the current period to the mid 21st century as
| (2) |
for location , month of the year , model , variant and scenario , where is the average of monthly temperatures within a specified period (2040 – 2059 for future scenarios, 1995 – 2014 for the historical-natural scenario) and is the average from the historical scenario for 1995 – 2014. We match variants between scenarios for each GCM when calculating , defaulting to the first variant available for all of the desired scenarios run for a GCM, or if no variant is available for all scenarios, a single variant for future scenarios and a separate variant for the historical-natural scenario. Details on variants used for each GCM and scenario are in Supplementary Table S2.
To calculate estimated temperature under different scenarios, we then add the estimated temperature change to debiased daily temperature data , and then aggregate to monthly temperatures as described above. To compare scenarios, we use observed temperatures from 1995 – 2014 for all locations.
Data extraction for moderators
For each country, health expenditure per capita expressed in international dollars at purchasing power parity in 2010 was from WHO Global Health Expenditure Database was downloaded from World Bank Open Data56. For the Gini index and health expenditure, we selected the year that is the average of all time series midpoints (2010). If this was missing, we took health expenditure from the closest date. We calculated population density using total population from WorldPop42 (as described above) and diving by the area of the administrative unit. To estimate average dengue incidence at the administrative units, we calculate annual average dengue incidence in the our case data, and scale by the ratio between observed country-level average annual dengue incidence in the case data and country-level incidence estimates (1990 – 2017) from the Global Burden of Diseases57 to account for potential differences in disease detection rates between countries while still allowing for variation in dengue incidence within countries.
Estimating dengue-temperature responses
To obtain an overall estimate of temperature dependence on dengue we used a panel regression. Our model takes the form
| (3) |
where is unit, is country, is month, and is year. This model includes an administrative unit fixed effect to control for differences between locations that are consistent over time, a country-year fixed effect to account for country-level patterns over time, and country-month fixed effect to remove seasonal patterns in dengue and temperature. The main specification included population weighting and used cubic polynomials of temperature with 1–3 months of lags, but we also considered functional forms with up to degree 5 and lags from 0 to 4 months. We also tested sensitivity of model estimates to including quadratic effects of precipitation rather than linear, no weighting of estimates, removing Brazil from the sample, fixed effects for country - month - year rather than country - month and country - year, and fixed effects for unit - month rather than country - month (Fig. S4). All models were run as Poisson regressions with the ‘fixest’ package in R58. For countries that had data from multiple sources, we removed any duplicate years from the earlier data source and treated each country-data source as a different “country” for the purpose of fixed effects to allow for potentially different reporting rates between data sources.
To understand how this relationship varies spatially, we interacted the estimated temperature relationship with administrative unit-level covariates including continental region, health expenditure, population density, average dengue incidence:
| (4) |
where is the covariate value for unit in country . For continent we divided into Asia and the Americas and for the remaining covariates, we split into terciles with health expenditure terciles being defined at the country-level due to availability of health expenditure data, and population density and dengue incidence terciles defined at the administrative-units. We defined these terciles only accounting for administrative units that reported dengue during the study.
We calculate confidence intervals on estimated temperature responses and marginal effects using stratified bootstraps and analytic confidence intervals based on the Delta method. Stratified bootstraps were conducted using countries as the strata, and sampling administrative units with each country with replacement, then using the full time series for each sampled administrative unit in the bootstrap sample. Bootstrapped confidence intervals were only slightly wider than analytic confidence intervals calculated with the delta method (Fig. S4, so alternative model specifications and models with heterogeneous effects are shown with analytic confidence intervals, while main model estimates and continent-specific estimates used in projections are bootstrapped.
Attributing existing dengue burden and projecting future impacts
Using the estimated temperature relationship , we calculate the change in dengue incidence due to temperature changes as
| (5) |
where indexes the scenario of interest (SSP1–2.6, SSP2–4.5, SSP3–7.0, historical-natural forcing). We use debiased monthly ERA5 data for observed temperatures and monthly climate projections (described above) for scenario temperatures. We estimate the percent change in dengue for the specified GCMs (22 for future scenarios, 10 for historical - natural forcing, see Table S2) using 50 of the bootstrapped estimated temperature response to incorporate uncertainty from both the model estimates and the climate scenarios. For each GCM and bootstrap, we calculate the average percent change in dengue over the 20 years of temperature data for each administrative unit. To estimate administrative unit-specific effects, we then calculate medians and 95% confidence intervals over 1100 estimates from the GCMs and bootstraps. Similarly, to estimate country-level and overall effects, for each bootstrap and GCM we calculate a population-weighted average of the administrative unit averages, then calculate the median and 95% CI over the 1100 estimates for each scenario. To compare the between two future climate scenarios (particularly between SSP1–2.6 and SSP3–7.), we use the scenario temperatures in equation 5 instead of observed temperatures. We project future impacts using both the main model bootstrapped estimates and the continent-specific estimates.
Supplementary Material
Acknowledgements
Some of the computing for this project was performed on the Sherlock cluster, and we would like to thank Stanford University and the Stanford Research Computing Center for providing computational resources and support that contributed to these research results. We would also like to acknowledge computational resources from Google Cloud for Earth Engine. We acknowledge the World Climate Research Programme, which, through its Working Group on Coupled Modelling, coordinated and promoted CMIP6. We thank the climate modeling groups for producing and making available their model output, the Earth System Grid Federation (ESGF) for archiving the data and providing access, and the multiple funding agencies who support CMIP6 and ESGF. MLC was supported by the Illich-Sadowsky Fellowship through the Stanford Interdisciplinary Graduate Fellowship program at Stanford University and by an Environmental Fellowship at the Harvard University Center for the Environment. KPL was supported by the NSF Postdoctoral Research Fellowships in Biology Program under Grant No. 2208947. MJH was supported by the Achievement Rewards for College Scientists Scholarship and the National Institutes of Health (R35GM133439). EAM was supported by the National Institutes of Health (R35GM133439, R01AI168097, R01AI102918), the National Science Foundation (DEB-2011147, with Fogarty International Center), and the Stanford Center for Innovation in Global Health, King Center on Global Development, and Woods Institute for the Environment.
Data availability
Data is available upon request and code to replicate all results in the main text and supplementary materials will be made available at https://github.com/marissachilds/global-dengue-temperature.
References
- [1].Knowlton Kim, Rotkin-Ellman Miriam, Geballe Linda, Max Wendy, and Solomon Gina M.. Six Climate Change–Related Events In The United States Accounted For About $14 Billion In Lost Lives And Health Costs. Health Affairs, 30(11):2167–2176, November 2011. ISSN 0278–2715, 1544–5208. doi: 10.1377/hlthaff.2011.0229. URL 10.1377/hlthaff.2011.0229. [DOI] [PubMed] [Google Scholar]
- [2].Watts Nick, Adger W. Neil, Agnolucci Paolo, Blackstock Jason, Byass Peter, Cai Wenjia, Chaytor Sarah, Colbourn Tim, Collins Mat, Cooper Adam, Cox Peter M., Depledge Joanna, Drummond Paul, Ekins Paul, Galaz Victor, Grace Delia, Graham Hilary, Grubb Michael, Haines Andy, Hamilton Ian, Hunter Alasdair, Jiang Xujia, Li Moxuan, Kelman Ilan, Liang Lu, Lott Melissa, Lowe Robert, Luo Yong, Mace Georgina, Maslin Mark, Nilsson Maria, Oreszczyn Tadj, Pye Steve, Quinn Tara, Svensdotter My, Venevsky Sergey, Warner Koko, Xu Bing, Yang Jun, Yin Yongyuan, Yu Chaoqing, Zhang Qiang, Gong Peng, Montgomery Hugh, and Costello Anthony. Health and climate change: policy responses to protect public health. The Lancet, 386(10006):1861–1914, November 2015. ISSN 0140–6736, 1474-547X. doi: 10.1016/S0140-6736(15)60854-6. URL https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)60854-6/fulltext. Publisher: Elsevier. [DOI] [PubMed] [Google Scholar]
- [3].Limaye Vijay S., Max Wendy, Constible Juanita, and Knowlton Kim. Estimating The Costs Of Inaction And The Economic Benefits Of Addressing The Health Harms Of Climate Change: Commentary describes illuminates the costs of inaction on the climate crisis and the economic savings of addressing this problem. Health Affairs, 39(12):2098–2104, December 2020. ISSN 0278–2715, 1544–5208. doi: 10.1377/hlthaff.2020.01109. URL 10.1377/hlthaff.2020.01109. [DOI] [PubMed] [Google Scholar]
- [4].Patz Jonathan A., Gibbs Holly K., Foley Jonathan A., Rogers Jamesine V., and Smith Kirk R.. Climate Change and Global Health: Quantifying a Growing Ethical Crisis. EcoHealth, 4(4):397–405, December 2007. ISSN 1612–9202, 1612–9210. doi: 10.1007/s10393-007-0141-1. URL 10.1007/s10393-007-0141-1. [DOI] [Google Scholar]
- [5].Ebi Kristie L., Aström Christofer, Boyer Christopher J., Harrington Luke J., Hess Jeremy J., Honda Yasushi, Kazura Eileen, Stuart-Smith Rupert F., and Otto Friederike E. L.. Using Detection And Attribution To Quantify How Climate Change Is Affecting Health. Health Affairs, 39(12):2168–2174, December 2020. ISSN 0278–2715. doi: 10.1377/hlthaff.2020.01004. URL 10.1377/hlthaff.2020.01004. Publisher: Health Affairs. [DOI] [PubMed] [Google Scholar]
- [6].Metcalf C. Jessica E., Walter Katharine S., Wesolowski Amy, Buckee Caroline O., Shevliakova Elena, Tatem Andrew J., Boos William R., Weinberger Daniel M., and Pitzer Virginia E.. Identifying climate drivers of infectious disease dynamics: recent advances and challenges ahead. Proceedings of the Royal Society B: Biological Sciences, 284(1860): 20170901, August 2017. ISSN 0962–8452, 1471–2954. doi: 10.1098/rspb.2017.0901. URL 10.1098/rspb.2017.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alcayna Tilly, Fletcher Isabel, Gibb Rory, Tremblay Léo, Funk Sebastian, Rao Bhargavi, and Lowe Rachel. Climate-sensitive disease outbreaks in the aftermath of extreme climatic events: A scoping review. One Earth, 5(4):336–350, April 2022. ISSN 2590–3322. doi: 10.1016/j.oneear.2022.03.011. URL https://www.sciencedirect.com/science/article/pii/S2590332222001440. [DOI] [Google Scholar]
- [8].Altizer Sonia, Ostfeld Richard S., Johnson Pieter T. J., Kutz Susan, and C. Drew Harvell. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science, 341(6145):514–519, August 2013. ISSN 0036–8075, 1095–9203. doi: 10.1126/science.1239401. URL 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- [9].Ogden Nick H., Mechai Samir, and Margos Gabriele. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Frontiers in Cellular and Infection Microbiology, 3, 2013. ISSN 2235–2988. doi: 10.3389/fcimb.2013.00046. URL . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brady Oliver J. and Hay Simon I.. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annual Review of Entomology, 65(1):191–208, January 2020. ISSN 0066–4170, 1545–4487. doi: 10.1146/annurev-ento-011019-024918. URL 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- [11].Mordecai Erin A., Caldwell Jamie M., Grossman Marissa K., Lippi Catherine A., Johnson Leah R., Neira Marco, Rohr Jason R., Ryan Sadie J., Savage Van, Shocket Marta S., Sippy Rachel, Ibarra Anna M. Stewart, Thomas Matthew B., and Villena Oswaldo. Thermal biology of mosquito-borne disease. Ecology Letters, 22(10):1690–1708, October 2019. ISSN 1461–023X, 1461–0248. doi: 10.1111/ele.13335. URL 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mordecai Erin A., Paaijmans Krijn P., Johnson Leah R., Balzer Christian, Ben-Horin Tal, de Moor Emily, McNally Amy, Pawar Samraat, Ryan Sadie J., Smith Thomas C., and Lafferty Kevin D.. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters, 16(1):22–30, January 2013. ISSN 1461023X. doi: 10.1111/ele.12015. URL 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- [13].Shocket Marta S, Verwillow Anna B, Numazu Mailo G, Slamani Hani, Cohen Jeremy M, El Moustaid Fadoua, Rohr Jason, Johnson Leah R, and Mordecai Erin A. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23°C and 26°C. eLife, 9:e58511, September 2020. ISSN 2050–084X. doi: 10.7554/eLife.58511. URL https://elifesciences.org/articles/58511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mordecai Erin A., Cohen Jeremy M., Evans Michelle V., Gudapati Prithvi, Johnson Leah R., Lippi Catherine A., Miazgowicz Kerri, Murdock Courtney C., Rohr Jason R., Ryan Sadie J., Savage Van, Shocket Marta S., Anna Stewart Ibarra Matthew B. Thomas, and Weikel Daniel P.. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLOS Neglected Tropical Diseases, 11(4): e0005568, April 2017. ISSN 1935–2735. doi: 10.1371/journal.pntd.0005568. URL 10.1371/journal.pntd.0005568. Publisher: Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ryan Sadie J., Carlson Colin J., Mordecai Erin A., and Johnson Leah R.. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Neglected Tropical Diseases, 13(3):e0007213, March 2019. ISSN 1935–2735. doi: 10.1371/journal.pntd.0007213. URL 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ryan Sadie J., Lippi Catherine A., and Zermoglio Fernanda. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malaria Journal, 19(1):170, December 2020. ISSN 1475–2875. doi: 10.1186/s12936-020-03224-6. URL 10.1186/s12936-020-03224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caldwell Jamie M., LaBeaud A. Desiree, Lambin Eric F., Stewart-Ibarra Anna M., Ndenga Bryson A., Mutuku Francis M., Krystosik Amy R., Ayala Efraın Beltrán, Anyamba Assaf, Borbor-Cordova Mercy J., Damoah Richard, Grossi-Soyster Elysse N., Heras Froilán Heras, Ngugi Harun N., Ryan Sadie J., Shah Melisa M., Sippy Rachel, and Mordecai Erin A.. Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nature Communications, 12(1):1233, February 2021. ISSN 2041–1723. doi: 10.1038/s41467-021-21496-7. URL https://www.nature.com/articles/s41467-021-21496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nova Nicole, Deyle Ethan R., Shocket Marta S., MacDonald Andrew J., Childs Marissa L., Rypdal Martin, Sugihara George, and Mordecai Erin A.. Susceptible host availability modulates climate effects on dengue dynamics. Ecology Letters, 24(3):415–425, March 2021. ISSN 1461–023X, 1461–0248. doi: 10.1111/ele.13652. URL 10.1111/ele.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laureano-Rosario Abdiel E., Garcia-Rejon Julian E., Gomez-Carro Salvador, Farfan-Ale Jose A., and Muller-Karger Frank E. . Modelling dengue fever risk in the State of Yucatan, Mexico using regional-scale satellite-derived sea surface temperature. Acta Tropica, 172: 50–57, August 2017. ISSN 0001706X. doi: 10.1016/j.actatropica.2017.04.017. URL https://linkinghub.elsevier.com/retrieve/pii/S0001706X1730089X. [DOI] [PubMed] [Google Scholar]
- [20].Rodó Xavier, Pascual Mercedes, Doblas-Reyes Francisco J., Gershunov Alexander, Stone Dáithí A., Giorgi Filippo, Hudson Peter J., Kinter James, Rodríguez-Arias Miquel-Àngel, Stenseth Nils Ch., Alonso David, García-Serrano Javier, and Dobson Andrew P.. Climate change and infectious diseases: Can we meet the needs for better prediction? Climatic Change, 118(3–4):625–640, June 2013. ISSN 0165–0009, 1573–1480. doi: 10.1007/s10584-013-0744-1. URL 10.1007/s10584-013-0744-1. [DOI] [Google Scholar]
- [21].Zhang Ying, Riera Jefferson, Ostrow Kayla, Siddiqui Sauleh, Harendra de Silva Sahotra Sarkar, Fernando Lakkumar, and Gardner Lauren. Modeling the relative role of human mobility, land-use and climate factors on dengue outbreak emergence in Sri Lanka. BMC Infectious Diseases, 20(1):649, December 2020. ISSN 1471–2334. doi: 10.1186/s12879-020-05369-w. URL 10.1186/s12879-020-05369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Anwar Asim, Khan Noman, Ayub Muhammad, Nawaz Faisal, Shah Asim, and Flahault Antoine. Modeling and Predicting Dengue Incidence in Highly Vulnerable Countries using Panel Data Approach. International Journal of Environmental Research and Public Health, 16(13):2296, June 2019. ISSN 1660–4601. doi: 10.3390/ijerph16132296. URL https://www.mdpi.com/1660-4601/16/13/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martínez-Bello Daniel, López-Quílez Antonio, and Prieto Alexander Torres. Spatiotemporal modeling of relative risk of dengue disease in Colombia. Stochastic Environmental Research and Risk Assessment, 32(6):1587–1601, June 2018. ISSN 1436–3240, 1436–3259. doi: 10.1007/s00477-017-1461-5. URL 10.1007/s00477-017-1461-5. [DOI] [Google Scholar]
- [24].Gibb Rory, Colón-González Felipe J., Lan Phan Trong, Huong Phan Thi, Nam Vu Sinh, Duoc Vu Trong, Hung Do Thai, Dong Nguyn Thanh, Chien Vien Chinh, Trang Ly Thi Thuy, Quoc Do Kien, Hoa Tran Minh, Tai Nguyen Hu, Hang Tran Thi, Tsarouchi Gina, Ainscoe Eleanor, Harpham Quillon, Hofmann Barbara, Lumbroso Darren, Brady Oliver J., and Lowe Rachel. Interactions between climate change, urban infrastructure and mobility are driving dengue emergence in Vietnam. preprint, Epidemiology, July 2023. URL 10.1101/2023.07.25.23293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].An Dao Thi Minh and Rocklöv Joacim. Epidemiology of dengue fever in Hanoi from 2002 to 2010 and its meteorological determinants. Global Health Action, 7(1):23074, December 2014. ISSN 1654–9716, 1654–9880. doi: 10.3402/gha.v7.23074. URL 10.3402/gha.v7.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Giesen Christine, Roche Jesús, Redondo-Bravo Lidia, Ruiz-Huerta Claudia, Gomez-Barroso Diana, Benito Agustin, and Herrador Zaida. The impact of climate change on mosquito-borne diseases in Africa. Pathogens and Global Health, 114(6):287–301, August 2020. ISSN 2047–7724, 2047–7732. doi: 10.1080/20477724.2020.1783865. URL 10.1080/20477724.2020.1783865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burke Marshall, González Felipe, Baylis Patrick, Sam Heft-Neal, Baysan Ceren, Basu Sanjay, and Hsiang Solomon. Higher temperatures increase suicide rates in the United States and Mexico. Nature Climate Change, 8(8):723–729, August 2018. ISSN 1758–6798. doi: 10.1038/s41558-018-0222-x. URL https://www.nature.com/articles/s41558-018-0222-x. Number: 8 Publisher: Nature Publishing Group. [DOI] [Google Scholar]
- [28].Sharmin Sifat, Glass Kathryn, Viennet Elvina, and Harley David. Interaction of Mean Temperature and Daily Fluctuation Influences Dengue Incidence in Dhaka, Bangladesh. PLOS Neglected Tropical Diseases, 9(7):e0003901, July 2015. ISSN 1935–2735. doi: 10.1371/journal.pntd.0003901. URL 10.1371/journal.pntd.0003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Colón-González Felipe J., Gibb Rory, Khan Kamran, Watts Alexander, Lowe Rachel, and Brady Oliver J.. Projecting the future incidence and burden of dengue in Southeast Asia. Nature Communications, 14(1):5439, September 2023. ISSN 2041–1723. doi: 10.1038/s41467-023-41017-y. URL https://www.nature.com/articles/s41467-023-41017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shabbir Waqas, Pilz Juergen, and Naeem Amna. A spatial-temporal study for the spread of dengue depending on climate factors in Pakistan (2006–2017). BMC Public Health, 20 (1):995, December 2020. ISSN 1471–2458. doi: 10.1186/s12889-020-08846-8. URL 10.1186/s12889-020-08846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Colón-González Felipe J., Bentham Graham, and Lake Iain R.. Climate Variability and Dengue Fever in Warm and Humid Mexico. The American Journal of Tropical Medicine and Hygiene, 84(5):757–763, May 2011. ISSN 0002–9637, 1476–1645. doi: 10.4269/ajtmh.2011.10-0609. URL 10.4269/ajtmh.2011.10-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kirk Devin, Straus Samantha, Childs Marissa L., Harris Mallory, Couper Lisa, Davies T. Jonathan, Forbes Coreen, Gehman Alyssa-Lois, Groner Maya L., Harley Christopher, Lafferty Kevin D., Savage Van, Skinner Eloise, Mary O’Connor, and Erin A. Mordecai. Temperature impacts on dengue incidence are nonlinear and mediated by climatic and socioeconomic factors. bioRxiv, page 2022.06.15.496305, January 2022. doi: 10.1101/2022.06.15.496305. URL http://biorxiv.org/content/early/2022/06/17/2022.06.15.496305.abstract. [DOI] [Google Scholar]
- [33].Ogden Nicholas H.. Climate change and vector-borne diseases of public health significance. FEMS Microbiology Letters, 364(19):fnx186, October 2017. ISSN 0378–1097. doi: 10.1093/femsle/fnx186. URL 10.1093/femsle/fnx186. [DOI] [PubMed] [Google Scholar]
- [34].Messina Jane P., Brady Oliver J., Golding Nick, Kraemer Moritz U. G., Wint G. R. William, Ray Sarah E., Pigott David M., Shearer Freya M., Johnson Kimberly, Earl Lucas, Marczak Laurie B., Shirude Shreya, Weaver Nicole Davis, Gilbert Marius, Velayudhan Raman, Jones Peter, Jaenisch Thomas, Scott Thomas W., Reiner Robert C., and Hay Simon I.. The current and future global distribution and population at risk of dengue. Nature Microbiology, 4(9):1508–1515, September 2019. ISSN 2058–5276. doi: 10.1038/s41564-019-0476-8. URL http://www.nature.com/articles/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Colón-González Felipe J, Odhiambo Sewe Maquins, Tompkins Adrian M, Sjödin Henrik, Casallas Alejandro, Rocklöv Joacim, Caminade Cyril, and Lowe Rachel. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. The Lancet Planetary Health, 5(7):e404–e414, July 2021. ISSN 25425196. doi: 10.1016/S2542-5196(21)00132-7. URL https://linkinghub.elsevier.com/retrieve/pii/S2542519621001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rogers David J.. Dengue: recent past and future threats. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1665):20130562, April 2015. ISSN 0962–8436, 1471–2970. doi: 10.1098/rstb.2013.0562. URL 10.1098/rstb.2013.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu Zhiwei, Bambrick Hilary, Frentiu Francesca D., Devine Gregor, Yakob Laith, Williams Gail, and Hu Wenbiao. Projecting the future of dengue under climate change scenarios: Progress, uncertainties and research needs. PLOS Neglected Tropical Diseases, 14 (3):e0008118, March 2020. ISSN 1935–2735. doi: 10.1371/journal.pntd.0008118. URL 10.1371/journal.pntd.0008118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Colón-González Felipe J., Fezzi Carlo, Lake Iain R., and Hunter Paul R.. The Effects of Weather and Climate Change on Dengue. PLoS Neglected Tropical Diseases, 7(11):e2503, November 2013. ISSN 1935–2735. doi: 10.1371/journal.pntd.0002503. URL 10.1371/journal.pntd.0002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Carlson Colin J., Carleton Tamma A., Odoulami Romaric C., and Trisos Christopher H.. The historical fingerprint and future impact of climate change on childhood malaria in Africa, July 2023. URL 10.1101/2023.07.16.23292713v1. Pages: 2023.07.16.23292713. [DOI] [Google Scholar]
- [40].Carleton Tamma, Jina Amir, Delgado Michael, Greenstone Michael, Houser Trevor, Hsiang Solomon, Hultgren Andrew, Kopp Robert E, McCusker Kelly E, Nath Ishan, et al. Valuing the global mortality consequences of climate change accounting for adaptation costs and benefits. The Quarterly Journal of Economics, 137(4):2037–2105, 2022. [Google Scholar]
- [41].Semenza Jan C. and Paz Shlomit. Climate change and infectious disease in Europe: Impact, projection and adaptation. The Lancet Regional Health - Europe, 9:100230, October 2021. ISSN 26667762. doi: 10.1016/j.lanepe.2021.100230. URL https://linkinghub.elsevier.com/retrieve/pii/S2666776221002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Edwards Robin, Bondarenko Maksym, Tatem Andrew, and Sorichetta Alessandro. Unconstrained national Population Weighted Density in 2000, 2005, 2010, 2015 and 2020 (1km resolution ), 2021. URL 10.5258/SOTON/WP00702. [DOI] [Google Scholar]
- [43].Gorelick Noel, Hancher Matt, Dixon Mike, Ilyushchenko Simon, Thau David, and Moore Rebecca. Google earth engine: Planetary-scale geospatial analysis for everyone. Remote Sensing of Environment, 2017. doi: 10.1016/j.rse.2017.06.031. URL 10.1016/j.rse.2017.06.031. [DOI] [Google Scholar]
- [44].United Nations Office for the Coordination of Humanitarian Affairs. Humanitarian Data Exchange. https://data.humdata.org/.
- [45].Hijmans Robert J.. Second-level administrative divisions, taiwan, 2015. [shapefile]. University of California, Berkeley. Museum of Vertebrate Zoology, 2015. Retrieved from https://earthworks.stanford.edu/catalog/stanford-fn648mm8787. [Google Scholar]
- [46].Socio-economic regions of Costa Rica — second.wiki. https://second.wiki/wiki/regiones_socioeconc3b3micas_de_costa_rica. [Accessed 08-01-2024].
- [47].Copernicus Climate Change Service (C3S) (2017). Era5: Fifth generation of ecmwf atmospheric reanalyses of the global climate. Copernicus Climate Change Service Climate Data Store (CDS), https://cds.climate.copernicus.eu/cdsapp#!/home. [Google Scholar]
- [48].Fick Stephen E. and Hijmans Robert J.. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12):4302–4315, October 2017. ISSN 0899–8418, 1097–0088. doi: 10.1002/joc.5086. URL 10.1002/joc.5086. [DOI] [Google Scholar]
- [49].Coupled model intercomparison project 6. Accessed from https://registry.opendata.aws/cmip6.
- [50].Intergovernmental Panel On Climate Change. Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, 1 edition, July 2023. ISBN 978–1-00–915789-6. doi: 10.1017/9781009157896. URL https://www.cambridge.org/core/product/identifier/9781009157896/type/book. [DOI] [Google Scholar]
- [51].Hausfather Zeke, Marvel Kate, Schmidt Gavin A., Nielsen-Gammon John W., and Zelinka Mark. Climate simulations: recognize the ‘hot model’ problem. Nature, 605(7908): 26–29, May 2022. ISSN 0028–0836, 1476–4687. doi: 10.1038/d41586-022-01192-2. URL https://www.nature.com/articles/d41586-022-01192-2. [DOI] [PubMed] [Google Scholar]
- [52].Meehl Gerald A., Senior Catherine A., Eyring Veronika, Flato Gregory, Lamarque Jean-Francois, Stouffer Ronald J., Taylor Karl E., and Schlund Manuel. Context for interpreting equilibrium climate sensitivity and transient climate response from the CMIP6 Earth system models. Science Advances, 6(26):eaba1981, June 2020. ISSN 2375–2548. doi: 10.1126/sciadv.aba1981. URL 10.1126/sciadv.aba1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Di Virgilio Giovanni, Ji Fei, Tam Eugene, Nishant Nidhi, Evans Jason P., Thomas Chris, Riley Matthew L., Beyer Kathleen, Grose Michael R., Narsey Sugata, and Delage Francois. Selecting CMIP6 GCMs for CORDEX Dynamical Downscaling: Model Performance, Independence, and Climate Change Signals. Earth’s Future, 10(4):e2021EF002625, April 2022. ISSN 2328–4277, 2328–4277. doi: 10.1029/2021EF002625. URL 10.1029/2021EF002625. [DOI] [Google Scholar]
- [54].Di Luca Alejandro, Pitman Andrew J., and de Elía Ramón. Decomposing Temperature Extremes Errors in CMIP5 and CMIP6 Models. Geophysical Research Letters, 47(14): e2020GL088031, July 2020. ISSN 0094–8276, 1944–8007. doi: 10.1029/2020GL088031. URL 10.1029/2020GL088031. [DOI] [Google Scholar]
- [55].Schoeman David S., Gupta Alex Sen, Harrison Cheryl S., Everett Jason D., Brito-Morales Isaac, Hannah Lee, Bopp Laurent, Roehrdanz Patrick R., and Richardson Anthony J.. Demystifying global climate models for use in the life sciences. Trends in Ecology & Evolution, 38(9):843–858, September 2023. ISSN 01695347. doi: 10.1016/j.tree.2023.04.005. URL https://linkinghub.elsevier.com/retrieve/pii/S016953472300085X. [DOI] [PubMed] [Google Scholar]
- [56].World Health Organization Global Health Expenditure database (apps.who.int/nha/database). Accessed on June 14, 2022 from https://data.worldbank.org/indicator/SH.XPD.EHEX.PP.CD.
- [57].Zeng Zhilin, Zhan Juan, Chen Liyuan, Chen Huilong, and Cheng Sheng. Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine, 32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bergé Laurent. Efficient estimation of maximum likelihood models with multiple fixed-effects: the R package FENmlm. CREA Discussion Papers, (13), 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request and code to replicate all results in the main text and supplementary materials will be made available at https://github.com/marissachilds/global-dengue-temperature.