Abstract
Background:
Malaria remains a major cause of morbidity in sub-Saharan Africa. Undetected asymptomatic falciparum malaria results in a large transmission reservoir and there is evidence of increasing non-falciparum malaria as malaria is controlled in Africa, both resulting in challenges for malaria control programs.
Methods:
We performed quantitative real time PCR for 4 malaria species in 4,596 individuals from the 2014–2015 Rwanda Demographic Health Survey. Bivariate models were used to determine species-specific associations with risk factors.
Results:
Asymptomatic falciparum malaria, P. ovale spp., and P. malariae infection had broad spatial distribution across Rwanda. P. vivax infection was rare. Overall infection prevalence was 23.6% (95%CI [21.7%, 26.0%]), with falciparum and non-falciparum at 17.6% [15.9%, 19.0%] and 8.3% [7.0%, 10.0%], respectively. Parasitemias tended to be low and mixed species infections were common, especially where malaria transmission was the highest. Falciparum infection was associated with socio-econiomic status, rural residence and low altitude. Few risk factors were associated with non-falciparum malaria.
Conclusions:
Asymptomatic falciparum malaria and non-falciparum malaria are common and widely distributed across Rwanda. Continued molecular monitoring of Plasmodium spp. is needed to monitor these threats to malaria control in Africa.
Keywords: Plasmodium, falciparum, malaria, asymptomatic, non-falciparum, ovale, vivax, malariae
Introduction
Malaria remains a major global health challenge, with 249 million malaria cases in 85 malaria endemic countries in 2022. This was an increase from 233 million in 2021, with most of this increase coming from countries in the WHO African Region.[1] Malaria infection is caused by five species in the Plasmodium genus, with most cases and morbidity attributed to Plasmodium falciparum. Diagnosis of malaria in Africa relies principally on rapid diagnostic tests (RDTs). RDTs primarily detect the antigen P. falciparum histidine rich protein 2 (HRP2), specific to falciparum malaria, often with a second less sensitive band for pan-species lactate dehydrogenase (LDH).[2] Unfortunately, these tests often miss low density falciparum infection and species other than falciparum malaria, leading to a reservoir of asymptomatic and non-falciparum malaria which contribute to continued transmission.
Molecular detection, including real time PCR, can detect lower levels of parasitemia than RDTs, which increases the number of falciparum malaria infections detected, particularly among asymptomatic community surveys.[3] Characterization of asymptomatic infections is vitally important. These infections are major reservoir of infection, contributing to the persistence of malaria.[4] It has also been argued that these infections have significant associated morbidity.[5] Additionally, asymptomatic malaria often is often followed by symptomatic infection.[6] Thus, characterizing the distribution of asymptomatic malaria is important for malaria control and prevention.
Molecular detection can also help us better understand the distribution of non-falciparum malaria, P. malariae, P. ovale curtisii, P. ovale walkieri, and P. vivax. Non-falciparum malaria species will complicate the challenge of malaria elimination in Africa. There is growing evidence that P. ovale spp. and P. malariae malaria become more common in Africa as P. falciparum is controlled and prevalence decreases.[7] In addition, P. vivax is being reported more frequently due to wider implementation of molecular diagnostics.[8–10] In contrast with falciparum infection, vivax and ovale contribute to malaria by causing relapse through the persistence of hypnozoites (dormant liver stage parasites).[11] Hypnozoites do not respond to blood-stage treatment, like artemisinin-combination therapies (ACTs), the primary treatment for severe malaria in most countries, and require radical cure. Thus, their presence may require national malaria control programs to alter therapeutic options in the country.
Rwanda has historically had strong malaria control, leveraging effective antimalarials, insecticide treated bed nets, and indoor residual spraying.[12] However, malaria cases in Rwanda increased from 48 cases per 1,000 in 2012 to 403 per 1,000 in 2016, while mortality increased 41% over the same time.[13] Emerging insecticide resistance, an increase in irrigated agriculture, and insufficient insecticide-treated mosquito net coverage, among other factors, have been attributed to these increases.[13] Malaria transmission in Rwanda is a constant occurrence, with peaks from April to May and November to December.[12] Transmission also varies with the geography of the country; northern and western Rwanda are prone to epidemics, while the south and east are considered endemic areas of stable transmission.[14] Areas of lower transmission (endemic areas) may have more instances of asymptomatic infection, which may result in under-reporting and lower prevalence estimates in these areas.
While transmission is variable across the country, the entire population of Rwanda is considered at risk for malaria.[15] In order to have a better picture of national malaria trends, large population based surveys are needed. Demographic Health Survey (DHS) studies in multiple countries have been used to understand the distribution of both falciparum and non-falciparum malaria by using molecular testing of malaria from residual blood spots collected for HIV testing.[9,16–18] Given there is currently limited data on non-falciparum malaria in Rwanda, and a need for better understanding of falciparum infections among asymptomatic individuals, leveraging DHS samples would provide a more robust picture of malaria in the country.
The 2014–15 Rwanda DHS occurred during the beginning of increased malaria rates in Rwanda and reported a 2% malaria prevalence among children age 6–59 months and 0.6% among women age 15–49, based primarily on microscopy.[19] Previous studies used this data to show that malaria was associated with lower socio-economic status, not using a insecticide treated bed net, and residing at lower altitude.[20,21] However, given the reliance on non-molecular diagnostics, these results likely do not reflect the full burden of malaria. For example, a study of school children in Huye District, that also occurred in 2014, showed a 22% prevalence of any malaria (19% P. falciparum prevalence) using a combined microscopy and PCR diagnostic.[22]
Data concerning non-falciparum malaria in Rwanda is sparse. A recent household survey estimated that P. falciparum is responsible for 97% of malaria infections in Rwanda, with P. malariae and P. ovale spp. each responsible for 1%–2% of total infections [16]. Reports of vivax malaria in Rwanda are few; a single case of P. vivax infection (previously misclassified as falciparum infection) was reported in 2018. [23] More recently, a cluster of P. vivax was reported in the Huye District. [24] An accurate estimate of malaria prevalence in Rwanda requires the use of molecular diagnostics in a nationally representative survey.
This paper presents data on the spatial distribution of asymptomatic falciparum and non-falciparum infection in Rwanda from the 2014–15 Rwanda DHS, determined by quantitative real time PCR. We also identified attributes associated with different species to inform targeted interventions. The results provide a broader understanding of the distribution of all malaria species at that time, illuminating the role of non-falciparum malaria in the country and providing a baseline for further comparisons in future surveys.
Methods
Study design and population
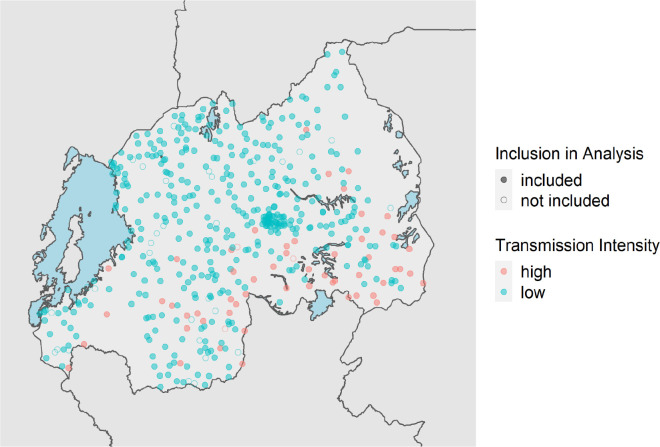
The 2014–2015 Rwanda DHS studied 12,699 households from 492 GPS-located clusters from all 30 recognized districts. In 50% of households, dried blood spot (DBS) specimens were collected for HIV testing from men aged 15–59 years and women aged 15–49 years, and a subsample of children 0–14 years; in the other 50% of households, rapid malaria diagnostic testing was completed on children aged 6–59 months.[25] Previously, we used these data to estimate clusters that would represent high and low malaria transmission areas.[26] High prevalence clusters had a RDT or microscopy positivity rate of >15%. We included 1,434 samples from these 55 high prevalence clusters in 3 regions. In addition to samples from high prevalence areas, a random subset of 3,161 samples from 402 low prevalence clusters were selected. A total of 4,595 samples from 457 out of 492 DHS clusters were analyzed for four species of malaria infection by real time PCR. (Figure 1).
Figure 1. Distribution of 2014–2015 Rwanda Demographic Health Survey Clusters.
Clusters are color coded based upon malaria transmission intensity based on DHS malaria testing results (high represents >15% positive by RDT or microscopy). Shape fill is based on the cluster’s inclusion in this analysis.
Species-specific real time PCR
DNA from each sample was extracted from three 6 mm DBS punches using Chelex and screened for four species of malaria infection using real-time PCR assays.[27] These assays (Supplementary Table 1) targeted the 18s genes for P. malariae, P. ovale, and P. vivax, and the varATS repeat in P. falciparum.[9,17,28,29] To allow for quantification of falciparum, mock DBS were created using whole blood and cultured 3d7 parasites (MRA-102, BEI Resources, Manasas, VA) and extracted with the same assay used for samples. Controls for non-falciparum species used serial dilutions of plasmid DNA (MRA-180, MRA-179, MRA-178; BEI Resources, Manassas VA), with estimates for parasitemia based on an estimated six 18s rRNA gene copies per parasite.[30] All assays were run for 45 cycles to enable detection of lower density infections. The high cycle number approach has been previously evaluated for P. ovale and P. vivax, where assays were tested against 390 negative controls (human DNA) with no false positives.[16] We had no false positive results in our non-template controls for this study. All positive samples were confirmed by manually reviewing the amplification curves in the machine software. Standard curves had a minimum r-squared value of 0.95 across all runs. A positive result for each species was determined using a 45-cycle cutoff, unless otherwise stated.
Spatial & ecological variables
Deidentified survey and geospatial data from the 2014–2015 Rwanda DHS were matched to PCR data using DBS sample barcodes. Clusters with individuals positive for any species of malaria infection were mapped using DHS geospatial coordinates. Individual level covariates assessed for association included sex, age group, wealth quintile, education level, livestock ownership, source of drinking water, bed net ownership, whether the household bed net has been treated with long-lasting insecticide (LLIN = long-lasting insecticide-treated net) and sleeping under a LLIN the night prior to the survey. Cluster level covariates included region, urban/rural status of place of residence, elevation, month of data collection, proportion of a given cluster living in a household with a bed net, proportion of the cluster that slept under an LLIN, land cover, average daily maximum temperature for the current month and precipitation for the prior month. Land cover estimates were taken from the Regional Center for Mapping Resources for Development and SERVIR-Eastern and Southern Africa and temperature and precipitation values were obtained from the Climate Hazards Center at the University of California, Santa Barbara.[31,32] Survey clusters were assigned GPS coordinate values within buffers as described previously in accordance with DHS specifications.[16,33]
Statistical analysis
We estimated species-specific prevalence, non-falciparum prevalence, and overall Plasmodium sp. prevalence, applying HIV sampling weights, inverse propensity for selection weights, and weights to account for selection by cluster transmission intensity and the skewed selection of samples from low and high transmission clusters. [34,35] We estimated bivariate associations between each Plasmodium species and a variety of covariates available in the DHS and investigated in other contexts,[16,36] using the same combination of weights. We report prevalence differences and 95% confidence intervals to assess precision. We analyzed data using the survey (4.2.1), srvyr (v1.2.0), and sf (v1.0–8) packages using R 4.2.1 (R Foundation for Statistical Computing). Shapefiles of Rwanda district boundaries taken from the OCHA Regional Office for Southern and Eastern Africa database.[37]
Results
Study population characteristics
The study population was 40% female, 14% aged 15–24 years, 76% lived in rural areas, and 80% had a primary school education or no education (60% reported primary education, 20% reported preschool/none). Overall, most (83%) individuals reported a household bed net and 68% reported sleeping under a long-lasting insecticide treated net the night before the survey. However, 41% of the study population lived in a household that did not meet the World Health organization’s criteria of at least 1 net per 1.8 household members.
Prevalence of falciparum and non-falciparum infection by real time PCR
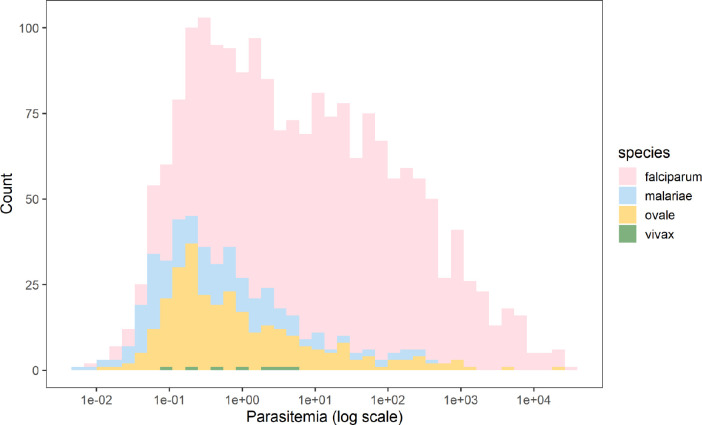
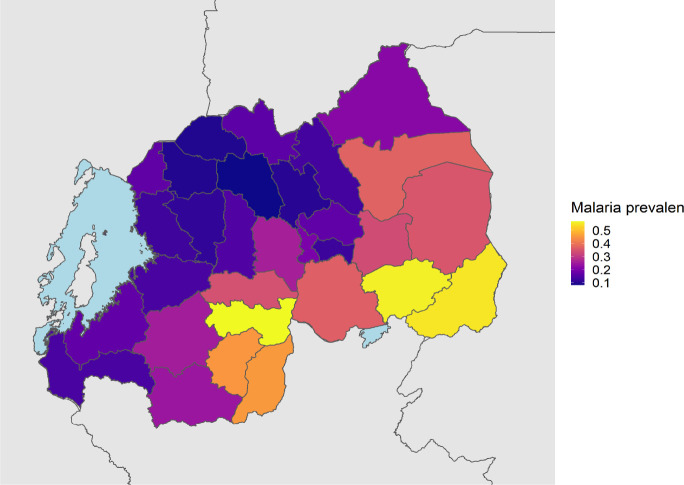
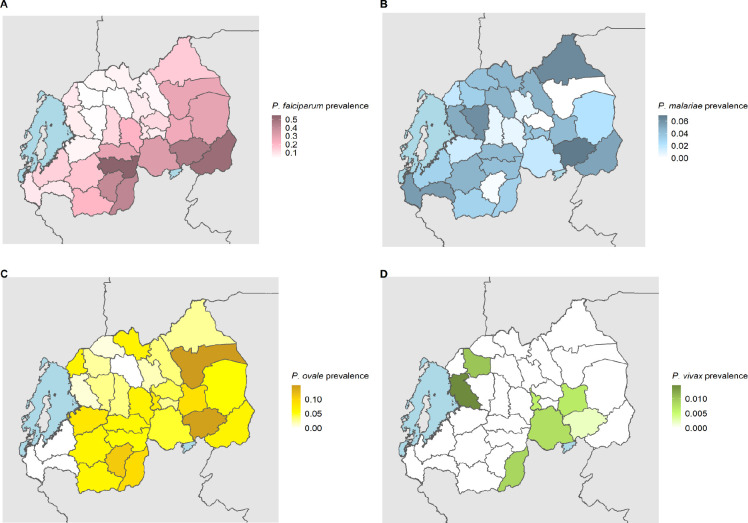
A total of 1,231 P. falciparum, 246 P. ovale, 168 P. malariae and 7 P. vivax infections were identified. The overall weighted prevalence of any non-falciparum malaria infection was 8.3% (95% CI: [7.0%, 10.0%]) compared to 17.6% [15.9%, 19.0%] for falciparum and 23.6% [21.7%, 26.0%] overall malaria prevalence with HIV sampling, inverse propensity of selection, and transmission intensity correction weights applied. Species specific weighted prevalences were 3.3% [2.7%, 4.0%] and 5.1% [4.0%, 7.0%] for P. malariae and P. ovale spp. Unweighted prevalence for P. vivax was 0.15%, with a manually calculated 95% CI [0.04%, 0.27%]. Using a more restrictive cut off of 40 cycles (requiring a higher parasitemia to be positive at approximately 1 parasite per microliter of template DNA), resulted in weighted overall prevalences of 4.3% [3.6%, 5.0%] for any non-falciparum malaria infection, compared to 14.3% [12.7%, 16.0%] for falciparum and 17.4% for [15.8%, 19.0%] overall malaria. Species specific weighted prevalences at this cut off were 2.7% [2.2%, 3.0%) and 1.7% [1.2%, 2.0%], and for P. malariae, and P. ovale spp. Unweighted prevalence for P. vivax was 0.09% [0.002%, 0.17%]. The largest difference in estimated prevalence was for P. ovale spp. This is not surprising given the distribution of estimated parasitemia values (Figure 2), showing a lower median parasitemia in the non-falciparum species compared to falciparum malaria. District level weighted prevalences and their differences by PCR cut off are shown in Table 2 and Supplemental Table 3. Falciparum, ovale and malariae infections were distributed across the country, while vivax infections were more localized (Supplemental Figure 1). District level overall malaria prevalence is shown in Figure 3, while district level prevalences for each species are illustrated in Figure 4. Among P. ovale spp., P. malariae, and P. vivax infections, 45%, 45% and 57% (unweighted counts) were infected with at least one other species of malaria (Supplemental Table 4).
Figure 2. Calculated Parasitemia Estimates for Falciparum and Non-falciparum Infections.
Overall, falciparum had a higher parasite density with a median of 10.90 (IQR 0.96–101.41). The median parasitemia level for P. malariae, P. ovale spp., and P. vivax malaria were 0.35 (IQR: 0.07–1.83), 0.48 (IQR: 0.16–3.25) and 0.94 (IQR: 0.20–3.44), respectively.
Table 2. Differences in District Level Prevalence by PCR Cutoff.
The difference in prevalence for malaria for each district between the 45 and 40 PCR cycle threshold is shown for overall malaria burden, as well as for each species. Darker shading denotes larger differences in prevalence estimates, and districts are ordered by the difference in overall malaria prevalence from highest to lowest difference in prevalence. Specific prevalence estimates are shown in Supplemental Table 3.
| District | Difference in overall malaria prevalence | Difference in Pf prevalence | Difference in Pm prevalence | Difference in Po prevalence | Difference in Pv prevalence |
|---|---|---|---|---|---|
|
| |||||
| Nyarugenge | 0.128 | 0.039 | 0.027 | 0.063 | 0.000 |
| Gatsibo | 0.120 | 0.047 | 0.000 | 0.112 | 0.000 |
| Ngoma | 0.102 | 0.054 | 0.002 | 0.091 | 0.002 |
| Burera | 0.090 | 0.037 | 0.018 | 0.034 | 0.000 |
| Huye | 0.089 | 0.073 | 0.002 | 0.033 | 0.000 |
| Rwamagana | 0.088 | 0.052 | 0.015 | 0.071 | 0.000 |
| Kirehe | 0.084 | 0.052 | 0.003 | 0.046 | 0.000 |
| Gasabo | 0.080 | 0.037 | 0.000 | 0.043 | 0.000 |
| Nyamagabe | 0.074 | 0.053 | 0.000 | 0.036 | 0.000 |
| Rubavu | 0.068 | 0.029 | 0.000 | 0.040 | 0.000 |
| Kamonyi | 0.063 | 0.024 | 0.007 | 0.047 | 0.000 |
| Nyaruguru | 0.063 | 0.049 | 0.000 | 0.039 | 0.000 |
| Kayonza | 0.061 | 0.048 | 0.000 | 0.020 | 0.000 |
| Karongi | 0.061 | 0.002 | 0.000 | 0.061 | 0.000 |
| Gakenke | 0.061 | 0.027 | 0.034 | 0.000 | 0.000 |
| Gicumbi | 0.060 | 0.017 | 0.009 | 0.033 | 0.000 |
| Ruhango | 0.059 | 0.030 | 0.021 | 0.038 | 0.000 |
| Gisagara | 0.058 | 0.052 | 0.003 | 0.068 | 0.000 |
| Nyabihu | 0.057 | 0.022 | 0.007 | 0.018 | 0.010 |
| Muhanga | 0.046 | 0.023 | 0.000 | 0.023 | 0.000 |
| Nyamasheke | 0.043 | 0.043 | 0.000 | 0.000 | 0.000 |
| Nyanza | 0.043 | 0.041 | 0.006 | 0.020 | 0.000 |
| Nyagatare | 0.041 | 0.041 | 0.000 | 0.018 | 0.000 |
| Kicukiro | 0.040 | 0.026 | 0.000 | 0.021 | 0.000 |
| Musanze | 0.036 | 0.022 | 0.006 | 0.008 | 0.000 |
| Rutsiro | 0.035 | 0.045 | 0.000 | 0.000 | 0.015 |
| Bugesera | 0.034 | 0.014 | 0.002 | 0.025 | 0.008 |
| Ngororero | 0.021 | 0.010 | 0.010 | 0.010 | 0.000 |
| Rusizi | 0.018 | 0.010 | 0.008 | 0.000 | 0.000 |
| Rulindo | 0.015 | 0.008 | 0.000 | 0.007 | 0.000 |
Figure 3. District Level Weighted Overall Malaria Prevalence.
Weighted prevalence of any malaria infection, using HIV sampling, inverse propensity for selection and transmission intensity weights (described above).
Figure 4. District Level Weighted Prevalence Estimates for Malaria Species.
The weighted prevalence estimate for each species is shown. Panel A, B, C and D represent falciparum, malariae, ovale spp. and vivax malaria, respectively.
Bivariate associations for infection
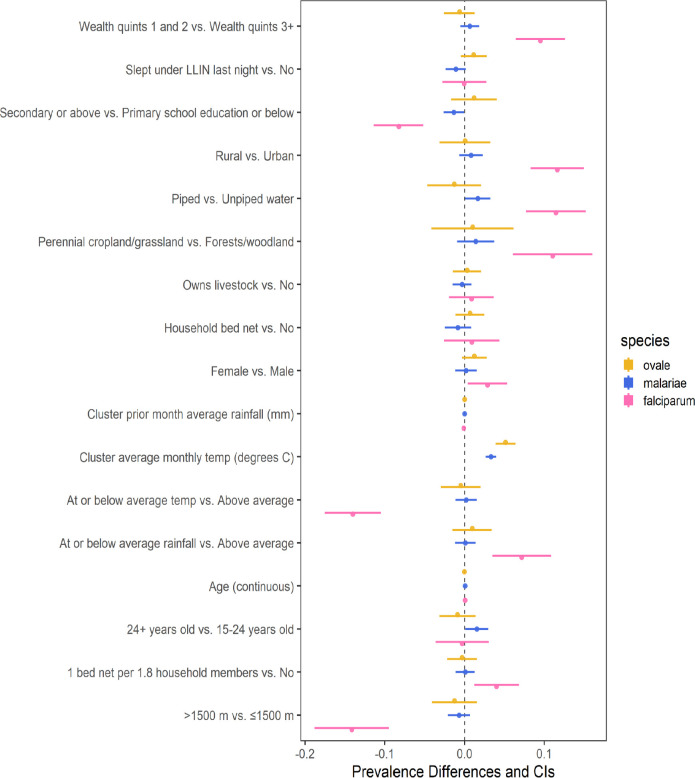
Bivariate regression models using weighted (as previously described) survey data found multiple associations for infection with falciparum malaria, but few for non-falciparum malaria (Figure 5). Similar to previous work, a higher prevalence of falciparum malaria in our dataset was significantly (at a 0.05 confidence level for all associations) associated with multiple study covariates related to socioeconomic status (e.g. lower wealth quintile, lower education status, and unpiped water). Secondary or higher education, access to piped drinking water, and higher altitude (>1,500m) were all associated with significantly lower prevalence of falciparum malaria. Clusters designated as perennial cropland, rural clusters compared to urban, lower wealth index (first and second quintiles compared to the upper three), and female participants were associated with higher falciparum prevalence. Few associations were found for non-falciparum malaria. Lower prevalence of P. malariae infection was significantly associated with piped water and secondary education, and both a 1-year increase in age and participants over 24 (compared to those 15–24 years old) were associated with higher P. malariae prevalence. No significant associations were found for P. ovale infection. Associations for P. vivax infection were not attempted due to the limited number of infections in the survey (n=7).
Figure 5. Bivariate associations and between demographic and environmental risk factors and Plasmodium spp. prevalence using weighted survey data.
Models incorporate 2014–15 Rwanda Demographic and Health Survey weights, inverse probability of selection weights, and cluster transmission intensity weights (described in Table 1). Point estimates of prevalence difference are surrounded by confidence intervals. The reference is the second variable listed.
Discussion
Information on the prevalence and distribution of asymptomatic falciparum malaria and non-falciparum malaria in Rwanda is essentially absent in the literature. We report the largest survey for non-falciparum malaria and asymptomatic falciparum malaria infection using molecular methods to have been conducted in Rwanda by leveraging the 2014–15 DHS. Given the DHS represents individuals taking surveys at home, it primarily reflects asymptomatic infections with low parasite density (Figure 2). Overall, malaria was much more common than expected based on RDT and microscopy data in the DHS, reaching 23.6% nationally. As expected, overall malaria was higher in the South and East than the North and West (Figure 3). Asymptomatic falciparum prevalence was 17.6% nationally. The rates of malaria infection were highest in the South and East as expected, but asymptomatic infection remained common in other areas (Figure 4). Non-falciparum malaria was detected in 8.3% of individuals nationally, a prevalence not previously appreciated in the country. P. ovale spp. and P. malariae were both common in Rwanda (5.1% and 3.3% prevalence, respectively) and distributed in regions of both high and low transmission (Figure 4). P. vivax is present but remains relatively uncommon (0.15% unweighted prevalence) and sporadic (Figure 4). Asymptomatic falciparum malaria has been shown to increase risk of symptomatic disease in the future (at one month) and significant morbidity has been associated with malaria parasite carriage.[5,6] The impact of low density, asymptomatic, non-falciparum malaria infection on clinical disease and future symptomatic disease remains unclear and requires further study. In addition, the higher than expected prevalence of P. ovale spp. and occasional P. vivax infection raises control concerns around the use of radical cure for relapse to reach malaria elimination.
Molecular diagnostics for malaria have higher sensitivity and detect lower density infections than RDTs or microscopy. However, there is increased concern about false positive detection with higher cycle cut offs in quantitative real time PCR. Our assays have been run extensively at 45 cycles with little to no evidence of false positives[16], but we also present prevalences with a lower cut off (40 cycles). Even with more conservative quantitative real time PCR cut-offs, national malaria prevalence is still high (17.4%). The largest change in prevalence occurred with P. ovale spp., where estimated prevalence dropped from 5.1% to 1.7% at different cycle cut offs, reflecting the high number of low density infections detected (Figure 2). The relative decrease in prevalence for each species was not always consistent, with some regions having no decline in falciparum prevalence with large declines in ovale malaria (e.g. Karongi near Lake Kivu) or the opposite with no decline in non-falciparum but lower falciparum prevalence estimates (e.g. Rutsiro and Nyamasheke) (Table 2).
Not surprisingly, mixed species infections were common and widely distributed, but occurred more commonly in clusters in the south and east where malaria transmission was the highest (Supplemental Figure 1). Among P. ovale spp., P. malariae, and P. vivax infections, 44%, 45% and 57% (unweighted) were infected with at least one other species of malaria (Supplemental Table 4). Mixed infections are often underappreciated and may lead to severe disease complications. A recent meta-analysis suggested that patients with mixed infections have a higher proportion of pulmonary complications and multiple organ failure than patients with P. falciparum infection alone.[38] The impact of mixed species infections on clinical malaria outcomes in Rwanda is unknown and requires additional evaluation in symptomatic infections, which were not included in this study.
Bivariate associations found between survey covariates and falciparum prevalence matched previous reports for RDT and microscopy prevalence in the DHS.[20,21] Like similar studies, associations of covariates for non-falciparum malaria were few and traditional risk factors for falciparum malaria did not appear as important for non-falciparum malaria.[9,16,17,36] This raises concern for how the control program can target non-falciparum infections without better diagnostics in the community. The reasons for the relative lack of risk factors, especially for P. ovale spp., remains unclear. Relapsing malaria, caused by ovale and vivax, may not be associated with typical covariates due to the inability to discern between incident or relapse infections in the study.
This is the first national survey in Rwanda to examine four species of human malaria infection using molecular methods. The use of existing samples and individual level data from a DHS is a highly informative method to gain insights into national malaria prevalence. However, these studies represent only a singular cross sectional time point. This analysis can be improved upon by the repeated use of multiple DHSs, with a new DHS completed in 2019–20, to assess trends in malaria as has been done in the DRC.[39] Additionally, the relative lack of data for children under 15 makes this survey nonrepresentative of the true age distribution in Rwanda. School aged children are a particularly vulnerable group and often have the highest rate of malaria infection in Africa.[40] Because this was a single cross sectional survey, we also could not determine if P. ovale spp. and P. vivax infections were newly acquired or the result of relapse from hypnozoites.
This study represents the first depiction of asymptomatic falciparum malaria and non-falciparum malaria infection nationally in Rwanda using molecular methods. The prevalence of asymptomatic falciparum malaria was significantly higher than estimates with RDT and microscopy.[41] P. ovale spp. and P. malariae were found across the country; however, few covariates were found to be significantly associated with non-falciparum infection. P. vivax was found, but infrequently. Most non-falciparum infections had low-density parasitemias and coinfection with P. falciparum was common, especially for P. ovale spp. and P. vivax. The high rate of P. ovale and P. malariae infection, with no discernable risk factors, indicates that the control program needs diagnostic plans for these species in communities where their prevalence is increasing. The data from this study is critical for national malaria control goals, given asymptomatic individuals comprise a large reservoir of falciparum infections and a high rate of relapsing malaria infection that requires radical cure. More up to date data, potentially from the 2019–20 DHS is urgently needed to understand what these data mean for control. However, the utility of large, nationally representative, molecular surveys is clear, as they provide insights into malaria surveillance often missed by routine data collection.
Supplementary Material
Table 1. Study Population Characteristics and Distribution of PCR Positives by Species.
Weighted counts and percentages for all individuals positive for each species of malaria. Weights applied are DHS sampling weights, propensity score weights, and weights to correct for over-selection of high transmission intensity clusters (transmission intensity weights). Characteristics of the included study population are also shown.
| Pf positive | Pm positive | Po positive | Pv positive | Included study population | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unweighted counts | 1231 | 168 | 246 | 7 | 4595 | ||||||
| Weighted counts | 813 | 152 | 237 | 7.5 | 4616 | ||||||
| Individual level covariates | |||||||||||
|
| |||||||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Sex of respondent | Male | 453.4 | 56% | 89.0 | 59% | 128.1 | 54% | 6.0 | 80% | 2755.4 | 60% |
| Female | 359.5 | 44% | 62.9 | 41% | 108.8 | 46% | 1.5 | 20% | 1860.6 | 40% | |
| Age group (years) | 0–15 | 24.9 | 3% | 1.5 | 1% | 29.5 | 12% | 0.0 | 0% | 121.4 | 3% |
| 15–24 | 114.4 | 14% | 14.2 | 9% | 3.0 | 1% | 0.8 | 11% | 657.8 | 14% | |
| 25–34 | 234.3 | 29% | 43.2 | 28% | 42.8 | 18% | 2.8 | 37% | 1450.1 | 31% | |
| 35–44 | 167.3 | 21% | 34.6 | 23% | 72.8 | 31% | 0.0 | 0% | 929.9 | 20% | |
| 45–54 | 150.3 | 16% | 42.4 | 24% | 50.3 | 20% | 2.4 | 32% | 842.5 | 18% | |
| 55+ | 121.5 | 15% | 16.1 | 11% | 38.5 | 16% | 1.5 | 20% | 614.4 | 13% | |
| Wealth Quintile | Poorest | 200.3 | 25% | 24.1 | 0.16 | 36.7 | 16% | 2.4 | 33% | 694.4 | 15% |
| Poorer | 163.4 | 20% | 32.4 | 0.21 | 34.3 | 14% | 0.3 | 4% | 820.4 | 18% | |
| Middle | 161.4 | 20% | 29.5 | 0.19 | 34.8 | 15% | 0.0 | 0% | 828.3 | 18% | |
| Richer | 166.7 | 21% | 35.8 | 0.24 | 53.0 | 22% | 4.7 | 63% | 1027.5 | 22% | |
| Richest | 121.0 | 15% | 30.1 | 0.20 | 78.0 | 33% | 0.0 | 0% | 1245.5 | 27% | |
| Education | None/preschool | 190.0 | 23% | 39.0 | 26% | 40.1 | 17% | 5.1 | 68% | 907.2 | 20% |
| Primary | 520.7 | 64% | 92.4 | 61% | 140.4 | 59% | 2.4 | 32% | 2779.0 | 60% | |
| Secondary | 72.5 | 9% | 11.6 | 8% | 45.2 | 19% | 0.0 | 0% | 723.7 | 16% | |
| Higher | 29.5 | 4% | 8.9 | 6% | 11.2 | 5% | 0.0 | 0% | 205.1 | 4% | |
| Owns livestock, herds, or farm animals | No | 354.3 | 44% | 71.7 | 47% | 102.6 | 43% | 4.8 | 64% | 2067.7 | 45% |
| Yes | 458.6 | 56% | 80.2 | 53% | 134.3 | 57% | 2.7 | 36% | 2548.3 | 55% | |
| Source of drinking water | Unpiped | 756.6 | 93% | 138.5 | 91% | 193.1 | 82% | 7.5 | 100% | 3911.7 | 85% |
| Piped | 55.3 | 7% | 13.4 | 9% | 43.8 | 18% | 0.0 | 0% | 701.8 | 15% | |
| Household bed net | No | 134.6 | 17% | 31.9 | 21% | 36.5 | 15% | 3.3 | 44% | 797.7 | 17% |
| Yes | 678.3 | 83% | 120.0 | 79% | 200.4 | 85% | 4.2 | 56% | 3818.3 | 83% | |
| Slept under LLIN last night | No | 257.2 | 32% | 59.1 | 39% | 63.5 | 27% | 3.3 | 44% | 1457.8 | 32% |
| Yes | 555.6 | 68% | 92.8 | 61% | 173.4 | 73% | 4.2 | 56% | 3158.2 | 68% | |
| Insecticide-treated household net | No | 0.9 | 0% | 0.0 | 0% | 2.6 | 1% | 0.0 | 0% | 6.7 | 0% |
| Yes | 616.7 | 76% | 115.8 | 76% | 180.4 | 76% | 4.2 | 56% | 3527.0 | 76% | |
| Missing data | 195.2 | 24% | 36.1 | 24% | 53.9 | 23% | 3.3 | 44% | 1082.3 | 23% | |
| 1 bed net per 1.8 household members | No | 291.2 | 36% | 61.0 | 41% | 101.8 | 43% | 3.3 | 44% | 1905.0 | 41% |
| Yes | 520.9 | 64% | 88.6 | 59% | 135.1 | 57% | 4.2 | 56% | 2699.2 | 59% | |
| Cluster level covariates | |||||||||||
|
| |||||||||||
| Region | Kigali City | 70.5 | 9% | 14.3 | 9% | 33.2 | 14% | 1.2 | 16% | 699.4 | 15% |
| South | 332.8 | 41% | 32.5 | 21% | 75.5 | 32% | 1.2 | 16% | 1176.2 | 25% | |
| West | 74.7 | 9% | 37.9 | 25% | 33.2 | 14% | 3.0 | 40% | 1026.3 | 22% | |
| North | 42.3 | 5% | 29.4 | 19% | 19.1 | 8% | 0.0 | 0% | 776.0 | 17% | |
| East | 292.6 | 36% | 37.9 | 25% | 75.9 | 32% | 2.1 | 28% | 938.1 | 20% | |
| Place of residence | Urban | 95.9 | 12% | 29.3 | 19% | 55.9 | 24% | 1.2 | 16% | 1096.0 | 24% |
| Rural | 717.0 | 88% | 122.6 | 81% | 181.0 | 76% | 6.3 | 84% | 3520.0 | 76% | |
| Elevation (m) | 500 – 1000 | 3.3 | 0% | 0.8 | 1% | 0.0 | 0% | 0.0 | 0% | 15.4 | 0% |
| 2 | 1001 – 1500 | 352.2 | 43% | 47.8 | 31% | 77.3 | 33% | 3.3 | 44% | 1263.3 | 27% |
| 3 | 1501 – 2000 | 412.8 | 51% | 67.2 | 44% | 121.0 | 51% | 1.2 | 16% | 2449.8 | 53% |
| 4 | 2000 – 2500 | 41.2 | 5% | 34.3 | 23% | 38.6 | 16% | 1.5 | 20% | 821.5 | 18% |
| 5 | > 2500 | 3.3 | 0% | 1.9 | 1% | 0.0 | 0% | 1.5 | 20% | 66.0 | 1% |
| Month of data collection | Jan-15 | 164.0 | 20% | 28.3 | 19% | 42.8 | 18% | 0.0 | 0% | 1066.8 | 23% |
| Feb-15 | 186.1 | 23% | 26.3 | 17% | 38.8 | 16% | 2.0 | 27% | 780.0 | 17% | |
| Mar-15 | 228.2 | 28% | 39.5 | 26% | 76.0 | 32% | 2.7 | 37% | 1100.1 | 24% | |
| Apr-15 | 5.5 | 1% | 1.3 | 1% | 1.5 | 1% | 0.0 | 0% | 32.3 | 1% | |
| Nov-14 | 95.7 | 9% | 21.0 | 11% | 16.9 | 6% | 1.2 | 12% | 456.8 | 8% | |
| Dec-14 | 133.4 | 16% | 35.5 | 23% | 60.9 | 26% | 1.5 | 20% | 1180.0 | 26% | |
| Landcover | Moderate forest | 5.8 | 0.01 | 6.4 | 4% | 13.3 | 6% | 0.0 | 0% | 304.2 | 7% |
| Sparse forest | 239.6 | 0.29 | 2.3 | 2% | 0.3 | 0% | 0.0 | 0% | 27.7 | 1% | |
| Woodland | 508.5 | 0.63 | 33.9 | 22% | 59.2 | 25% | 1.3 | 17% | 774.8 | 17% | |
| Closed grassland | 33.7 | 0.04 | 100.4 | 66% | 149.2 | 63% | 6.2 | 83% | 3099.2 | 67% | |
| (Perennial) cropland | 25.1 | 0.03 | 8.9 | 6% | 15.0 | 6% | 0.0 | 0% | 410.1 | 9% | |
| Cluster level averages | |||||||||||
|
| |||||||||||
| Pf positive | Pm positive | Po positive | Pv positive | overall study | |||||||
| mean | SE | mean | SE | mean | SE | mean | SE | mean | SE | ||
| Proportion of cluster with household bed nets | 0.853 | 0.011 | 0.787 | 0.023 | 0.855 | 0.018 | 0.680 | 0.073 | 0.827 | 0.008 | |
| Proportion of cluster that slept under a net last night | 0.697 | 0.014 | 0.631 | 0.027 | 0.741 | 0.024 | 0.560 | 0.064 | 0.684 | 0.010 | |
| Current month’s average daily max temp (degrees C) | 27.16 | 0.1 | 26.40 | 0.2 | 26.84 | 0.2 | 25.96 | 1.0 | 26.39 | 0.05 | |
| Prior month’s precipitation (mm) | 80.60 | 2.4 | 91.05 | 4.2 | 86.02 | 4.7 | 94.51 | 21.1 | 90.38 | 1.38 | |
Acknowledgements:
The following reagent was obtained through BEI Resources, NIAID, NIH: Plasmodium falciparum, Strain 3D7, MRA-102, contributed by Daniel J. Carucci. The following reagent was obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium vivax, MRA-178, contributed by Peter A. Zimmerman. The following reagent was obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium malariae, MRA-179, contributed by Peter A. Zimmerman. The following reagent was obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium ovale, MRA-180, contributed by Peter A. Zimmerman.
Funding:
This work was funded by the National Institutes for Health (R01AI156267 to JAB, JBM and JJJ and K24AI134990 to JJJ). The funding for the DHS was provided by the government of Rwanda, the United States Agency for International Development (USAID), the One United Nations (One UN), the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), World Vison International, the Swiss Agency for Development and Cooperation (SDC), and the Partners in Health (PIH). ICF International provided technical assistance through The DHS Program, a USAID-funded project providing support and technical assistance in the implementation of population and health surveys in countries worldwide. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Conflict of Interest/Ethics: Authors do not declare conflicts of interest associated with this work. Dried Blood Spots (DBS) were provided by the Ministry of Health- Rwanda. Clinical and offset GPS data was downloaded from DHS-MEASURE. The University of North Carolina and Brown University IRBs deemed this non-human subjects research.
Data Availability:
Clinical and spatial data are available through the DHS MEASURE website. PCR data is available upon request. Code used for analysis is available at: https://github.com/claudiagaither.
REFERENCES
- 1.World malaria report 2023 [Internet]. [cited 2023 Sep 15]. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023
- 2.Boyce MR, O’Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health. 2017; 17(1):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L, Hoogen LL van den, Slater H, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature. Nature Publishing Group; 2015; 528(7580):S86–S93. [DOI] [PubMed] [Google Scholar]
- 4.Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. Elsevier; 2014; 30(4):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen I, Clarke SE, Gosling R, et al. “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated. PLoS Med [Internet]. PLOS; 2016. [cited 2023 Sep 15]; 13(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4718522/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumner KM, Mangeni JN, Obala AA, et al. Impact of asymptomatic Plasmodium falciparum infection on the risk of subsequent symptomatic malaria in a longitudinal cohort in Kenya. eLife Sciences Publications Limited; 2021. [cited 2023 Sep 15];. Available from: https://elifesciences.org/articles/68812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yman V, Wandell G, Mutemi DD, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl Trop Dis. Public Library of Science; 2019; 13(5):e0007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet. Elsevier; 2019; 394(10195):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazeau NF, Mitchell CL, Morgan AP, et al. The epidemiology of Plasmodium vivax among adults in the Democratic Republic of the Congo. Nat Commun. 2021; 12(1):4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twohig KA, Pfeffer DA, Baird JK, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019; 13(1):e0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter J, Franken G, Mehlhorn H, Labisch A, Häussinger D. What is the evidence for the existence of Plasmodium ovale hypnozoites? Parasitol Res. 2010; 107(6):1285–1290. [DOI] [PubMed] [Google Scholar]
- 12.Karema C, Wen S, Sidibe A, et al. History of malaria control in Rwanda: implications for future elimination in Rwanda and other malaria-endemic countries. Malar J [Internet]. BMC; 2020. [cited 2023 Sep 15]; 19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7539391/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.[No title] [Internet]. [cited 2023 Nov 2]. Available from: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2022/01/FY-2022-RDMA-MOP.pdf
- 14.[No title] [Internet]. [cited 2023 Nov 2]. Available from: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2017-rwanda-malaria-operational-plan.pdf
- 15.[No title] [Internet]. [cited 2023 Nov 2]. Available from: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2017-rwanda-malaria-operational-plan.pdf
- 16.Gumbo A, Topazian HM, Mwanza A, et al. Occurrence and Distribution of Nonfalciparum Malaria Parasite Species Among Adolescents and Adults in Malawi. J Infect Dis [Internet]. J Infect Dis; 2022. [cited 2023 Sep 15]; 225(2). Available from: https://pubmed.ncbi.nlm.nih.gov/34244739/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell CL, Brazeau NF, Keeler C, et al. Under the Radar: Epidemiology of Plasmodium ovale in the Democratic Republic of the Congo. J Infect Dis [Internet]. J Infect Dis; 2021. [cited 2023 Sep 15]; 223(6). Available from: https://pubmed.ncbi.nlm.nih.gov/32766832/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SM, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One [Internet]. PLoS One; 2011. [cited 2023 Sep 15]; 6(1). Available from: https://pubmed.ncbi.nlm.nih.gov/21305011/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.[No title] [Internet]. [cited 2023 Sep 15]. Available from: https://dhsprogram.com/pubs/pdf/FR316/FR316.pdf [Google Scholar]
- 20.Kubana E, Munyaneza A, Sande S, et al. “A comparative analysis of risk factors of malaria” case study Gisagara and Bugesera District of Rwanda. RDHS 2014/2015. A retrospective study. BMC Public Health. BioMed Central; 2023; 23(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudasingwa G, Cho S-I. Determinants of the persistence of malaria in Rwanda. Malar J. BioMed Central; 2020; 19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sifft KC, Geus D, Mukampunga C, et al. Asymptomatic only at first sight: malaria infection among schoolchildren in highland Rwanda. Malar J [Internet]. Malar J; 2016. [cited 2023 Sep 15]; 15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/27842542/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaffery JN, Munyaneza T, Uwimana A, et al. Symptomatic Infection in Rwanda. Open Forum Infect Dis. 2022; 9(3):ofac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loon W van, Oliveira R, Bergmann C, et al. Plasmodium vivax Malaria in Duffy-Positive Patients in Rwanda. Am J Trop Med Hyg [Internet]. Am J Trop Med Hyg; 2023. [cited 2023 Sep 15]; 109(3). Available from: https://pubmed.ncbi.nlm.nih.gov/37549894/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.[No title] [Internet]. [cited 2023 Nov 17]. Available from: https://dhsprogram.com/pubs/pdf/FR316/FR316.pdf
- 26.Kirby R, Giesbrecht D, Karema C, et al. Examining the Early Distribution of the Artemisinin-Resistant R561H Mutation in Areas of Higher Transmission in Rwanda. Open Forum Infect Dis. 2023; 10(4):ofad149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh PS, Metzger DA, Higushi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10(4): 506–13 (April 1991). Biotechniques. 2013; 54(3):134–139. [DOI] [PubMed] [Google Scholar]
- 28.Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004; 42(12):5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015; 12(3):e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercereau-Puijalon O, Barale J-C, Bischoff E. Three multigene families in Plasmodium parasites: facts and questions. Int J Parasitol. 2002; 32(11):1323–1344. [DOI] [PubMed] [Google Scholar]
- 31.RCMRD Open Data Site [Internet]. [cited 2023 Sep 15]. Available from: https://opendata.rcmrd.org/pages/servir-esa
- 32.Funk C, Peterson P, Landsfeld M, et al. The climate hazards infrared precipitation with stations--a new environmental record for monitoring extremes. Sci Data. 2015; 2:150066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.[No title] [Internet]. [cited 2023 Nov 2]. Available from: https://spatialdata.dhsprogram.com/references/DHS%20Covariates%20Extract%20Data%20Description%202.pdf
- 34.Guide to DHS Statistics [Internet]. [cited 2023 Nov 2]. Available from: https://dhsprogram.com/data/Guide-to-DHS-Statistics/index.cfm
- 35.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. 1982.
- 36.Mitchell CL, Topazian HM, Brazeau NF, et al. Household Prevalence of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale in the Democratic Republic of the Congo, 2013–2014. Clin Infect Dis. 2021; 73(11):e3966–e3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rwanda - Subnational Administrative Boundaries [Internet]. [cited 2023 Sep 15]. Available from: https://data.humdata.org/dataset/cod-ab-rwa?
- 38.Kotepui M, Kotepui KU, De Jesus Milanez G, Masangkay FR. Plasmodium spp. mixed infection leading to severe malaria: a systematic review and meta-analysis. Sci Rep. 2020; 10(1):11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deutsch-Feldman M, Aydemir O, Carrel M, et al. The changing landscape of Plasmodium falciparum drug resistance in the Democratic Republic of Congo. BMC Infect Dis. 2019; 19(1):872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sendor R, Mitchell CL, Chacky F, et al. Similar Prevalence of Plasmodium falciparum and Non-P. falciparum Malaria Infections among Schoolchildren, Tanzania. Emerg Infect Dis. 2023; 29(6):1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.[No title] [Internet]. [cited 2023 Nov 11]. Available from: https://dhsprogram.com/pubs/pdf/FR316/FR316.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical and spatial data are available through the DHS MEASURE website. PCR data is available upon request. Code used for analysis is available at: https://github.com/claudiagaither.