Abstract
Sera from calves vaccinated with the recombinant Schistosoma bovis-derived 28-kDa glutathione S-transferase (28GST) and subsequently naturally or experimentally exposed to Schistosoma mattheei were studied for their content of specific immunoglobulin G (IgG) and IgA antibodies to recombinant S. bovis 28GST as well as for their capacity to inhibit the enzymatic activity of the antigen. The results were analyzed in regard to the presence (natural infection) or absence (experimental infection) of a protective effect(s) (reductions in worm burden, egg load, fecal egg counts, and excretion of viable eggs) toward S. mattheei challenge. Under such conditions, no differences in the IgG- and IgA-specific antibodies to recombinant S. bovis 28GST or in the ability to block the catalytic function of the antigen between the two groups were recorded. Nevertheless, correlation analysis between the specific antibody responses to recombinant S. bovis 28GST and the inhibition of GST activity suggested an association with IgG in experimentally infected vaccinated animals, while in naturally infected vaccinated calves, the inhibitory activity appeared to be linked to a greater degree with IgA. These results suggest that in contrast to schistosomiasis in humans, IgG antibodies in calves with schistosomiasis may exhibit inhibitory functions toward GST enzymatic activity or have a modulatory effect on IgA antibody properties. Furthermore, sera from animals immunized with recombinant S. bovis 28GST recognized the native S. mattheei 28GST and achieved comparable levels of inhibition of activity of recombinant S. bovis 28GST and S. matthei 28GST, indicating the presence of cross-reactive epitopes on these two molecules.
During the past few years, the increasing interest devoted to the development of the immunological control of schistosome infections has led, through the introduction of monoclonal antibody and molecular biology methodologies, to the characterization of a number of schistosome antigens exhibiting protective properties towards schistosome challenge infections. Among these many vaccine candidates, schistosome 28-kDa glutathione S-transferases (28GSTs) perhaps represent the most promising molecules.
Since the first characterization of 28GST in S. mansoni (1, 2), the protein has been cloned, sequenced, and expressed in both Escherichia coli and Saccharomyces cerevisiae (2). The native and recombinant proteins were shown to induce highly significant levels of protection in various animal models, such as mice, rats, hamsters, and baboons (2, 3, 4, 10). This protection led to a reduction of worm burden (3) and/or an impairment of parasite fecundity, the latter having potentially major consequences for the development of egg-related pathology, e.g., granuloma formation (4).
Protective effects of GST were also demonstrated against Schistosoma bovis experimental infections in ruminants (5, 7). Immunization of calves with native S. bovis GSTs induced a reduction of the egg burden without any effect on the number of worms (7), whereas vaccination of goats with the recombinant S. bovis-derived 28GST (recombinant S. bovis 28GST) affected worm counts with no impairment of fecundity (5).
The mechanisms underlying the protection induced by immunization with the S. mansoni 28GST have been studied with monoclonal antibodies. The effect upon fecundity seems to be linked to the inactivation of the enzymatic activity, whereas the reduction of the worm burden appears to be independent of the GST enzyme activity (32). In human schistosomiasis, specific immunoglobulin A (IgA) antibodies to S. mansoni 28GST, which displayed a neutralizing effect on the enzymatic activity of the molecule, have been shown to significantly impair in vitro the egg laying of female worms and the hatching of S. mansoni eggs (12). The existence of a link between the inactivation of the enzymatic activity of S. mansoni 28GST and the effect on fecundity is further supported by the data collected from immunization experiments involving synthetic peptides derived from the primary structure of S. mansoni 28GST. Immunization with the N- or C-terminal peptides involved in the catalytic site of the molecule mainly affects worm fertility, whereas immunization with the central peptide from positions 115 to 131 induces a reduction of the worm burden (22, 31, 33). Comparative analysis of the 28GST sequences undertaken with different species of schistosomes revealed slight amino acid variations in the central peptide from positions 115 to 131, supporting the species specificity and a highly conserved structure for the C- and N-terminal peptides (29). The latter could explain the significant decrease of Schistosoma haematobium egg production recorded for primates (Erythrocebus patas) after immunization with S. mansoni 28GST (6).
Recently, we were able to demonstrate that immunization of calves with recombinant S. bovis 28GST induced significant reductions in the female worm burdens, fecal egg counts, and excretion of viable eggs, as determined by miracidial counts, in animals exposed to natural Schistosoma mattheei infection in the field (8). In contrast, the same immunization had no protective effect against a heavy experimental challenge with S. mattheei. In the present paper, we report our study of the molecular basis of the cross protection recorded for the natural infection in which we examined at the humoral level the susceptible parameters to explain why there were differences in protective effects between the two experiments.
MATERIALS AND METHODS
Immunization trials and blood samples.
The serum samples were collected in Zambia during two immunization trials aimed at testing the potential of recombinant S. bovis 28GST to protect cattle against S. mattheei infection (8). These studies involved a total of 28 castrated male calves (Friesian) aged 4 to 6 months. The calves were divided by live weight into two equal groups of 14 calves each. The first group received two intramuscular injections of 0.250 mg of recombinant S. bovis 28GST in phosphate-buffered saline (PBS) emulsified in an equal volume of complete Freund’s adjuvant (CFA; Sigma) at a 3-week interval (vaccinated group). The second group also received two injections but with PBS emulsified in CFA only (control group). All calves were then challenged 2 weeks after the second inoculation (vaccinated calves; n = 14) or adjuvants alone (controls; n = 14). In the first experiment, seven vaccinated and seven control animals were exposed to 10,000 S. mattheei cercariae percutaneously. All animals developed clinical schistosomiasis 7 to 8 weeks after challenge. At the time of perfusion 12 weeks after challenge, no significant differences between the vaccinated and control groups in the fecal egg counts (vaccinated, 887 ± 497 eggs/g of feces [mean ± standard deviation]; control, 541 ± 497 eggs/g), tissue egg counts (vaccinated, 4,236 × 103 ± 1,625 × 103 eggs; control, 3,397 × 103 ± 3,261 × 103 eggs), or worm burdens (vaccinated, 6,515 ± 2,199 worms; control, 5,989 ± 538 worms) were observed. These results indicate that the immunization protocol used did not protect calves against the single massive experimental challenge. In the second experiment, another two groups of seven vaccinated and seven control animals were challenged naturally over a period of 9 months on a farm known for S. mattheei endemicity. Natural infections were much weaker in intensity, as indicated by the mean fecal egg count (13 eggs/g), worm count (139 worms), and tissue egg count (294,999 eggs) in nonvaccinated controls. In the vaccinated calves, significant reductions in female worm burdens (50%), fecal egg counts (89%), and miracidial counts (93%) were recorded. Total tissue egg counts were also reduced by 42% in vaccinated animals. It was concluded that in cattle, recombinant S. bovis 28GST could provide significant protection against S. mattheei under conditions of low to moderate natural infection.
During the course of the two vaccination trials, blood samples were collected from the jugular vein on a weekly basis from experimentally infected animals and every fortnight during the natural infection. Sera were stored at −20°C and transported to the Institut Pasteur at Lille, France, for further analysis.
Experimental setup.
Animals were vaccinated with a heterologous antigen. Therefore, two major series of serological tests were considered. In the first series conducted on all samples, the serological responses to the recombinant S. bovis 28GST were evaluated by measuring both the IgG- and IgA-specific antibody responses and the neutralizing effects of the bovine sera on the enzymatic activity of the recombinant molecule. In the second series of tests, the cross-reactivity between recombinant S. bovis 28GST and the native S. mattheei 28GST was examined by using the Western blot, enzyme-linked immunosorbent assay (ELISA), and inhibition of enzymatic activity techniques that are outlined in detail below. Because of the limited supply of S. mattheei 28GST, these tests were carried out only on sera collected from calves involved in the natural infection trial at the end of the immunization period (day 0) and after 6 and 32 weeks of residence on the farm. In addition, the antibody response to S. mansoni soluble egg antigens (SEA) in sera from naturally infected calves was monitored.
Antigens.
Recombinant S. bovis 28GST was prepared as described previously (29). Native S. mattheei 28GST was prepared from S. mattheei adult worms collected in Zambia from experimentally infected cattle by using a glutathione (GSH)-agarose column as described previously (15). The purity of the preparation was controlled by electrophoresis, and the enzyme-specific activity was determined as described previously (29). S. mansoni SEA were prepared as described before (24).
Electrophoresis and Western blot analysis.
Schistosome antigens were fractionated and analyzed on a sodium dodecyl sulfate–13% polyacrylamide gel electrophoresis (SDS–13% PAGE) gel under reducing conditions (18). After transfer to nitrocellulose sheets (28), strips were incubated overnight at 4°C with bovine sera diluted 1/500 in PBS containing 3% (wt/vol) bovine serum albumin (PBS-BSA). Detection of antibody binding was performed with mouse anti-bovine isotype monoclonal antibodies (Sigma Inc., St. Louis, Mo.) specific for bovine IgG or IgA (Sigma) diluted in PBS-BSA at 1/500 and 1/100, respectively, overnight at 4°C. The strips were then washed six times with PBS-BSA and incubated with horseradish peroxidase-labelled anti-mouse total immunoglobulin (Sigma; 1/500 in PBS-BSA), and the enzymatic reaction was visualized by using 4-chloro-1-naphthol as the substrate (Sigma).
Analysis of humoral responses.
The specific IgA and IgG antibody responses to schistosome GSTs were analyzed by specific ELISA methods. Maxisorb polystyrene plates (Nunc, Roskilde, Denmark) were coated with 100 μl of recombinant S. bovis 28GST antigen diluted (1 μl/ml) in Na2CO3 buffer (0.1 M, pH 9.5). After 18 to 24 h of incubation at 4°C, the antigen solution was removed and the wells were saturated for 2 h at room temperature with a 5% milk solution in PBS (0.1 M, pH 7.4) and then washed twice with PBS before the addition of 100 μl of diluted bovine sera (1/100 for IgG and 1/20 for IgA). After a second incubation for 18 to 24 h at 4°C, the plates were washed five times with a 0.3% Tween 20 solution in PBS (TPBS) and incubated with mouse anti-bovine isotype monoclonal antibodies (Sigma) diluted in PBS (100 μl/well) at 1/1,000 for IgG and 1/500 for IgA. After an overnight incubation at 4°C and five washes with TPBS (100 μl/well), 100 μl of horseradish peroxidase-labelled anti-mouse total immunoglobulins (Sigma) diluted in PBS (1/5,000) was added, and the plates were incubated for 4 to 5 h at room temperature. The plates were washed five times in TPBS, and the enzymatic reaction was performed in the presence of the substrate orthophenylenediamine (Sigma) supplemented with 0.03% (vol/vol) H2O2 in phosphate citric buffer (pH 7.5). The reaction was stopped after 20 min by the addition of 1.0 N H2SO4 (100 μl/well), and the optical density was read at 492 nm. A similar protocol was applied for the evaluation of the anti-SEA immune response by using plates coated with a 5-μg/ml antigen solution.
Inhibition of enzymatic activity.
Inhibition of the GST catalytic activity was performed as described previously (13) by using chloro-2,4-dinitrobenzene (CDNB; Sigma) as the substrate for GST activity. For the inhibition, 0.1 μg of recombinant or native GST was incubated with 20 μl of bovine serum for 1 h at 37°C and for another 4 h at 4°C. After incubation, the residual enzymatic activity was evaluated after the addition of 200 μl of potassium phosphate solution (50 mM, pH 6.5), 5 mM GSH, and 1 mM CDNB. The absorbance was measured at 340 nm every 15 s for up to 2 min. Results were compared to those obtained in parallel with the appropriate controls (normal bovine sera or buffer).
Statistical analysis.
Relationships between specific IgG and IgA antibody responses to recombinant S. bovis 28GST and levels of inhibition of GST enzymatic activity achieved by serum samples were examined by using Spearman’s rank correlation test. Statistical significance was inferred as P of <0.05 at least.
RESULTS
IgG and IgA antibody responses.
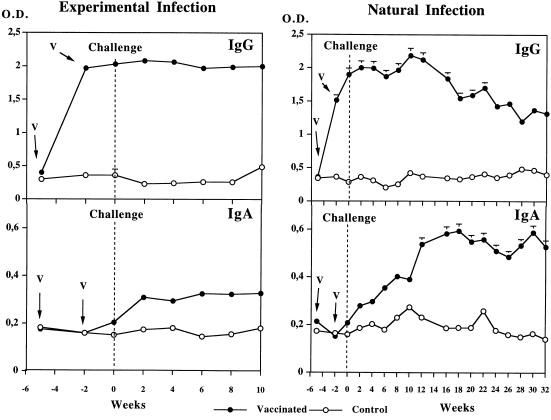
The changes in IgG and IgA antibody responses to recombinant S. bovis 28GST were monitored over 15 weeks for the experimentally infected calves and over 37 weeks for those infected naturally (Fig. 1).
FIG. 1.
Specific IgG and IgA antibody responses in cattle vaccinated with recombinant S. bovis 28GST and in nonvaccinated animals receiving adjuvant alone (CFA). Animals were exposed to experimental or natural challenge with S. mattheei 2 weeks after the second immunization. Results are expressed as the mean value for seven animals in each group. V, date of vaccination; O.D., optical density.
In both trials, the first injection of recombinant S. bovis 28GST elicited a strong specific IgG response which was not significantly amplified by the second injection. After the challenge, the average level of IgG antibodies remained stable until week 10 in experimentally infected calves. In naturally infected calves, it fluctuated until week 13 and then decreased significantly. Between weeks −5 and +10 of the experiment, no marked differences in IgG antibody titers between calves infected experimentally and those infected naturally were recorded.
The IgA antibody responses showed a different pattern (Fig. 1). During the immunization period, the signal observed remained very close to background levels. The specific IgA antibody response to recombinant S. bovis 28GST increased after experimental exposure to S. mattheei and remained stable up to week 10. In the naturally infected animals, a gradual increase in IgA antibody levels was observed after the introduction of calves onto the farm, with the highest titers recorded around week 20. Control animals did not display IgG or IgA antibody responses to recombinant S. bovis 28GST even after 32 weeks of natural exposure to S. mattheei infection.
Antibody response to S. mansoni egg antigens.
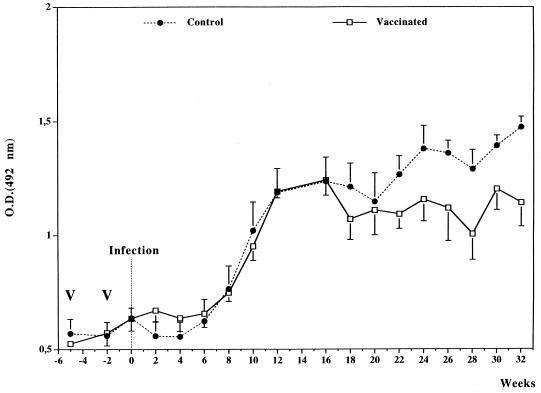
The changes in IgG antibody responses to S. mansoni egg antigens in vaccinated and control calves exposed to natural infection are shown in Fig. 2. The specific IgG antibody responses increased in both groups between weeks 6 and 11 after the introduction onto the farm and remained stable thereafter.
FIG. 2.
Specific IgG antibody response to S. mansoni SEA in calves vaccinated with recombinant S. bovis 28GST and in nonvaccinated animals receiving adjuvant alone (CFA). Animals were exposed to S. mattheei natural challenge over a period of 32 weeks starting 2 weeks after the second immunization. Results are expressed as the mean value for seven animals. V, date of vaccination, O.D., optical density.
Inhibition of GST enzymatic activity and correlation with antibody responses.
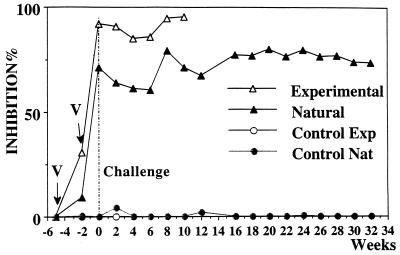
Analyses of the neutralizing effects of sera on the GST activity of recombinant S. bovis 28GST indicated a strong inhibitory effect in vaccinated animals when compared to that of nonvaccinated controls (Fig. 3). Despite individual fluctuations, levels of inhibitory activity were comparable for the two groups of vaccinated animals (inhibition values between 40 and 100%).
FIG. 3.
Analysis of the blocking effect of sera from calves upon recombinant S. bovis 28GST enzymatic activity. Calves vaccinated with recombinant S. bovis 28GST and control animals receiving the adjuvant alone (CFA) were challenged either experimentally (Exp) or naturally (Nat) with S. mattheei. Recombinant S. bovis 28GST (0.1 μg) was incubated with 20 μl of sera. The percentage of inhibition was calculated by comparison with inhibition by normal calf serum. Results are expressed as the mean value of seven samples. V, date of vaccination.
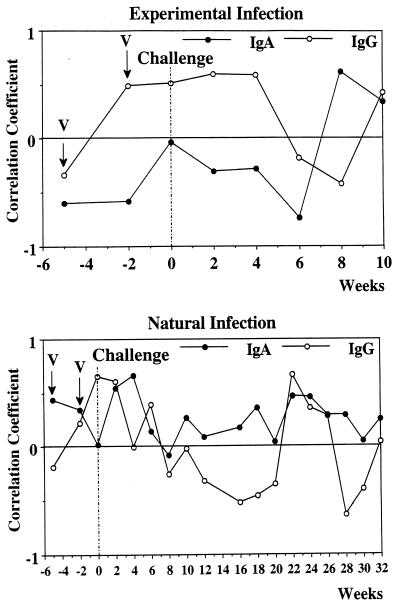
To evaluate the respective involvement of IgG and IgA antibodies in the neutralization of recombinant S. bovis 28GST catalytic activity, a correlation analysis between the respective amounts of IgG- and IgA-specific antibodies to recombinant S. bovis 28GST and the levels of inhibition achieved by the different bovine samples was undertaken (Fig. 4).
FIG. 4.
Correlation coefficients between IgG and IgA antibodies to recombinant S. bovis 28GST and inhibition of GST enzymatic activity in calves vaccinated with recombinant S. bovis 28GST and challenged either experimentally or naturally with S. mattheei. V, date of vaccination.
For the experimental infection, a positive association with IgG antibodies (r = 0.210, P > 0.05) and a negative coefficient value for IgA antibody response (r = 0.203, P > 0.05) were recorded. For the natural infection, the pattern appears to be more complex. However, globally, the inhibitory activity seems to be supported by IgA antibodies (r = 0.266, P < 0.05) whereas a nonsignificant correlation coefficient of 0.003 at a P of > 0.05 was observed for the IgG counterpart. Fluctuations occur, and the correlation values recorded for IgG and IgA appear at some points to fluctuate in opposite manners.
Cross-reactive analysis between recombinant S. bovis 28GST and native S. matthei 28GST.
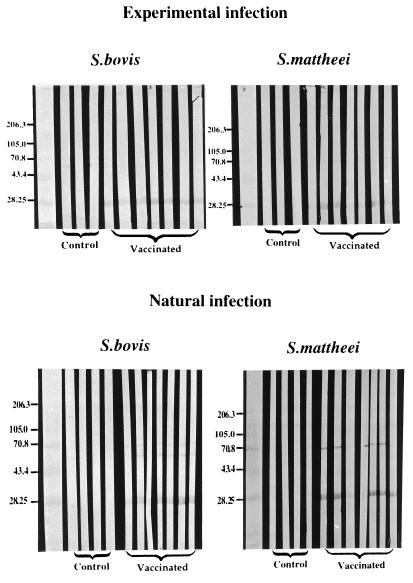
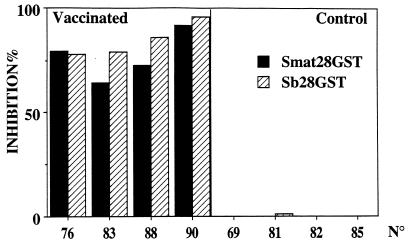
Results of the Western blot analysis of sera collected on day 0, when calves were transported to the farm, are presented in Fig. 5. Western blot strips obtained after SDS-PAGE separation of recombinant S. bovis 28GST or S. matthei 28GST were probed with sera from vaccinated or control calves. Results show that both S. mattheei and S. bovis GSTs are recognized by the sera of calves immunized with recombinant S. bovis 28GST. Similar results were obtained with sera collected after 6 and 32 weeks of natural exposure. This suggests the existence of cross-reactive epitopes on the two molecules, an assumption that was further supported by the results of an ELISA in which comparable signals for the different serum samples on plates coated with one or the other antigen were recorded (data not shown). The cross-reactivity of the sera was also visualized at the enzymatic level during a test of the inhibition of the GST enzymatic activity (Fig. 6) in which the same serum samples achieved comparable levels of inhibition of the GST activity of either recombinant S. bovis 28GST or S. matthei 28GST.
FIG. 5.
Western blot analysis of sera from calves immunized with recombinant S. bovis 28GST and from control animals receiving adjuvant alone (CFA). Animals were challenged either experimentally or naturally with S. mattheei. Western blot strips were obtained after an SDS–13% PAGE migration of either recombinant S. bovis 28GST or native S. mattheei 28GST. Molecular masses of standards (in kilodaltons) are indicated to the left of the panels.
FIG. 6.
Analysis of the blocking effect of the sera from calves on recombinant S. bovis 28GST (Sb28GST) and native S. bovis 28GST (Smat 28GST) enzymatic activities. Calves vaccinated with the recombinant S. mansoni 28GST and control animals receiving the adjuvant alone (CFA) were challenged either experimentally or naturally with S. mattheei. The recombinant S. bovis 28GST (0.1 μg) or native S. mattheei 28GST (0.1 μg) were incubated with 20 μl of sera. The percentage of inhibition was calculated by comparison to inhibition by normal calf serum. Results are expressed as the mean value of two different experiments carried out on individual samples.
DISCUSSION
The kinetic analysis of the antibody response to recombinant S. bovis 28GST carried out for the two groups of vaccinated calves has demonstrated the presence of specific IgA and IgG antibodies to this antigen. The IgG antibody profiles appeared to be very similar in the two groups of calves before and after challenge with S. mattheei, whereas the IgA antibody levels increased only after challenge.
It is unfortunate that the comparative study of the IgA responses in the experimental and natural infection groups was limited to 10 weeks after challenge. In the natural infection group, a continuous increase in IgA was observed after introduction of the calves onto the farm, suggesting that repeated contacts with the parasite could act as a potent boost of the IgA response. It is known that IgA responses are preferentially induced when antigen is delivered via the mucosal surfaces (21). It is generally assumed that cattle become infected mainly by cercarial penetration of the skin when they enter water for drinking. However, it has been demonstrated that the peroral route of infection could also be of importance in cattle (14, 19, 30), and it is quite possible that the naturally challenged animals came in contact with the parasite while drinking. The delayed appearance of IgA in cattle as compared to that of its IgG counterpart has also been reported for murine schistosomiasis, where schistosome eggs were described as an essential factor in the induction of the IgA antibody response via the induction of appropriate cytokines (24). It is possible that egg deposition also amplified the IgA antibody response in calves naturally infected with S. mattheei.
The production in all naturally infected calves of antibodies against S. mansoni SEA 6 weeks after introduction onto the farm suggests an early contact with schistosome infection. However, the nonvaccinated control animals did not display any antibody response to recombinant S. bovis 28GST during the 32 weeks of exposure to natural S. mattheei challenge. This is perhaps not surprising since antibody responses against schistosome 28GSTs in other animal models infected with schistosomes have been reported to be low as compared to the one observed in animals immunized parenterally (5–7).
The inhibitory activity of the sera from vaccinated calves was studied in the context of the relationship observed in human S. mansoni infections between IgA antibodies to S. mansoni 28GST, the inhibition of the enzymatic activity, and the impairment of parasite fecundity (12). The levels of inhibition achieved in the two groups of animals naturally and experimentally exposed to S. mattheei appear to be very similar and range between 40 and 100%. Nevertheless, this inhibitory effect does not seem to be supported by the same isotype (Fig. 4). In experimentally infected calves, the inhibition of the GST activity seems to be globally linked with the presence of specific IgG antibodies, whereas in naturally infected animals, impairment of the catalytic activity of the antigen appears to be essentially associated with IgA antibodies. In addition, IgG antibodies appear at some points during the period of natural exposure to also contribute to the inhibitory activity, more precisely at the beginning of the experiment and again between weeks 21 and 25. Interestingly, the correlation profiles for IgG and IgA fluctuated in opposite manners during the period of natural exposure to S. mattheei. A decrease in the correlation value for one isotype coincided with the increase of the same parameter for the other isotype, except for the period between weeks 21 and 25, in which both IgG and IgA were positively associated with the inhibition. This suggests that in contrast to antibodies in human schistosomiasis (12), IgG antibodies in cattle also exhibit inhibition activity in the neutralization of a GST(s) or may express at some time points a modulatory effect toward IgA antibodies. Indeed, interactions between IgG and IgA antibodies have been previously reported in various experimental models (11, 25–27). Furthermore, another explanation for the participation of IgG in the impairment of the enzymatic activity could be related to the fact that the dominant mucosal immunoglobulin in ruminants is thought to be an IgG1 subclass that is selectively transported into secretions by a mechanism involving secretory component (16) and thus may share some of the properties of the ruminant IgA antibodies (16).
Since animals were immunized with a heterologous protein (recombinant S. bovis 28GST), it was essential to analyze the molecular basis of the cross protection observed. Sera collected from vaccinated calves before challenge with S. mattheei recognized both recombinant S. bovis 28GST and S. matthei 28GST. This indicates the presence of cross-reactive epitopes between the two antigens. Trottein et al. (29) have pointed out a high degree of homology in the primary structure of different species of schistosome 28GSTs, e.g., S. mansoni, S. haematobium, and S. bovis. This may explain the positive protective effects recorded in the heterologous vaccination trial performed with patas monkeys infected with S. haematobium and previously immunized with recombinant S. mansoni 28GST (6). In that model, the protective effect was targeted towards worm fecundity. In the present experiment, vaccination induced significant reductions in female worm burdens, fecal egg counts, and miracidial counts in naturally infected calves. Although the existence of a vaccine-induced decline in egg viability could not be demonstrated, a comparison of the levels of reduction in female worm burdens (50%), tissue egg counts (42%), and miracidial counts (93%) suggests that such a decline in egg viability may have also occurred (8). This coincided with the expression of a strong inhibitory activity of sera towards the catalytic property of GSTs, which suggests that at least in the naturally infected groups, the enzymatic site plays the predominant role in the elicitation of the antiviability effect on eggs.
Recent studies on the ultrastructural localization of 28GST in the female worm genital system of S. mansoni have demonstrated an association between the intensity of S. mansoni 28GST expression and the process of egg maturation. This suggests the participation of this antigen in egg formation (20). The reduction in excretion of viable eggs observed in our experiment, and attributed at least partly to reduced egg hatching, could be a consequence of the blocking effect on the functional properties of the GST molecule. This reduction may also be linked to the binding of antibodies to S. mansoni 28GST “liberated” from the egg through the shell (23) and thus limiting the detoxification essential for egg survival and subsequent hatching. Indeed, it has been reported that a rat monoclonal antibody to S. mansoni 28GST which inhibits the catalytic property of the antigen was able to reduce in vitro and/or in vivo egg laying and egg hatching (32). Moreover, antibodies have been found on the eggshells during natural S. haematobium infection in humans (17) and antiembryonation effects have been described in schistosomiasis japonica (9).
No clear explanation for the lack of any protective effect recorded in the experimentally challenged animals was found. On the one hand, serological responses were monitored only for 10 weeks after experimental challenge, and it is possible that the animals were slaughtered before the mechanisms involved in protection started to act. Such a delay in the expression of protective effects has been observed in patas monkeys experimentally infected with S. haematobium after vaccination with S. mansoni 28GST (6). Boulanger et al. (6) recorded the maximum differences between vaccinated and control animals at 42 weeks postinfection, in comparison to similar levels of fecal egg excretion at 12 weeks postinfection. On the other hand, the lack of a protective effect in the experimentally challenged animals may be linked to the high recovery rates due to the high susceptibility of calves to S. mattheei infection. Therefore, the sudden and massive infection may have overcome the immune response elicited by vaccination. This raises questions of the biological relevance of massive experimental challenges in the evaluation of protective immunity to schistosome infections.
In conclusion, despite the differences recorded between the models of infection, our data confirm the protective properties of schistosome 28GST and provide new perspectives for the development of vaccines able to reduce transmission of infection.
ACKNOWLEDGMENTS
This work received financial support from European Union contracts Bio2CT-CT930111 and STD3-910030. This work was also supported by the Institut Pasteur de Lille, IFR No. 17, Unité INSERM U-167, and the Région Nord-Pas de Calais.
We thank Gilles Riveau and D. J. Shaw for helpful discussions.
REFERENCES
- 1.Balloul J M, Pierce R J, Grzych J-M, Capron A. In vitro synthesis of a 28 kilodalton antigen present on the surface of the schistosomulum of Schistosoma mansoni. Mol Biochem Parasitol. 1985;17:105–114. doi: 10.1016/0166-6851(85)90131-8. [DOI] [PubMed] [Google Scholar]
- 2.Balloul J M, Sondermeyer P, Dreyer D, Capron M, Grzych J-M, Pierce R J, Carvallo D, Lecocq J P, Capron A. Molecular-cloning of a protective antigen of schistosomes. Nature (London) 1987;326:149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- 3.Balloul J M, Grzych J-M, Pierce R J, Capron A. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J Immunol. 1987;138:3448–3453. [PubMed] [Google Scholar]
- 4.Boulanger D, Reid G D, Sturrock R F, Wolowczuk I, Balloul J M, Grezel D, Pierce R J, Otieno M F, Guerret S, Grimaud J A, Butterworth A E, Capron A. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991;13:473–490. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 5.Boulanger D, Trottein F, Mauny F, Bremond P, Couret D, Pierce R J, Kadri I S, Godin C, Sellin E, Lecocq J P, Capron A. Vaccination of goats against the trematode Schistosoma bovis with a recombinant homologous schistosome-derived glutathione S-transferase. Parasite Immunol. 1994;16:399–406. doi: 10.1111/j.1365-3024.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger D, Warter A, Mauny F, Bremond P, Audibert F, Couret D, Kadri I S, Godin C, Sellin E, Pierce R J, Lecocq J P, Sellin B, Capron A. Vaccination of patas monkeys experimentally infected with Schistosoma haematobium using a recombinant glutathione S-transferase cloned from S. mansoni. Parasite Immunol. 1995;17:361–369. doi: 10.1111/j.1365-3024.1995.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 7.Bushara H O, Bashir M E N, Malik K H E, Mukhtar M M, Trottein F, Capron A, Taylor M G. Suppression of Schistosoma bovis egg production in cattle by vaccination with either glutathione S-transferase or keyhole limpet hemocyanin. Parasite Immunol. 1993;15:383–390. doi: 10.1111/j.1365-3024.1993.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 8.De Bont J, Vercruysse J, Grzych J-M, Meeus P F M, Capron A. Potential of a recombinant Schistosoma bovis-derived glutathione S-transferase to protect cattle against experimental and natural S. mattheei infection. Parasitology. 1997;115:249–255. doi: 10.1017/s0031182097001352. [DOI] [PubMed] [Google Scholar]
- 9.Gracia E G, Mitchell G F, Rivera P T, Levardone R E, Tiu W U. Evidence for anti-embryonation immunity and egg destruction in mice sensitized with immature eggs of Schistosoma japonicum. Asian Pac J Allergy Immunol. 1987;5:137–141. [PubMed] [Google Scholar]
- 10.Grezel D, Capron M, Grzych J-M, Fontaine J, Lecocq J P, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28 GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23:454–460. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 11.Griffiss J M, Broub D, Bertram M A. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975;114:1779–1784. [PubMed] [Google Scholar]
- 12.Grzych J-M, Grezel D, Xu C B, Neyrinck J-L, Capron M, Ouma J H, Butterworth A E, Capron A. IgA antibodies to a protective antigen in human schistosomiasis mansoni. J Immunol. 1993;150:527–535. [PubMed] [Google Scholar]
- 13.Habig W H, Pubst M J, Jacoby W B. Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:730. [PubMed] [Google Scholar]
- 14.Kassuku A, Nansen P, Christensen N O. A comparison of the efficiency of percutaneous and per-oral routes of infection in caprine Schistosoma bovis infections. J Helminthol. 1985;59:23–28. doi: 10.1017/s0022149x0003443x. [DOI] [PubMed] [Google Scholar]
- 15.Ketterer B, Tan K H, Meyer D J, Coles B. A possible role in detoxification of the DNA and lipid hydroperoxydes. In: Mantle T J, Pisckett C D, Hayes J D, editors. Glutathion S-transferases and carcinogenesis. London, United Kingdom: Taylor and Francis; 1987. pp. 149–163. [Google Scholar]
- 16.Kilian M, Russel M W. Function of mucosal immunoglobulins. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 127–137. [Google Scholar]
- 17.Koech D K, Hirata M, Shimada M, Wambayi E. Precipitates found around Schistosoma haematobium eggs from human urine prior to circumoval precipitin test. Ann Trop Med Parasitol. 1984;78:627–632. doi: 10.1080/00034983.1984.11811874. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence, J. A., and J. B. Condy. 1970. The developing problem of schistosomiasis in domestic stock in Rhodesia. Cent. Afr. J. Med. 7(Suppl.):19–22. [PubMed]
- 20.Liu J L, Fontaine J, Capron A, Grzych J-M. Ultrastructural localization of Sm28GST protective antigen in Schistosoma mansoni adult worms. Parasitology. 1996;113:377–391. doi: 10.1017/s003118200006652x. [DOI] [PubMed] [Google Scholar]
- 21.Mestecky J, Abraham R, Ogra P L. Common mucosal immune system and strategies for the development of vaccines effective at the mucosal surfaces. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 357–372. [Google Scholar]
- 22.Pancré V, Wolowczuk I, Bossus M, Gras-Masse H, Guerret S, Delanoye A, Capron A, Auriault C. Evaluation of the effect of Sm28GST-derived peptides in murine hepatosplenic schistosomiasis: interest of the lipopeptidic form of the C-terminal peptide. Mol Immunol. 1994;31:1247–1256. doi: 10.1016/0161-5890(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 23.Porchet E, McNair A, Caron A, Kusnierz J P, Zemzoumi K, Capron A. Tissue expression of the Schistosoma mansoni 28 kDa glutathione S-transferase. Parasitology. 1994;109:565–572. doi: 10.1017/s0031182000076447. [DOI] [PubMed] [Google Scholar]
- 24.Poulain-Godefroy O, Gaubert S, Lafitte S, Capron A, Grzych J-M. Immunoglobulin A response in murine schistosomiasis: stimulatory role of egg antigen. Infect Immun. 1996;64:763–768. doi: 10.1128/iai.64.3.763-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell M W, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fabα fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–2249. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 26.Russell-Jones G J, Ey P L, Reynolds B L. The ability of IgA to inhibit the complement-mediated lysis of target red blood cells sensitized with IgG antibody. Mol Immunol. 1980;17:1173–1180. doi: 10.1016/0161-5890(80)90114-5. [DOI] [PubMed] [Google Scholar]
- 27.Russell-Jones G J, Ey P L, Reynolds B L. Inhibition of cutaneous anaphylaxis and Arthus reactions in mouse by antigen specific IgA. Int Arch Allergy Immunol. 1981;66:316–325. doi: 10.1159/000232836. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trottein F, Godin C, Pierce R J, Sellin B, Taylor M G, Gorillot I, Sampaio Silva M, Lecocq J P, Capron A. Interspecies variation of schistosome 28-kDa glutathione S-transferases. Mol Biochem Parasitol. 1992;54:63–72. doi: 10.1016/0166-6851(92)90095-2. [DOI] [PubMed] [Google Scholar]
- 30.Van Wyk J A, Bartsch R C, Van Rensburg L J, Heitmann L P, Goosen P. Studies on schistosomiasis. 6. A field outbreak of bilharzia in cattle. Onderstepoort J Vet Res. 1974;41:39–50. [PubMed] [Google Scholar]
- 31.Wolowczuk I, Auriault C, Bossus M, Boulanger D, Gras-Masse H, Mazingue C, Pierce R J, Grezel D, Reid G D, Tartar A, Capron A. Antigenicity and immunogenicity of a multiple peptidic construction of the Schistosoma mansoni Sm28GST antigen in rat, mouse, and monkey. J Immunol. 1990;146:1987–1995. [PubMed] [Google Scholar]
- 32.Xu C B, Verwaerde C, Grzych J-M, Fontaine J, Capron A. A monoclonal antibody blocking the Schistosoma mansoni 28-kDa glutathione S transferase activity reduces female worm fecundity and egg viability. Eur J Immunol. 1991;21:1801–1807. doi: 10.1002/eji.1830210804. [DOI] [PubMed] [Google Scholar]
- 33.Xu C B, Verwaerde C, Gras-Masse H, Fontaine J, Bossus M, Trottein F, Wolowczuk I, Tartar A, Capron A. Schistosoma mansoni 28-kDa glutathione S-transferase and immunity against parasite fecundity and egg viability. Role of the amino- and carboxyl-terminal domains. J Immunol. 1993;150:940–949. [PubMed] [Google Scholar]