Abstract
Objective:
To determine if machine learning (ML) can predict acute brain injury (ABI) and identify modifiable risk factors for ABI in venoarterial extracorporeal membrane oxygenation (VA-ECMO) patients.
Design:
Retrospective cohort study of the Extracorporeal Life Support Organization (ELSO) Registry (2009–2021).
Setting:
International, multicenter registry study of 676 ECMO centers.
Patients:
Adults (≥18 years) supported with VA-ECMO or extracorporeal cardiopulmonary resuscitation (ECPR).
Interventions:
None.
Measurements and Main Results:
Our primary outcome was ABI: central nervous system (CNS) ischemia, intracranial hemorrhage (ICH), brain death, and seizures. We utilized Random Forest, CatBoost, LightGBM and XGBoost ML algorithms (10-fold leave-one-out cross-validation) to predict and identify features most important for ABI. We extracted 65 total features: demographics, pre-ECMO/on-ECMO laboratory values, and pre-ECMO/on-ECMO settings.
Of 35,855 VA-ECMO (non-ECPR) patients (median age=57.8 years, 66% male), 7.7% (n=2,769) experienced ABI. In VA-ECMO (non-ECPR), the area under the receiver-operator characteristics curves (AUC-ROC) to predict ABI, CNS ischemia, and ICH was 0.67, 0.67, and 0.62, respectively. The true positive, true negative, false positive, false negative, positive, and negative predictive values were 33%, 88%, 12%, 67%, 18%, and 94%, respectively for ABI. Longer ECMO duration, higher 24h ECMO pump flow, and higher on-ECMO PaO2 were associated with ABI.
Of 10,775 ECPR patients (median age=57.1 years, 68% male), 16.5% (n=1,787) experienced ABI. The AUC-ROC for ABI, CNS ischemia, and ICH was 0.72, 0.73, and 0.69, respectively. The true positive, true negative, false positive, false negative, positive, and negative predictive values were 61%, 70%, 30%, 39%, 29% and 90%, respectively, for ABI. Longer ECMO duration, younger age, and higher 24h ECMO pump flow were associated with ABI.
Conclusions:
This is the largest study predicting neurological complications on sufficiently powered international ECMO cohorts. Longer ECMO duration and higher 24h pump flow were associated with ABI in both non-ECPR and ECPR VA-ECMO.
Keywords: machine learning, extracorporeal membrane oxygenation, acute brain injury, Extracorporeal Life Support Organization, neurological complications
Introduction
Extracorporeal membrane oxygenation (ECMO) is increasingly used for cardiopulmonary support.(1) Acute brain injury (ABI), which includes central nervous system (CNS) ischemia, intracranial hemorrhage (ICH) and hypoxic-ischemic brain injury, (HIBI) is reported to occur in up to 20% of adult venoarterial (VA)-ECMO patients(2) in the Extracorporeal Life Support Organization (ELSO) Registry. Furthermore, this rate is as high as 33% in VA-ECMO patients using noninvasive multimodal neuromonitoring at a single institution.(3) With greater ECMO usage and more cases of ABI, accurately predicting ABI with modifiable risk factors such as hyperoxia(4), low pulse pressure (PP)(5, 6), and hypercarbia(7) is important to lessen its occurrence.
In VA-ECMO, there have been several scoring systems developed to predict survival outcomes,(8–15) but their generalizability is limited as they stem from single-center studies, are focused in a specific subset of patients (e.g., only cardiogenic shock), and were created from logistic regression. Machine learning (ML) leverages big data to explore patterns and interactions without explicit programming from humans, thus offering distinct advantages to traditional regression.(16) Furthermore, coupled with the large sample size of the ELSO Registry, ML may be the most promising technique to adequately synthesize demographic and laboratory information to effectively predict ABI.(17) Additionally, identifying variables in the ML model that impact clinical outcomes will inform ECMO clinicians for mitigation of key risk factors for ABI.
Current literature applying ML to predict outcomes in ECMO patients is sparse and primarily focused on non-neurological outcomes such as thrombosis/hemorrhage and mortality.(18–20) An ELSO Registry analysis of VA-ECMO patients (n = 23,812) demonstrated ML yielded better prediction for in-hospital mortality (AUC-ROC = 0.80) versus the SAVE score (AUC-ROC = 0.61).(19) This study demonstrated the power of ML when applied to the ELSO Registry, and provided the impetus for this study designed to test the capability of ML to predict ABI.
Herein, we aimed to leverage ML to predict ABI in a large international cohort (the ELSO Registry) of ECMO patients.
Methods
Study design and population
The Johns Hopkins Hospital Institutional Review Board approved this retrospective observational study (IRB00216321) with a waiver of informed consent. “Retrospective Analysis of Outcomes of Patients on Extracorporeal Membrane Oxygenation” is the study title. All procedures were followed in accordance with the Helsinki Declaration of 1975 and the ethical standards of the responsible committee on human experimentation (institutional or regional). The ELSO Registry is an international multicenter database from over several hundred ECMO centers worldwide.(21) It collects clinical characteristics and demographics, pre-ECMO and on-ECMO laboratory values such as arterial blood gas (ABG), on-ECMO complications, and outcomes like in-hospital mortality.16 Comorbidity information was captured using the International Classification of Diseases, 10th Revision (ICD-10) codes.
We included patients who were 1) 18 years of age or older; and 2) supported with VA-ECMO for extracorporeal cardiopulmonary resuscitation (ECPR) and non-ECPR indications from 2009–2021. We excluded repeat ECMO runs within the same patient to avoid bias and complexity. VA-ECMO and ECPR cohorts were analyzed separately.
Data collection
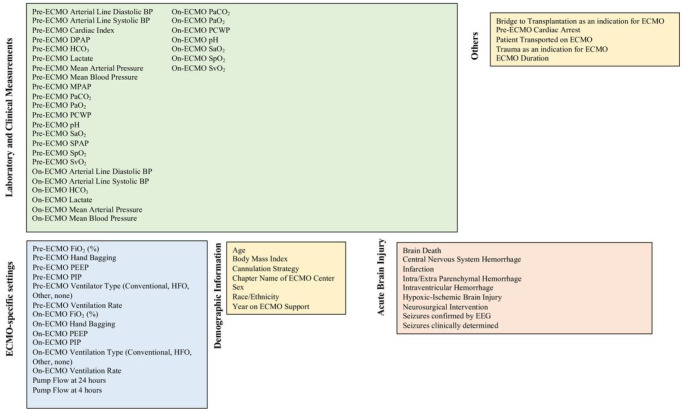
In total, 65 variables were collected (Fig. 1) for ML. The ELSO Registry collects ABG and hemodynamics pre-ECMO support and on-ECMO. Both pre-ECMO ventilator settings and ABGs were drawn within 6 hours of starting ECMO cannulation. If multiple ABGs existed within a specific period, the pre-ECMO ABG that was nearest to the start of ECMO cannulation was chosen. On-ECMO hemodynamic and ABG information were drawn closest to 24 hours of ECMO support. Values that were meant to be obtained simultaneously such as systolic and diastolic blood pressure and oxygen saturation by pulse oximetry and by arterial blood gas were abstracted by a trained ELSO data manager/abstracter from each center and were collected concurrently.
Figure 1.
All 65 variables incorporated into our machine learning models including laboratory values, ECMO settings, demographics, other variables, and our primary outcome (acute brain injury). BP: blood pressure. CI: cardiac index. DBP: diastolic blood pressure. DPAP: diastolic pulmonary arterial pressure. ECMO: extracorporeal membrane oxygenation. EEG: electroencephalogram. FiO2: fraction of inspired oxygen. HFV: high frequency ventilator. MPAP: mean pulmonary arterial pressure. PaO2: partial pressure of oxygen. PaCO2: partial pressure of carbon dioxide. PCWP: pulmonary capillary wedge pressure. PEEP: positive-end expiratory pressure. PIP: peak inspiratory pressure. SPAP: systolic pulmonary arterial pressure. SaO2: arterial blood gas oxygen saturation. SpO2: peripheral oxygen saturation. SvO2: mixed venous oxygen saturation.
Definitions
ABI was defined as the presence of infarction (ischemic stroke), diffuse ischemia (HIBI), intra/extra parenchymal hemorrhage, intraventricular hemorrhage, seizures determined by electroencephalograph or clinically, and neurosurgical intervention (examples include intracranial pressure monitor, external ventricular drain, and craniotomy) during ECMO support. CNS ischemia was defined as ischemic stroke (determined by ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI))) and HIBI (determined by CT or MRI). ICH was defined as intra/extra parenchymal hemorrhage and intraventricular hemorrhage (both determined by CT or MRI). Definitions for other variables included in our analysis are in the Supplemental Methods.
Outcomes
The primary outcome was the occurrence of ABI during ECMO support. Secondary outcomes included subtypes of ABI such as CNS ischemia and ICH.
Statistical analysis
Continuous variables were represented as median with interquartile range. Categorical variables were presented as frequency with percentages. The Wilcoxon rank-sum and Pearson’s chi-square tests were utilized to compare continuous and categorical variables, respectively. Statistical significance was set at a p-value < 0.05.
Data Pre-Processing
All categorical variables were one hot-encoded prior to running ML algorithms. Multiple imputation was used for missing data. All missing variables are shown in Supplemental Table 1.
Machine Learning Algorithm and Pipeline
We examined the suitability of 4 ML algorithms in predicting ABI from the ELSO Registry containing variables from pre-ECMO support and during ECMO support: Random Forest, CatBoost, LightGBM and XGBoost. For each algorithm, we fine-tuned the hyperparameters and used a Bayesian optimization onto our dataset split randomly into training (70%) and test (30%) sets. Further details are noted in the Supplemental Methods.
Feature Importance Scores in ML
To better understand how these ML models were constructed and to determine which variables were most important in predicting ABI, we analyzed which variables were of highest importance in correctly predicting ABI. Specifically, we examined the ranked feature importance in the best performing models, which discloses the contribution of each variables in the composition of the boosted decision trees within the model. Furthermore, Feature Importance Scores and Shapley Additive Explanations (SHAP) values depict the contribution of a variables on the predictions of the model (Supplemental Methods). Both Feature Importance Scores and SHAP values add interpretability to the model framework and reveal pertinent clinical variables associated with ABI. All statistical analyses were performed using R Studio (R 4.1.2, www.r-project.org) and Python.
Results
VA-ECMO (non-ECPR)
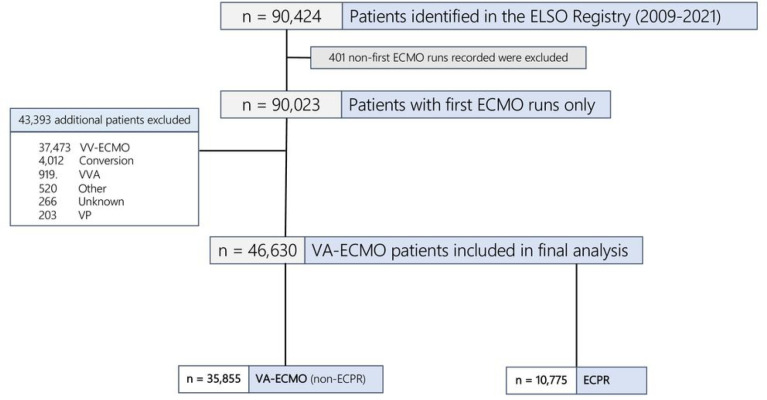
Of 35,855 VA-ECMO (non-ECPR) patients, 2,769 (8%) had ABI (Supplemental Table 2, Fig. 2). The median age was 57.8 years (interquartile range, IQR:45.9–66.4) and 66% (n = 23,542) were male. The median duration of ECMO support was 4.3 days (IQR:2–7.7).
Figure 2.
Flowchart of study cohort (VA-ECMO and ECPR patients) from the ELSO Registry in 2009–2020. ECMO = extracorporeal membrane oxygenation, VA = venoarterial, VV = venovenous, Conversion = VA -> VV or VV -> VA, ECPR = extracorporeal cardiopulmonary resuscitation, VVA = venovenoarterial, Other = mode not defined, VP = venopulmonary
Model Performance
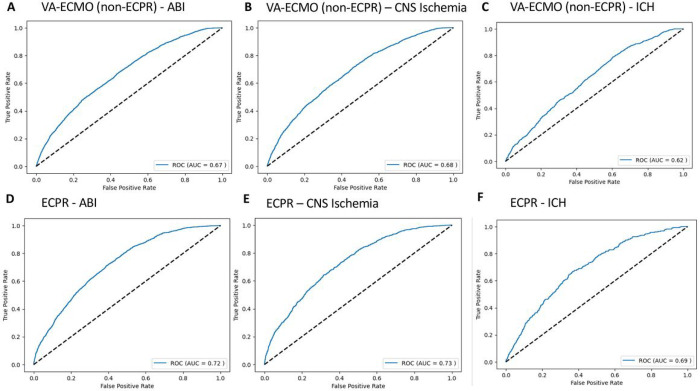
Using the leave-one-out-cross-validation (LOOCV) 10-fold approach, for predicting ABI in VA-ECMO patients, the model achieved an AUC-ROC of 0.67 (Fig. 3A). The accuracy of the model was 83%. The true positive rate, true negative rate, false positive rate, and false negative rate were 33%, 88%, 12%, and 67%, respectively (Table 1). The PPV and NPV were 18% and 94%, respectively.
Figure 3.
Receiver-operating characteristic curves for predicting A) acute brain injury (ABI), B) central nervous system (CNS) ischemia, and C) intracranial hemorrhage (ICH) in venoarterial extracorporeal membrane oxygenation (VA-ECMO) patients and for predicting D) ABI, E) CNS ischemia, and F) ICH in extracorporeal cardiopulmonary resuscitation (ECPR) patients.
Table 1.
Model performance in venoarterial extracorporeal membrane oxygenation patients for predicting acute brain injury, central nervous system ischemia, and intracranial hemorrhage.
| AUC-ROC | Acc | TPR | TNR | FPR | FNR | PPV | NPV | Precision | Recall | F1 | Brier Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABI | 0.67 | 83% | 33% | 88% | 12% | 67% | 18% | 94% | 0.18 | 0.34 | 0.24 | 0.175 |
| CNS Ischemia | 0.67 | 86% | 33% | 88% | 12% | 67% | 11% | 97% | 0.11 | 0.34 | 0.16 | 0.21 |
| ICH | 0.62 | 97% | 5% | 99% | 1% | 95% | 8% | 98% | 0.10 | 0.06 | 0.08 | 0.095 |
AUC-ROC: area under the receiver-operating characteristic curve. Acc: Accuracy. TPR: True Positive Rate. TNR: True Negative Rate. FPR: False Positive Rate. FNR: False Negative Rate. PPV: Positive Predictive Value. NPV: Negative Predictive Value. ABI: acute brain injury. CNS: central nervous system. ICH: intracranial hemorrhage.
For predicting CNS ischemia, the model achieved an AUC-ROC of 0.67 (Fig. 3B). The accuracy of the model was 86%. The true positive rate, true negative rate, false positive rate, and false negative rate were 33%, 88%, 12%, and 67%, respectively. The PPV and NPV were 11% and 97%, respectively.
For ICH, the model achieved an AUC-ROC of 0.62 (Fig. 3C). The accuracy of the model was 97%. The true positive rate, true negative rate, false positive rate, and false negative rate were 5%, 99%, 1%, and 95%, respectively. The PPV and NPV were 8% and 98%, respectively.
Feature Importance
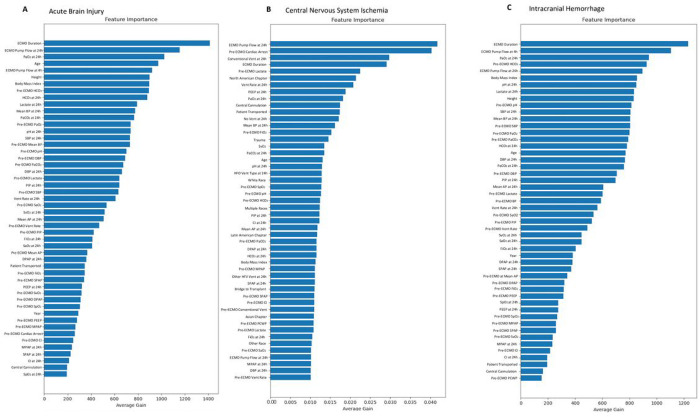
We identified the top 3 most important variables per Feature Importance Scores and depict the remaining variables (Fig. 4A, Supplemental Fig. 1A, Supplemental Table 3). The top 3 variables in predicting ABI were duration of ECMO support, ECMO pump flow rate at 24 hours, and on-ECMO PaO2.
Figure 4.
Feature Importance Scores for A) acute brain injury, B) central nervous system ischemia, and C) intracranial hemorrhage in VA-ECMO (non-ECPR) patients. AP: arterial pressure. BP: blood pressure. CI: cardiac index. DBP: diastolic blood pressure. DPAP: diastolic pulmonary arterial pressure. ECMO: extracorporeal membrane oxygenation. EEG: electroencephalogram. FiO2: fraction of inspired oxygen. HFV: high frequency ventilator. MPAP: mean pulmonary arterial pressure. PaO2: partial pressure of oxygen. PaCO2: partial pressure of carbon dioxide. PCWP: pulmonary capillary wedge pressure. PEEP: positive-end expiratory pressure. PIP: peak inspiratory pressure. SPAP: systolic pulmonary arterial pressure. SaO2: arterial blood gas oxygen saturation. SpO2: peripheral oxygen saturation. SvO2: mixed venous oxygen saturation. Vent: ventilator.
The median ECMO duration was higher in patients with ABI versus patients without ABI (4.8 versus 4.3 days, p < 0.001). The median ECMO pump flow rate at 24 hours was higher in patients with ABI versus patients without ABI (4 versus 3.95 liters per minute, p < 0.001). The median on-ECMO PaO2 was higher in patients with ABI versus patients without ABI (162 versus 141 mmHg, p < 0.001). The top 3 variables in predicting CNS ischemia were ECMO pump flow rate at 24 hours, pre-ECMO cardiac arrest, and conventional ventilation at 24 hours of ECMO support (Fig. 4B, Supplemental Fig. 1B, Supplemental Table 4). The median ECMO pump flow rate at 24 hours was higher in patients with CNS ischemia versus patients without CNS ischemia (4 versus 3.95 liters per minute, p < 0.001). The prevalence of CNS ischemia in patients with pre-ECMO cardiac arrest was higher than patients without cardiac arrest (5.8% versus 3.3%, p < 0.001). The prevalence of CNS ischemia in patients with conventional venting at 24 hours of ECMO support was higher than patients without conventional venting at 24 hours of ECMO support (8.6% versus 2.7%, p < 0.001). The top 3 variables in predicting ICH were duration of ECMO support, ECMO pump flow rate at 4 hours, and on-ECMO PaO2 (Fig. 4C, Supplemental Fig. 1C, Supplemental Table 5). The median ECMO duration was higher in patients with ICH versus patients without ICH (6 versus 4.3 days, p < 0.001). The median ECMO pump flow rate at 4 hours was higher in patients with ICH versus patients without ICH (3.98 versus 3.82 liters per minute, p < 0.001). The median on-ECMO PaO2 was similar between patients with ICH versus patients without ICH (151 versus 142 mmHg, p = 0.27).
ECPR
Of 10,775 ECPR patients, 1,787 (16.5%) had ABI (Fig. 1, Supplemental Table 6). The median age of the ECPR cohort was 57.1 years (IQR:45.5–65.9) and 68% (n = 7,388) were male. The median duration of ECMO support was 2.63 days (IQR:0.88–5.33).
Model Performance
For predicting ABI in ECPR patients, the model achieved an AUC-ROC of 0.72 (Fig. 3D). The accuracy of the model was 69%. The true positive rate, true negative rate, false positive rate, and false negative rate were 61%, 70%, 30%, and 39%, respectively (Table 2). The PPV and NPV were 29% and 90%, respectively.
Table 2.
Model performance in extracorporeal cardiopulmonary resuscitation patients for predicting acute brain injury, central nervous system ischemia, and intracranial hemorrhage.
| AUC-ROC | Acc | TPR | TNR | FPR | FNR | PPV | NPV | Precision | Recall | F1 | Brier Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABI | 0.72 | 69% | 61% | 70% | 30% | 39% | 29% | 90% | 0.28 | 0.61 | 0.39 | 0.14 |
| CNS Ischemia | 0.73 | 81% | 41% | 85% | 15% | 59% | 18% | 95% | 0.18 | 0.41 | 0.25 | 0.14 |
| ICH | 0.69 | 88% | 28% | 89% | 11% | 72% | 7% | 98% | 0.07 | 0.28 | 0.11 | 0.25 |
AUC-ROC: area under the receiver-operating characteristic curve. Acc: Accuracy. TPR: True Positive Rate. TNR: True Negative Rate. FPR: False Positive Rate. FNR: False Negative Rate. PPV: Positive Predictive Value. NPV: Negative Predictive Value. ABI: acute brain injury. CNS: central nervous system. ICH: intracranial hemorrhage.
For predicting CNS ischemia, the model achieved an AUC-ROC of 0.73 (Fig. 3E). The accuracy of the model was 81%. The true positive rate, true negative rate, false positive rate, and false negative rate were 41%, 85%, 15%, and 59%, respectively. The PPV and NPV were 18% and 95%, respectively.
For ICH, the model achieved an AUC-ROC of 0.69 (Fig. 3F). The accuracy of the model was 88%. The true positive rate, true negative rate, false positive rate, and false negative rate were 28%, 89%, 11%, and 72%, respectively. The PPV and NPV were 7% and 98%, respectively.
Feature Importance
The top 3 variables for predicting ABI were duration of ECMO support, age, and ECMO pump flow rates at 24 hours and further details are depicted in the Supplement (Supplemental Fig. 2, Supplemental Fig. 3, Supplemental Tables 7–9, Supplemental Results).
Discussion
This is the first ML study leveraging a large international database to predict ABI in ECMO patients, conveying novelty and generalizability of our study’s results. In 35,855 VA-ECMO (non-ECPR) and 10,775 ECPR patients, ML predicted ABI during ECMO support with an AUC-ROC of 0.67 and 0.70 in VA-ECMO (non-ECPR) and ECPR patients, respectively, while identifying risk factors for their occurrence.
VA-ECMO vs. Venovenous (VV)-ECMO risk factors
ML uniquely identified longer duration of ECMO support, higher ECMO pump flow rate at 24 hours of ECMO support, and higher on-ECMO 24-hour PaO2 as the top 3 most important variables associated with ABI. In another study applying ML to predict ABI in VV-ECMO, (22)ECMO duration was also a top 3 most important variable for ABI; however, a longer duration of ECMO support was associated with lower risk of ABI in VV-ECMO patients while it was associated with a higher risk in VA-ECMO. As VV-ECMO patients have been shown to be cannulated longer than VA-ECMO patients,(23–25) the longer ECMO duration and lower risk of ABI associated may be attributed to the withdrawal of life-sustaining therapy for severely sick patients.(26, 27) Accordingly, this may have created a selection bias for patients who did undergo ABI and survived on ECMO support for longer. Furthermore, a higher ECMO pump flow rate and likely corresponding hemolysis(28) was uniquely important for ABI in VA-ECMO and ECPR, but not in VV-ECMO. This finding may reflect the different hemodynamic/physiological states(28, 29) and use/disuse of an aortic cannula(30) in VA-versus VV-ECMO populations and warrants further study. Although cross-sectionally the ECMO pump flow rates were small and may not be clinically meaningful, over the duration of ECMO support these differences may accrue to substantial differences. While pre-ECMO cardiac arrest is a known risk factor for CNS ischemia in ECPR patients,(2, 31) likely related to reperfusion injury and associated reactive oxygen species formation,(31, 32) we also note that this factor was highly important in VV-ECMO patients(33) which has not been previously reported. These comparisons suggest there are similar underlying but overall divergent risk factors between these populations, which necessitates further investigation with prospective observational studies. Finally, although PaCO2 and PP have been previously shown to be important factors for ABI in regression analyses using the ELSO Registry,(4, 6) they were not in the top 3 most important variables in ML for any population but remained within the top 20.
Machine learning methodologies
We chose tree-based ML algorithms to predict ABI, which are becoming more commonly used in healthcare studies(34) as they provide an effective way to consider all different possible outcomes in a model. Furthermore, these tree-based ML models demonstrate high power, good accuracy, and provide interpretability to the models. (35) The primary difference between using Random Forest vs. gradient boosting tree methods is that Random Forest trees are constructed in an independent fashion while gradient boosting methods are created sequentially. Accordingly, Random Forest can determine their outputs without restriction of order while gradient boosting methods like XGBoost are restricted in a more fixed manner. There are also key differences within boosting methods: CatBoost may be most optimal for categorical data and can generate output more quickly than XGBoost or LightGBM. LightGBM demonstrates better accuracy and speed than XGBoost, but XGBoost is the more established ML algorithm, perhaps making it a very reliable ML tree-based method. Nevertheless, despite implementing these 4 powerful and innovative methods with oversampling to enhance statistical power, ML could still not accurately predict ABI in the ELSO Registry. This finding may suggest that the ELSO Registry does not capture causative variables for ABI over the entire duration of ECMO support which are needed to fully glean the insights and advantages of ML and ultimately identify modifiable risk factors for ABI. Finally, we note that while ML did not predict ABI with high accuracy, it did produce a strong NPV (94% and 90% for ABI in VA-ECMO and ECPR, respectively), suggesting our models’ true utility may lie in its high sensitivity and capability to rule out patients who truly did not have ABI.
Lack of standardized neurological monitoring
Given the relatively mediocre performance in predicting ABI and its subtypes in both cohorts, we reveal certain limitations using a heterogenous, large dataset such as the ELSO Registry to predict ABI with ML. Specifically, unlike the institutional protocol at Johns Hopkins Hospital which uses standardized neurological monitoring with proven efficacy,(3) the protocols used to determine ABI across ECMO centers are neither standardized nor adjudicated/validated, and thus vary considerably. Accordingly, we only observed a 7.7% prevalence of ABI in VA-ECMO patients and 16.5% prevalence of ABI in ECPR patients within the ELSO Registry, which is considerably less than the prevalence of 33% at an experienced tertiary care ECMO center.(3) Therefore, this study calls for more sensitive and accurate detection of ABI and more granular collection of variables across ECMO centers. ABI can precede mortality and therefore identifying risk factors for ABI can help clinicians mitigate their occurrence and their associated mortality risk. In fact, a single-center study of 106 VA-ECMO and 68 VV-ECMO pediatric patients using ML to predict CNS ischemia and ICH showed a superior AUC-ROC (0.76) than ours with the ELSO Registry (0.67).(36) This result may not be surprising given the institution’s rigorous advanced neuroimaging technique to determine ABI and adjudication system by multiple clinicians. Accordingly, their prevalence of ABI (51% in VA/VV-ECMO mixed population) was much higher than ours with the ELSO Registry (7.7% in VA-ECMO and 16.5% in ECPR). Overall, an ELSO Registry addendum for neurological monitoring and imaging protocols may improve performance for ML to predict ABI.
Limitations
The primary limitation of our analysis was the lack of standardized neurological monitoring protocols across ECMO centers and lack of ABI adjudication in the ELSO Registry. Correspondingly, we observed underdiagnoses of ABI. Likely due to low prevalence of ABI and lack of granularity in variables in the ELSO Registry, predicting this outcome using ML was challenging and resulted in suboptimal performance. Furthermore, the ELSO Registry lacks granularity with laboratory measurements as ABGs are only collected at a singular time point instead of multiple times throughout the ECMO run and were not collected at the same exact time point at each center. Finally, as this was a retrospective study, only associations could be determined.
Conclusions
Using the largest database of ECMO patients globally, we present the first study to predict neurological outcomes on sufficiently powered international ECMO patient cohorts. Machine learning identified ECMO duration and higher pump flow rates as the most important risk factors for ABI in both VA-ECMO and ECPR cohorts. Overall, performance of ML models to predict ABI in VA-ECMO and ECPR patients was suboptimal likely due to lack of standardization of neuromonitoring protocols and data granularity in the ELSO Registry. This finding suggests that the detection and sensitivity rates for capturing ABI in ECMO patients across ECMO centers worldwide is substandard. Accordingly, standardized neurological monitoring and imaging protocols are urgently needed.
Key Points.
Question:
Can machine learning (ML) accurately predict acute brain injury (ABI) in venoarterial-extracorporeal membrane oxygenation (VA-ECMO) patients?
Findings:
In a retrospective cohort study of 35,855 VA-ECMO and 10,775 extracorporeal cardiopulmonary resuscitation (ECPR) patients from the Extracorporeal Life Support Organization (ELSO) Registry (2009–2021), 7.7% (n=2,769) and 16.5% (n=1,787) experienced ABI, respectively. ML predicted ABI with an AUC-ROC of 0.67 and 0.72 in VA-ECMO and ECPR patients, respectively. Longer ECMO duration and higher pump flow were associated with ABI for both cohorts.
Meaning:
Clinicians should closely monitor ECMO duration and pump flow rates for VA-ECMO/ECPR patients at risk of ABI.
Acknowledgment
We would like to thank the ELSO for allowing us to analyze and publish this important data and the participating ELSO centers worldwide.
Abbreviations
- ABG
arterial blood gas
- ABI
acute brain injury
- AUC-ROC
area under the receiver-operating characteristic curve
- BMI
body mass index
- CI
confidence interval
- CNS
central nervous system
- DBP
diastolic blood pressure
- DPAP
diastolic pulmonary arterial pressure
- ECMO
extracorporeal membrane oxygenation
- ELSO
Extracorporeal Life Support Organization
- ICH
intracranial hemorrhage
- IQR
interquartile range
- LOOCV
leave-one-out-cross-validation
- ML
machine learning
- MPAP
mean positive airway pressure
- PaCO2
partial pressure of carbon dioxide
- PaO2
partial pressure of oxygen
- PCWP
positive capillary wedge pressure
- PEEP
positive end-expiratory pressure
- PIP
positive inspiratory pressure
- PP
pulse pressure
- SBP
systolic blood pressure
- SD
standard deviation
- SHAP
Shapley Additive Explanations
- SPAP
systolic pulmonary arterial pressure
- SvO2
venous oxygen saturation
- VA
venoarterial
- VV
venovenous
Footnotes
Financial/nonfinancial disclosures:
Dr. Brodie receives research support from and consults for LivaNova. He has been on the medical advisory boards for Xenios, Medtronic, Inspira and Cellenkos. He is the President-elect of the Extracorporeal Life Support Organization (ELSO) and the Chair of the Executive Committee of the International ECMO Network (ECMONet), and he writes for UpToDate. Dr. Ventetuolo has been a consultant or served on advisory boards for Merck, Janssen, and Regeneron, outside of the submitted work. The authors do not have any additional conflicts of interest to declare. SMC is supported by NHLBI (1K23HL157610) and Hyperfine (SAFE MRI ECMO study).
Declarations
IRB Approval: This study was approved by the Johns Hopkins Hospital Institutional Review Board (IRB00216321) on 10/22/2019. The study title is “Retrospective Analysis of Outcomes of Patients on Extracorporeal Membrane Oxygenation”. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
ISHLT Ethics Statement: This study is in compliance with the ISHLT Ethics statement.
Guarantor statement:
Andrew Kalra is responsible for the data analysis and all content of the manuscript.
Names of collaborators:
David Hager
Bo Soo Kim
Steven P. Keller
Errol L. Bush
R. Scott Stephens
Shivalika Khanduja
Jin Kook Kang
Ifeanyi David Chinedozi
Zachary Darby
Hannah J. Rando
Trish Brown
Jiah Kim
Christopher Wilcox
Albert Leng
Andrew Geeza
Armaan F. Akbar
Chengyuan Alex Feng
David Zhao
Marc Sussman
Pedro Alejandro Mendez-Tellez
Philip Sun
Karlo Capili
Ramon Riojas
Diane Alejo
Scott Stephen
Harry Flaster
Other contributions: N/A.
Additional Declarations: The authors declare no competing interests.
Contributor Information
Andrew Kalra, Johns Hopkins University School of Medicine.
Preetham Bachina, Johns Hopkins University School of Medicine.
Benjamin L. Shou, Johns Hopkins University School of Medicine.
Jaeho Hwang, Johns Hopkins University School of Medicine.
Meylakh Barshay, Warren Alpert Medical School of Brown University.
Shreyas Kulkarni, Warren Alpert Medical School of Brown University.
Isaac Sears, Warren Alpert Medical School of Brown University.
Carsten Eickhoff, University of Tübingen.
Christian A. Bermudez, Perelman School of Medicine at the University of Pennsylvania, Philadelphia.
Daniel Brodie, Johns Hopkins University School of Medicine.
Corey E. Ventetuolo, Warren Alpert Medical School of Brown University.
Bo Soo Kim, Johns Hopkins University School of Medicine.
Glenn J. R. Whitman, Johns Hopkins University School of Medicine.
Adeel Abbasi, Warren Alpert Medical School of Brown University.
Sung-Min Cho, Johns Hopkins University School of Medicine.
References
- 1.Thiagarajan RR, Barbaro RP, Rycus PT, et al. : Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017; 63(1):60–67 [DOI] [PubMed] [Google Scholar]
- 2.Cho SM, Canner J, Chiarini G, et al. : Modifiable Risk Factors and Mortality From Ischemic and Hemorrhagic Strokes in Patients Receiving Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med 2020; 48(10):e897–e905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong CS, Etchill E, Dong J, et al. : Neuromonitoring detects brain injury in patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg 2021 [DOI] [PubMed] [Google Scholar]
- 4.Shou BL, Ong CS, Premraj L, et al. : Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: Analysis of the extracorporeal life support organization registry. J Heart Lung Transplant 2023; 42(4):503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shou BL, Wilcox C, Florissi I, et al. : Early Low Pulse Pressure in VA-ECMO Is Associated with Acute Brain Injury. Neurocrit Care 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra A, Kang JK, Wilcox C, Brown P, Rycus P, Anders MA, Zaaqoq AM, Brodie D, Whitman GJR, Cho SM: Impact of Pulse Pressure on Acute Brain Injury in Venoarterial ECMO Patients with Cardiogenic Shock During the First 24 Hours of ECMO Cannulation: Analysis of the Extracorporeal Life Support Organization Registry. REPRINT (Version 1) available at Research Square 2023 [Google Scholar]
- 7.Shou BL, Ong CS, Zhou AL, et al. : Arterial Carbon Dioxide and Acute Brain Injury in Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J 2022; 68(12):1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Zogheib E, Rozé H, et al. : The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39(10):1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Burrell A, Roberts L, et al. : Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36(33):2246–2256 [DOI] [PubMed] [Google Scholar]
- 10.Akin S, Caliskan K, Soliman O, et al. : A novel mortality risk score predicting intensive care mortality in cardiogenic shock patients treated with veno-arterial extracorporeal membrane oxygenation. J Crit Care 2020; 55:35–41 [DOI] [PubMed] [Google Scholar]
- 11.Chen WC, Huang KY, Yao CW, et al. : The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care 2016; 20(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wengenmayer T, Duerschmied D, Graf E, et al. : Development and validation of a prognostic model for survival in patients treated with venoarterial extracorporeal membrane oxygenation: the PREDICT VA-ECMO score. Eur Heart J Acute Cardiovasc Care 2019; 8(4):350–359 [DOI] [PubMed] [Google Scholar]
- 13.Peigh G, Cavarocchi N, Keith SW, et al. : Simple new risk score model for adult cardiac extracorporeal membrane oxygenation: simple cardiac ECMO score. J Surg Res 2015; 198(2):273–279 [DOI] [PubMed] [Google Scholar]
- 14.Becher PM, Twerenbold R, Schrage B, et al. : Risk prediction of in-hospital mortality in patients with venoarterial extracorporeal membrane oxygenation for cardiopulmonary support: The ECMO-ACCEPTS score. J Crit Care 2020; 56:100–105 [DOI] [PubMed] [Google Scholar]
- 15.Muller G, Flecher E, Lebreton G, et al. : The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016; 42(3):370–378 [DOI] [PubMed] [Google Scholar]
- 16.Yoon JH, Pinsky MR, Clermont G: Artificial Intelligence in Critical Care Medicine. Critical Care 2022; 26(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamdani M, Slutsky AS: Artificial intelligence in intensive care medicine. Intensive Care Medicine 2021; 47(2):147–149 [DOI] [PubMed] [Google Scholar]
- 18.Ayers B, Wood K, Gosev I, et al. : Predicting Survival After Extracorporeal Membrane Oxygenation by Using Machine Learning. Ann Thorac Surg 2020; 110(4):1193–1200 [DOI] [PubMed] [Google Scholar]
- 19.Stephens AF, Šeman M, Diehl A, et al. : ECMO PAL: using deep neural networks for survival prediction in venoarterial extracorporeal membrane oxygenation. Intensive Care Med 2023; 49(9):1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbasi A, Karasu Y, Li C, et al. : Machine learning to predict hemorrhage and thrombosis during extracorporeal membrane oxygenation. Critical Care 2020; 24(1):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorusso R, Alexander P, Rycus P, et al. : The Extracorporeal Life Support Organization Registry: update and perspectives. Ann Cardiothorac Surg 2019; 8(1):93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalra A., Bachina P S, BS, et al. : Utilizing Machine Learning to Predict Neurological Injury in Venovenous Extracorporeal Membrane Oxygenation Patients: An Extracorporeal Life Support Organization Registry Analysis. PREPRINT (Version 1) available at Research Square [Google Scholar]
- 23.Aubron C, Cheng AC, Pilcher D, et al. : Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Critical Care 2013; 17(2):R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaber B, Bembea MM, Loftis LL, et al. : Venovenous Versus Venoarterial Extracorporeal Membranous Oxygenation in Inotrope Dependent Pediatric Patients With Respiratory Failure. ASAIO Journal 2021; 67(4):457–462 [DOI] [PubMed] [Google Scholar]
- 25.Kalra A, Shou BL, Zhao D, et al. : Racial and ethnical discrepancy in hypoxemia detection in patients on extracorporeal membrane oxygenation. JTCVS Open [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson JM, Etchill E, Whitman G, et al. : Early withdrawal of life sustaining therapy in extracorporeal cardiopulmonary resuscitation (ECPR): Results from the Extracorporeal Life Support Organization registry. Resuscitation 2022; 179:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson JM, Etchill EW, Enriquez CAG, et al. : Population Characteristics and Markers for Withdrawal of Life-Sustaining Therapy in Patients on Extracorporeal Membrane Oxygenation. J Cardiothorac Vasc Anesth 2022; 36(3):833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra A, Shou BL, Zhao D, et al. : ECMO Physiological Factors Influence Pulse Oximetry and Arterial Oxygen Saturation Discrepancies. The Annals of Thoracic Surgery [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makdisi G, Wang IW: Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015; 7(7):E166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlushkov E, Berman M, Valchanov K: Cannulation techniques for extracorporeal life support. Ann Transl Med 2017; 5(4):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox C, Choi CW, Cho S-M: Brain injury in extracorporeal cardiopulmonary resuscitation: translational to clinical research. Journal of Neurocritical Care 2021; 14(2):63–77 [Google Scholar]
- 32.Hafner S, Beloncle F, Koch A, et al. : Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Annals of Intensive Care 2015; 5(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booke H, Zacharowski K, Adam EH, et al. : Cardiopulmonary resuscitation in veno-venous-ECMO patients-A retrospective study on incidence, causes and outcome. PLoS One 2023; 18(8):e0290083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Li L: Using Tree-Based Machine Learning for Health Studies: Literature Review and Case Series. Int J Environ Res Public Health 2022; 19(23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habehh H, Gohel S: Machine Learning in Healthcare. Curr Genomics 2021; 22(4):291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah N, Farhat A, Tweed J, et al. : Neural Networks to Predict Radiographic Brain Injury in Pediatric Patients Treated with Extracorporeal Membrane Oxygenation. J Clin Med 2020; 9(9) [DOI] [PMC free article] [PubMed] [Google Scholar]