Abstract
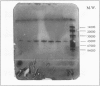
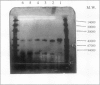
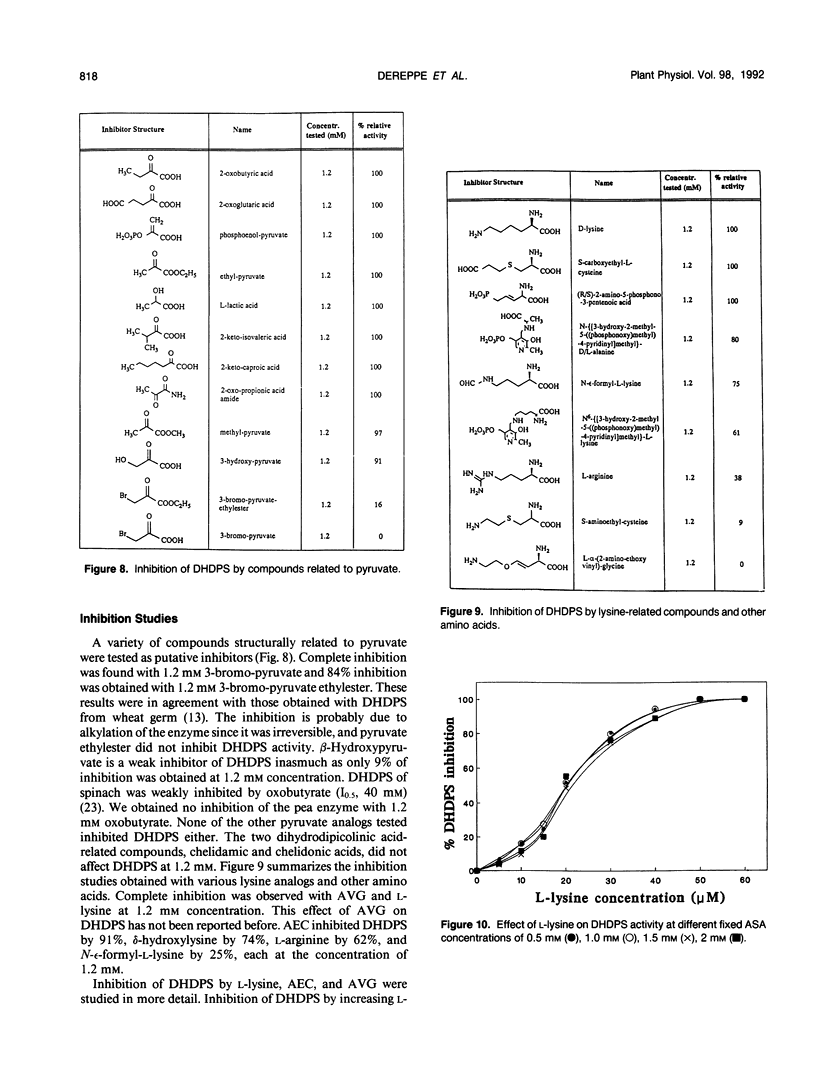
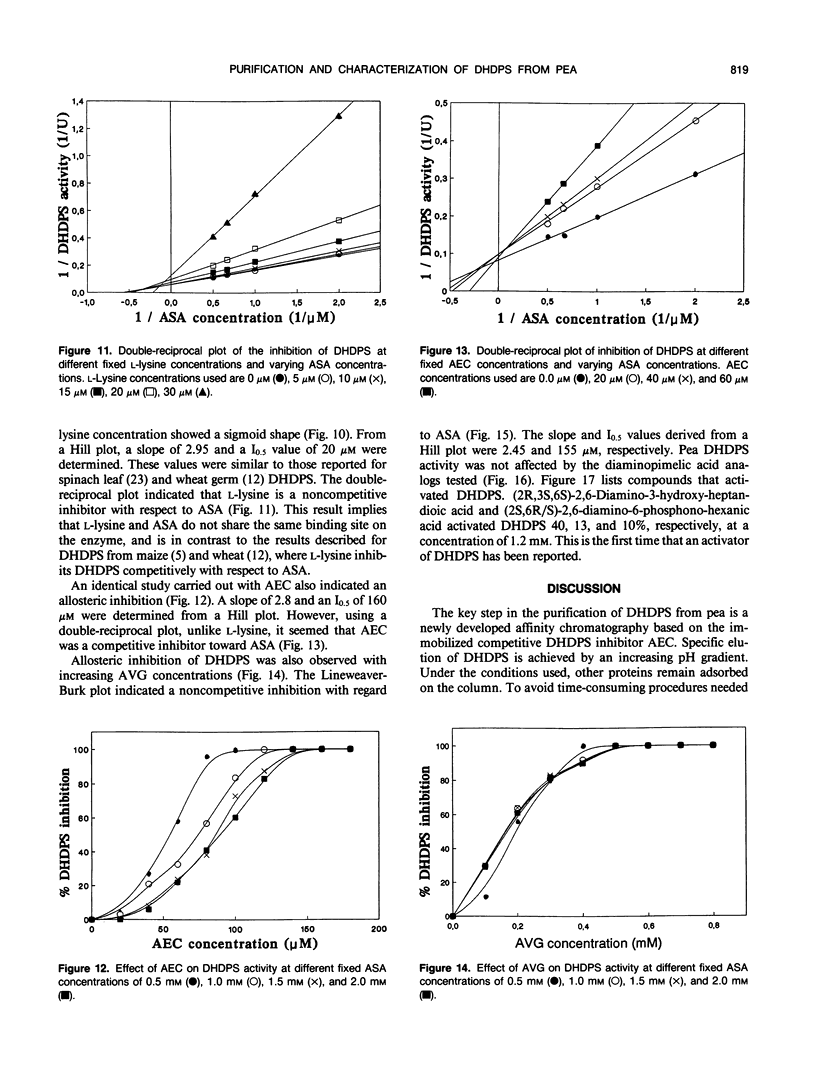
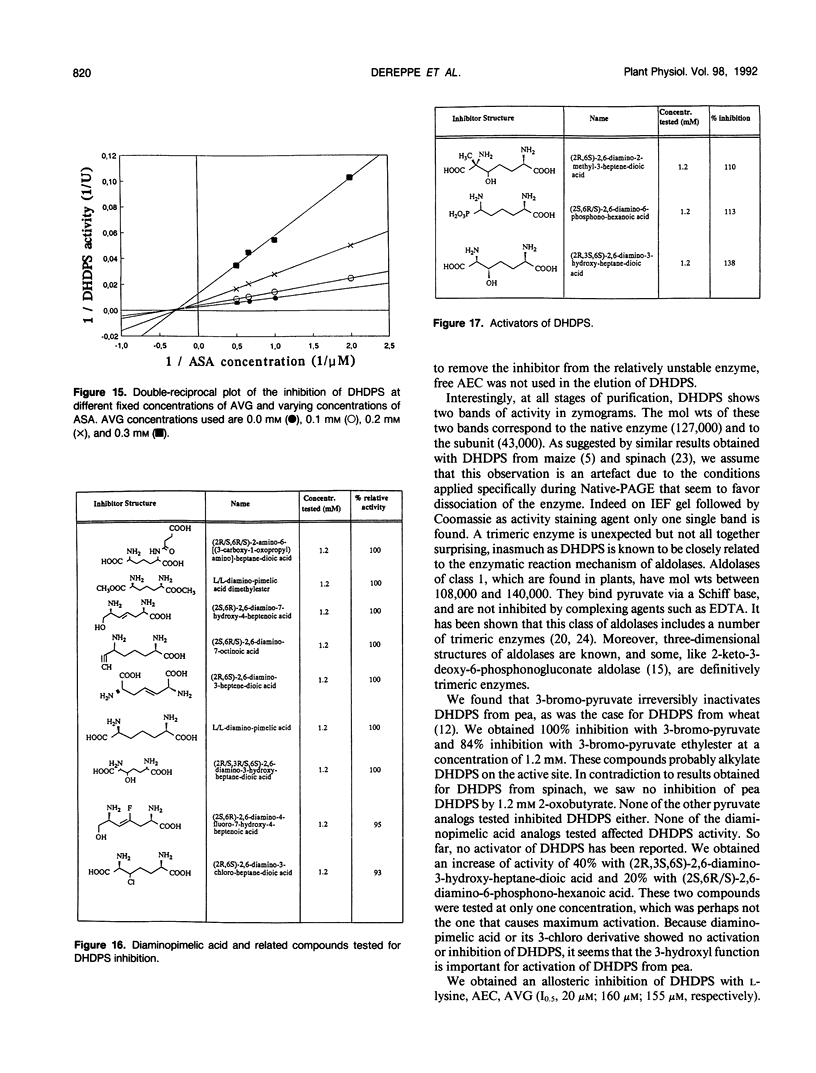
Dihydrodipicolinate synthase (EC 4.2.1.52), the first enzyme unique to lysine biosynthesis in bacteria and higher plants, has been purified to homogeneity from etiolated pea (Pisum sativum) seedlings using a combination of conventional and affinity chromatographic steps. This is the first report on a homogeneous preparation of native dihydrodipicolinate synthase from a plant source. The pea dihydrodipicolinate synthase has an apparent molecular weight of 127,000 and is composed of three identical subunits of 43,000 as determined by gel filtration and cross-linking experiments. The trimeric quaternary structure resembles the trimeric structure of other aldolases, such as 2-keto-3-deoxy-6-phosphogluconic acid aldolase, which catalyze similar aldol condensations. The amino acid compositions of dihydrodipicolinate synthase from pea and Escherichia coli are similar, the most significant difference concerns the methionine content: dihydrodipicolinate synthase from pea contains 22 moles of methionine residue per mole of native protein, contrary to the E. coli enzyme, which does not contain this amino acid at all. Dihydrodipicolinate synthase from pea is highly specific for the substrates pyruvate and l-aspartate-β-semialdehyde; it follows Michaelis-Menten kinetics for both substrates. The pyruvate and l-aspartate-β-semialdehyde have Michaelis constant values of 1.70 and 0.40 millimolar, respectively. l-Lysine, S-(2-aminoethyl)-l-cysteine, and l-α-(2-aminoethoxyvinyl)glycine are strong allosteric inhibitors of the enzyme with 50% inhibitory values of 20, 160, and 155 millimolar, respectively. The inhibition by l-lysine and l-α-(2-aminoethoxyvinyl)glycine is noncompetitive towards l-aspartate-β-semialdehyde, whereas S-(2-aminoethyl)-l-cysteine inhibits dihydrodipicolinate synthase competitively with respect to l-aspartate-β-semialdehyde. Furthermore, the addition of (2R,3S,6S)-2,6-diamino-3-hydroxy-heptandioic acid (1.2 millimolar) and (2S,6R/S)-2,6-diamino-6-phosphono-hexanic acid (1.2 millimolar) activates dihydrodipicolinate synthase from pea by a factor of 1.4 and 1.2, respectively. This is the first reported activation process found for dihydrodipicolinate synthase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Miflin B. J. Regulatory isoenzymes of aspartate kinase and the control of lysine and threonine biosynthesis in carrot cell suspension culture. Plant Physiol. 1978 Oct;62(4):536–541. doi: 10.1104/pp.62.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch D. A., Gengenbach B. G., Tommey A. M., Sellner J. M., Somers D. A., Myers D. E. Isolation and characterization of dihydrodipicolinate synthase from maize. Plant Physiol. 1991 Jun;96(2):444–452. doi: 10.1104/pp.96.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Regulatory Structure of the Biosynthetic Pathway for the Aspartate Family of Amino Acids in Lemna paucicostata Hegelm. 6746, with Special Reference to the Role of Aspartokinase. Plant Physiol. 1989 Aug;90(4):1584–1599. doi: 10.1104/pp.90.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Stahly D. P. Dihydrodipicolinic acid synthase of Bacillus licheniformis. Quaternary structure, kinetics, and stability in the presence of sodium chloride and substrates. Biochim Biophys Acta. 1976 Dec 8;452(2):580–596. doi: 10.1016/0005-2744(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Hoganson D. A., Stahly D. P. Regulation of dihydrodipicolinate synthase during growth and sporulation of Bacillus cereus. J Bacteriol. 1975 Dec;124(3):1344–1350. doi: 10.1128/jb.124.3.1344-1350.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman J. K. Covalent linkage of functional groups, ligands, and proteins to polyacrylamide beads. Methods Enzymol. 1974;34:30–58. doi: 10.1016/s0076-6879(74)34006-2. [DOI] [PubMed] [Google Scholar]
- Knecht R., Chang J. Y. Liquid chromatographic determination of amino acids after gas-phase hydrolysis and derivatization with (dimethylamino)azobenzenesulfonyl chloride. Anal Chem. 1986 Oct;58(12):2375–2379. doi: 10.1021/ac00125a006. [DOI] [PubMed] [Google Scholar]
- Kumpaisal R., Hashimoto T., Yamada Y. Purification and characterization of dihydrodipicolinate synthase from wheat suspension cultures. Plant Physiol. 1987 Sep;85(1):145–151. doi: 10.1104/pp.85.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Widholm J. M. Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Z Naturforsch C. 1979 Dec;34(12):1177–1185. doi: 10.1515/znc-1979-1216. [DOI] [PubMed] [Google Scholar]
- Mavridis I. M., Hatada M. H., Tulinsky A., Lebioda L. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase at 2 . 8 A resolution. J Mol Biol. 1982 Dec 5;162(2):419–444. doi: 10.1016/0022-2836(82)90536-8. [DOI] [PubMed] [Google Scholar]
- Mazelis M., Whatley F. R., Whatley J. The enzymology of lysine biosynthesis in higher plants. The occurrence, characterization and some regulatory properties of dihydrodipicolinate synthase. FEBS Lett. 1977 Dec 15;84(2):236–240. doi: 10.1016/0014-5793(77)80696-0. [DOI] [PubMed] [Google Scholar]
- Shedlarski J. G., Gilvarg C. The pyruvate-aspartic semialdehyde condensing enzyme of Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1362–1373. [PubMed] [Google Scholar]
- Stahly D. P. Dihydrodipicolinic acid synthase of Bacillus licheniformis. Biochim Biophys Acta. 1969 Nov 4;191(2):439–451. doi: 10.1016/0005-2744(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Vold B., Szulmajster J., Carbone A. Regulation of dihydrodipicolinate synthase and aspartate kinase in Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):970–974. doi: 10.1128/jb.121.3.970-974.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Mazelis M. The enzymology of lysine biosynthesis in higher plants: complete localization of the regulatory enzyme dihydrodipicolinate synthase in the chloroplasts of spinach leaves. FEBS Lett. 1980 Jul 28;116(2):189–192. doi: 10.1016/0014-5793(80)80640-5. [DOI] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]