Abstract
Plasmid cp8.3 of Borrelia afzelii IP21 carries several open reading frames (ORFs) and a 184-bp inverted repeat (IR) element. It has been speculated that this plasmid may encode factors involved in virulence or infectivity. In this report, we have characterized the distribution, molecular variability, and organization of ORFs 1, 2, and 4 and the IR elements among isolates of the Borrelia burgdorferi sensu lato complex. ORFs 1 and 2 are contained within a segment of cp8.3 that is bordered by the IR elements, while ORF 4 resides just outside of the IR-bordered region. By PCR, ORF 4 was amplified from most isolates while ORFs 1 and 2 were amplified from only some B. afzelii isolates. However, Southern hybridization analyses with ORF 1, 2, and 4 probes detected related sequences even in some isolates that were PCR negative. The ORF restriction fragment length polymorphism patterns varied widely even among isolates of the same species. Two-dimensional contour-clamped homogeneous electric field–pulsed-field gel electrophoresis and Southern hybridization detected ORF 1-, 2-, and 4-related sequences on linear and circular plasmids. In addition, an ORF 4-related sequence was detected on a previously uncharacterized, circular plasmid that is greater than 70 kb in size. The IR elements originally identified on plasmid cp8.3 of B. afzelii IP21 were also analyzed by Southern hybridization. Related sequences were detected in some but not all B. burgdorferi sensu lato isolates. These sequences are carried on plasmids in addition to cp8.3 in some isolates. Single-primer PCR analyses demonstrated that in some isolates these sequences exist with IR orientation. The data presented here demonstrate that the IR elements and the ORF 1-, 2-, and 4-related sequences are multicopy and are variable in organization and in genomic location among isolates of the B. burgdorferi sensu lato complex. These analyses provide additional evidence for the highly variable organization of the plasmid component of the B. burgdorferi sensu lato genome.
Lyme disease develops upon infection with pathogenic spirochete species of the Borrelia burgdorferi sensu lato complex (9, 51). This complex includes the pathogenic species B. burgdorferi, Borrelia garinii, and Borrelia afzelii (3, 9, 24, 28, 29, 31, 41, 42, 57, 59) and two species of uncertain pathogenic potential, Borrelia japonica and Borrelia andersonii (17, 31). Recent work has suggested that there may be additional genomic groups and species (2, 17–19, 24, 40), including the recently proposed Borrelia valaisiana sp. nov. (58) and Borrelia lusitaniae sp. nov. (16) (previously referred to as genomic groups VS116 and PotBi, respectively).
The Borrelia genome is unique (7), being composed of a linear chromosome and a series of linear and circular plasmids (LPs and CPs, respectively) (4, 14). Numerous genes have been localized to these plasmids (6, 12, 13, 25, 32, 38, 53, 56). The Borrelia genome is variable among isolates in both the number and size of the plasmids present (4, 33, 47, 48, 60). Mechanisms associated with the generation of plasmid variability include plasmid loss (38, 47), lateral exchange of plasmids (23, 33), recombination (26, 30, 33, 43), and dimer formation (22, 27, 55). Some plasmids carry repeated sequences (11, 20, 21, 35, 39, 50, 52, 63) which may allow for homologous recombination among plasmids. It has been suggested that plasmid cp8.3 of B. afzelii (13) or similarly sized small CPs of other isolates may carry genes that encode factors necessary for infectivity and/or virulence (47). Coincident with the loss of plasmids in this size range, it has been reported that some isolates lose their infective potential (47). An 8.3-kb CP (cp8.3) from B. afzelii IP21 has been sequenced and carries nine open reading frames (ORFs) and a 184-bp inverted repeat (IR) element (13). However, the potential role of the ORFs of cp8.3 remains unclear, and these sequences do not exhibit significant homology with the sequences of any known genes of other pathogenic organisms. Assessment of their potential importance is further complicated by the fact that some of the cp8.3 ORFs are multicopy and are carried on plasmids other than cp8.3 (5, 11, 52, 63). It has been suggested that the 184-bp IR elements on cp8.3 may have triggered recombination and integration events that have resulted in the multicopy state of cp8.3 ORFs found in some isolates (63). Hence, an assessment of the distribution of the IR elements may provide information as to the potential role of these elements in generating organizational diversity of the ORFs of cp8.3. In view of the extensive genomic variability of B. burgdorferi sensu lato isolates, it cannot, de facto, be concluded that the ORFs of cp8.3 are carried by all B. burgdorferi sensu lato species or that they exhibit the same genetic organization as that seen in the few isolates that have been studied to date (5, 10, 63). An assessment of the organization and molecular variability of genes carried on these plasmids among the different B. burgdorferi sensu lato complex species will prove important in elucidating the possible involvement of these ORFs in the biology of these pathogens. The goals of this study were (i) to assess the distribution, molecular organization, and possible multicopy state of cp8.3 ORFs 1, 2, and 4 among B. burgdorferi sensu lato species and isolates; (ii) to determine if B. burgdorferi sensu lato isolates carry sequences related to the IR element of cp8.3; and (iii) to identify the genomic elements that carry copies of these ORFs and IR elements.
MATERIALS AND METHODS
Bacterial cultivation and nucleic acid isolation.
Bacteria (Table 1) were cultivated at 32°C in BSK-H medium (Sigma) supplemented to 6% with rabbit serum (Sigma). Cells were harvested by centrifugation and washed twice with phosphate-buffered saline (pH 7.0). RNA and DNA were isolated as previously described (33, 36).
TABLE 1.
B. burgdorferi sensu lato complex isolates investigated
| Isolate | Origina and location | No. of passages | Summary of PCR resultsb
|
spPCR results | |||
|---|---|---|---|---|---|---|---|
| rrs | ORF 1 | ORF 2 | ORF 4 | ||||
| B. burgdorferi | |||||||
| LP7 | Human EM; Connecticut | 3 | + | − | − | + | − |
| CA9 | Ixodes pacificus tick; California | 3 | + | − | − | + | + |
| 297 | Human CSF; Connecticut | ?c | + | − | − | ± | − |
| B. garinii | |||||||
| FRG | Ixodes ricinus tick; Germany | ? | + | − | − | + | − |
| N34 | Ixodes ricinus tick; Germany | ? | + | − | − | + | + |
| VSBP | Ixodes ricinus tick; Switzerland | 9 | + | − | − | ± | − |
| B. valaisiana sp. nov. VS116 | Ixodes ricinus tick; Switzerland | 10 | + | − | − | − | − |
| B. afzelii | |||||||
| IP21 | Ixodes persulcatus tick; Russia | >20 | + | + | + | + | + |
| ECM1 | Human EM; Sweden | ? | + | − | +d | + | − |
| UMO1 | Human EM; Sweden | ? | + | + | + | + | + |
| B. andersonii | |||||||
| 19857 | Sylvilagus floridans (rabbit) kidney; New York | 7 | + | − | − | + | − |
| 21038 | Ixodes dentatus tick; New York | 6 | + | − | − | ± | − |
| B. japonica | |||||||
| IKA2 | Ixodes ovatus tick; Japan | ? | + | − | − | ± | − |
| HO14 | Ixodes ovatus tick; Japan | ? | + | − | − | + | − |
Abbreviations: EM, erythema migrans; CSF, cerebrospinal fluid.
+, positive; −, negative; ±, weak product (relative to that of the other isolates).
?, unknown.
The amplicon obtained from this isolate was approximately 300 bp shorter than predicted.
PCR analyses.
PCR was performed by using isolated DNA and Taq polymerase (Promega) as described previously (33). Primers were used at a final concentration of 0.5 pmol/μl. In single-primer PCR analyses (spPCR), the final concentration of the primer was 1.0 pmol/μl. Cycle conditions consisted of 30 cycles of 95°C for 45 s, 50°C for 30 s, and 72°C for 90 s. Amplicons were analyzed in 1% agarose gels in 40 mM Tris-acetate–2 mM EDTA (TAE; pH 8.5). Primer binding sites are depicted in Fig. 1, and the primer sequences are as follows: primer O1F1, TTATCTAATGTTAACAAAACTCG; primer O1R1, CGAGTTTTGTTAACATTAGATAA; primer O1R2, GTAACAAATACATTATTGTATTC; primer O2F1, CATGGAGAATTTATTGAAAAC; primer O2R2, TTAGTCCCTTATCAGAAT; primer O4F1, CAAGCAGAAATTCACTTTATA; primer O4R1, TATAAAGTGAATTTCTGCTTG; primer O4R3, TATCCATATCCTTTAAGA; primer O7R1, TTTAAGCACTCTATTTACCAATT; and primer IRA-F1, TTGATTAATTTCTTGTGGATT.
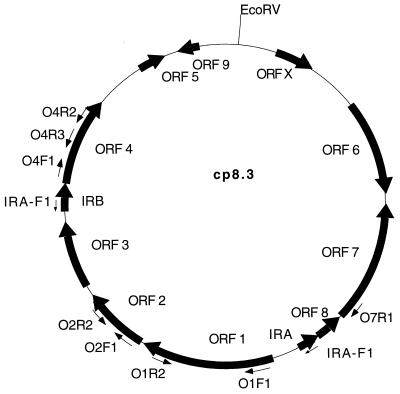
FIG. 1.
Map of B. afzelii IP21 cp8.3 indicating the location of ORFs, IR elements, and oligonucleotide binding sites. The sequence of cp8.3 was determined by Dunn et al. (13). The direction of possible transcription of each ORF is indicated by arrows. Oligonucleotides with designations including the letter R (reverse) that are not shown in the figure target the identical sites as their forward (F) primer counterparts except that they bind to the opposite strand. The map was generated with the MacPlasmap (Jindong Liu, University of Utah) and Canvas (Deneba) programs.
Southern blot analyses.
DNA (4 μg) was digested to completion with EcoRV, electrophoresed in 0.8% genetic-technology-grade agarose gels, vacuum blotted onto a Hybond N membrane (Amersham), and UV cross-linked to the membrane as described previously (33). Oligonucleotides were labeled by using polynucleotide kinase and [γ-32P]ATP (6,000 Ci/mmol; DuPont-NEN). PCR-generated probes were labeled by random primer methods (Boehringer Mannheim) and with [α-32P]dATP (3,000 Ci/mmol; DuPont-NEN). Hybridizations with oligonucleotides and PCR probes were conducted at 32 to 37°C and at 42 to 50°C, respectively. The hybridization buffer consisted of 0.2% bovine serum albumin, 0.2% polyvinylpyrrolidone (molecular weight, 40,000), 0.2% Ficoll (molecular weight, 400,000), 50 mM Tris-HCl (pH 7.5), 0.1% sodium pyrophosphate, 1% sodium dodecyl sulfate (SDS), 10% dextran sulfate, 100 μg of herring sperm DNA per ml, and 1 M NaCl. With PCR probes, formamide was added to the hybridization buffer at a final concentration of 50%. Washes were performed at temperatures ranging from 32 to 60°C, depending on the probe. A variety of wash and hybridization temperatures were employed in these analyses since interspecies sequence divergence in the target sequences was expected. Two 10-min washes with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS and a 60-min wash with 0.1× SSC–0.1% SDS, followed by a 5-min wash with 0.1× SSC–0.1% SDS with vigorous agitation, were performed. Membranes were exposed to film at −70°C.
Two-dimensional (2D) CHEF-PFGE.
DNA from cells lysed in agarose (27) was fractionated in 1% genetic-technology-grade agarose gels by using the contour-clamped homogeneous electric field mapper–pulsed-field gel electrophoresis (CHEF-PFGE) system (Bio-Rad). The algorithm used to separate the DNA by CHEF-PFGE in the first dimension was generated by using the auto-algorithm program with the following parameters: run time, 20 h 16 min; buffer, 0.5× Tris-borate-EDTA (TBE; pH 8.0); temperature, 14°C; ramping constant, −1.107; initial switch time, 0.47 s; final switch time, 35.46 s; angle, 120°; gradient, 6 V/cm. After electrophoresis in the first dimension, the gels were rotated 90° and electrophoresed for 3 h in 0.5× TBE buffer at 80 V (constant field).
The DNA was stained with 1.0 μg of ethidium bromide per ml for 30 min, UV irradiated with 60 mJ of energy, destained, and photographed. The gels were soaked in 0.4 N NaOH–1.5 M NaCl for 15 min. Transfer onto a Hybond N+ membrane (Amersham) was accomplished by either capillary action with 0.4 N NaOH–1.5 M NaCl over a period of 2 to 3 days or by vacuum blotting with a 6-h transfer time. Membranes were neutralized in 0.5 M Tris-HCl (pH 7.0) and rinsed in 2× SSC prior to hybridization.
DNA sequence analysis.
Briefly, PCR-generated sequencing templates were purified (Wizard PCR Prep system; Promega) and sequenced with the fmol DNA cycle sequencing system (Promega). Reactions were run in 6% acrylamide–8 M urea gels (85 W).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the O1R1-O7R1 amplicon sequences from B. andersonii 21038 and B. afzelii UMO1 and IP21 are AD001532, AD001533, and AD001534, respectively; the B. afzelii ECM1 and UMO1 ORF 2 amplicons generated with the O2F1-O2R2 primer set have been assigned the numbers AD001535 and AD001536, respectively.
RESULTS AND DISCUSSION
PCR analysis of ORFs originally identified on cp8.3.
cp8.3 from B. afzelii IP21 carries nine ORFs and a 184-bp IR element (13). Although the functional role of the ORFs is currently unknown, it has been suggested that plasmids in the 8- to 9-kb range may carry genes encoding factors necessary for infectivity or virulence (47). This study was undertaken to investigate the distribution of these putative virulence factors and their organization in other B. burgdorferi sensu lato species and isolates. This information will aid in future studies designed to assess the potential role of these ORFs in the overall biology of these bacteria. As a first step towards assessing the distribution of ORFs 1, 2, and 4 among B. burgdorferi sensu lato isolates, PCR analyses were conducted. These particular ORFs were analyzed since they represent ORFs that reside on two different segments of cp8.3 whose borders are defined by the presence of 184-bp IR elements (Fig. 1). The reason for analyzing sequences derived from these two different segments of cp8.3 stems from the influence that IR elements can have on the stability and organization of surrounding sequences. All isolates investigated except B. garinii VSBP carry plasmids in the 7- to 9-kb size range that are visible by ethidium bromide staining of DNA fractionated in 0.4% agarose gels (data not shown) (61). To verify that all templates were free of inhibitors, a positive-control PCR primer set targeting the 16S rRNA gene (rrs) was used. rrs-derived amplicons were obtained from all templates (PCR data are presented in Table 1). An ORF 4-directed primer set also yielded amplicons from all isolates, demonstrating that ORF 4 is carried by all species of the B. burgdorferi sensu lato complex tested (B. burgdorferi, B. garinii, B. afzelii, B. japonica, B. andersonii, and B. valaisiana sp. nov). B. garinii VSBP, which lacks a 7- to 9-kb CP, was also PCR positive. In contrast to ORF 4, ORFs 1 and 2 were amplified from only 2 of 14 and 3 of 14 isolates, respectively. PCR analyses of additional B. burgdorferi sensu lato isolates revealed that only 5 of 34 isolates were positive for ORF 2 while 28 of 34 were positive for ORF 4 (data not shown). All isolates positive for ORF 2 were B. afzelii. These data suggest that either ORFs 1 and 2 are absent from the genomes of most isolates or they are less conserved across species lines than ORF 4 and were therefore not detected by PCR with the primers used. These analyses also suggest that isolate VSBP may carry an ORF 4-related sequence on a genomic element other than cp8.3.
With the exception of a truncated ORF 2 amplicon obtained from B. afzelii ECM1, all ORF amplicons were of the predicted size, indicating that these genes are conserved in size. The ORF 2 amplicon from B. afzelii ECM1 was 173 bp instead of the predicted 500-bp product (Table 1). Sequence analysis of the ORF 2 amplicon revealed an in-frame coding sequence deletion of 327 nucleotides (109 amino acids).
Southern blot analysis of cp8.3 ORFs.
Southern hybridization analyses were performed to assess restriction fragment length polymorphism (RFLP) patterns of each ORF and to determine if ORFs 1, 2, and 4 are multicopy. DNA, digested to completion with EcoRV, was hybridized with PCR-generated ORF probes amplified from B. afzelii IP21 or with ORF-specific oligonucleotide probes.
An ORF 2 PCR-generated probe hybridized with one or more DNA fragments from some, but not all, B. burgdorferi, B. garinii, and B. afzelii isolates (Fig. 2). Similar results were obtained for ORF 1 (Southern hybridization data are summarized in Table 2). To determine if the multiple hybridizing bands were due to incomplete digestion of the DNA, the blots were stripped and probed with an rrs (16S rRNA gene)-targeting oligonucleotide. Consistent with rrs being a single-copy gene (49), a single hybridizing fragment was detected in each isolate (data not shown). This demonstrates that incomplete digestion is not responsible for the multiple ORF 1 and ORF 2 hybridizing fragments. Regarding hybridization specificity, it is important to note that some isolates did not hybridize with either the ORF 1 or the ORF 2 probe. This indicates that the hybridization conditions were sufficiently stringent to prevent general nonspecific binding of the probes to the AT-rich DNA of the Borrelia species. This suggests that the multiple hybridizing bands observed do in fact reflect specific binding of the probes to repeated ORF-related sequences. The data presented here are consistent with a recent report by Barbour et al., who detected two ORF 1 homologs on a 17-kb LP of B. burgdorferi B31 (5).
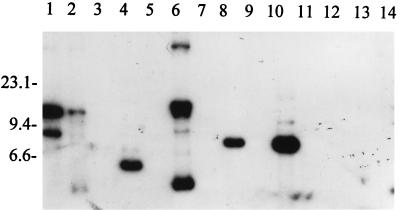
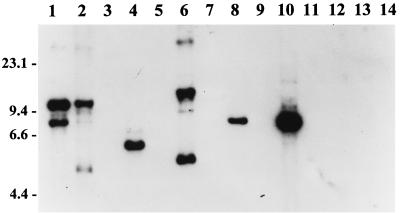
FIG. 2.
RFLP pattern analysis of ORF 2 among B. burgdorferi sensu lato isolates. DNA from each isolate was digested to completion with EcoRV, fractionated, blotted, and probed with an ORF 2 PCR-generated probe as discussed above. Isolates analyzed were B. burgdorferi LP7, CA9, and 297 (lanes 1 to 3, respectively); B. garinii FRG and N34 (lanes 4 and 5, respectively); B. valaisiana VS116 (lane 6); B. garinii VSBP (lane 7); B. afzelii IP21, ECM1, and UMO1 (lanes 8 to 10, respectively); B. andersonii 19857 and 21038 (lanes 11 and 12, respectively); and B. japonica IKA2 and HO14 (lanes 13 and 14, respectively). Molecular size standards (in kilobases) are indicated on the left.
TABLE 2.
Summary of Southern hybridization results
| Species and isolate | Hybridization results for target sequencea
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF 1
|
ORF 2
|
ORF 4
|
IR element
|
|||||||||||||||
| O1F1 oligo | O1R2 oligo | PCR probe (IP21)b | PCR probe (2D CHEF)
|
O2F1 oligo | O2R2 oligo | PCR probe (IP21)b | PCR probe (2D CHEF)
|
O4R3 oligo | PCR probe (IP21)b | PCR probe (CA9)b | PCR probe (2D CHEF)
|
IR-AF1 oligo | 2D CHEF (IR-AF1 oligo)
|
|||||
| LP | CP | LP | CP | LP | CP | LP | CP | |||||||||||
| B. burgdorferi | ||||||||||||||||||
| LP7 | − | − | − | ND | ND | − | − | +M | ND | ND | + | +M | +M | ND | ND | +M | ND | ND |
| CA9 | − | − | + | ND | ND | − | − | +M | ND | ND | +M | +M | +M | ND | ND | +M | ND | ND |
| 297 | − | − | +M | ND | ND | − | − | ± | ND | ND | +M | +M | +M | ND | ND | − | ND | ND |
| B. garinii | ||||||||||||||||||
| FRG | − | − | +M | +M | + | − | − | +M | + | +M | +M | +M | +M | +M | +M | +M | +M | +M |
| N34 | − | − | +M | ND | ND | − | − | − | ND | ND | +M | +M | +M | ND | ND | − | ND | ND |
| VSBP | − | − | +M | ND | ND | − | − | − | ND | ND | +M | +M | ± | ND | ND | − | ND | ND |
| B. valaisiana VS116 | − | − | − | ± | − | ±M | − | +M | − | +M | + | +M | +M | − | ± | +M | +M | +M |
| B. afzelii | ||||||||||||||||||
| IP21 | + | + | + | +M | + | + | + | + | + | +M | + | +M | + | + | +M | + | − | − |
| ECM1 | + | + | + | ND | ND | + | + | − | ND | ND | +M | +M | +M | ND | ND | − | ND | ND |
| UMO1 | + | + | + | ND | ND | + | + | +M | ND | ND | +M | +M | +M | ND | ND | +M | ND | ND |
| B. andersonii | ||||||||||||||||||
| 19857 | − | − | − | ND | ND | − | − | − | ND | ND | − | +M | − | ND | ND | − | ND | ND |
| 21038 | − | − | − | ND | ND | − | − | − | ND | ND | − | +M | − | ND | ND | − | ND | ND |
| B. japonica | ||||||||||||||||||
| IKA2 | − | − | − | ND | ND | − | − | − | ND | ND | − | − | − | ND | ND | − | ND | ND |
| HO14 | − | − | − | ND | ND | − | − | − | ND | ND | − | +M | +M | ND | ND | − | ND | ND |
With the exception of the Southern hybridizations involving 2D CHEF gels (as indicated at the top of the appropriate columns), all other hybridizations were conducted with EcoRV-digested DNA. 2D CHEF hybridization results are divided into two columns to indicate whether LPs or CPs hybridized with the probes. Hybridization results are scored as either +, ±, or −, for strong, weak, or no hybridization, respectively. Superscript M indicates that multiple hybridizing fragments or plasmids were detected. ND, not done; oligo, oligonucleotide.
The isolate from which the probe was generated is indicated in parentheses.
Several isolates that hybridized with ORF 1 or ORF 2 PCR-generated probes were PCR negative for these ORFs. When the ORF 1 and ORF 2 PCR primers were used as probes, strong hybridization was observed only with B. afzelii-derived DNA (Table 2). Weak hybridization with B. valaisiana VS116-derived DNA was observed with the O2F1 oligonucleotide. The lack of hybridization of the oligonucleotide probes with most isolates likely reflects sequence divergence in the primer binding sites among isolates and explains why so few were PCR positive for ORF 1 or 2. The selective hybridization with B. afzelii isolates is not surprising since the probes were designed based on the B. afzelii IP21 cp8.3 sequence.
Sequence analysis of the truncated B. afzelii ECM1 ORF 2 amplicon revealed a deletion in the coding sequence of 327 nucleotides. Oligonucleotides targeting the 5′ and 3′ ends of ORF 2 hybridized with an 8.3-kb fragment in ECM1 (Table 2). This was expected since the binding sites of these probes are not contained within the deletion. In contrast, the ORF 2 PCR probe did not hybridize with the ECM1 DNA (Fig. 2, lane 9). However, in view of the deletion, this is not surprising since the deletion would destabilize hybridization of the full-length gene probe. This result also indicates that ECM1 does not carry a second, full-length, conserved copy of ORF 2 elsewhere in its genome. It had been postulated, based on the homology of the amino acid sequence deduced from ORF 2 with that of RepC, a protein involved in rolling-circle plasmid replication (13), that ORF 2 may also play a role in plasmid replication. However, in light of the fact that a full-length copy of ORF 2 is not carried by all isolates, an essential role in plasmid replication or during growth in vitro seems unlikely.
Although most isolates were hybridization positive with ORF 4 PCR probes, significant differences in the RFLP patterns and overall intensities of hybridization were observed with ORF 4 amplicons derived from different B. burgdorferi sensu lato isolates (Fig. 3A and B). The B. afzelii IP21-derived probe hybridized with greater intensity to an 8.3-kb fragment in B. afzelii isolates than did the probe generated from B. burgdorferi CA9 (Fig. 3A and B, lanes 9 to 11). This likely reflects ORF 4 sequence divergence at the interspecies level. Interspecies sequence divergence has been reported for virtually all of the characterized genes of the B. burgdorferi sensu lato complex. Even the multiple ORF 4 homologs carried by an isogeneic population of B. burgdorferi B31 exhibit significant sequence divergence (63). It is interesting to note that while the IP21-derived probe hybridized with multiple fragments in B. andersonii 19857 and 21038 DNA (albeit faintly), the CA9-derived probe did not under identical hybridization conditions. Hence, the B. andersonii ORF 4-related sequences are more related to the ORF 4 sequence of B. afzelii than to that of B. burgdorferi. The lack of hybridization of the ORF 4 probes with B. japonica IKA2 also serves to illustrate that the probes are binding with specificity, since if general nonspecific binding were occurring, it would have occurred with this isolate as well. The ORF 4 hybridization analyses also demonstrate that there is intraspecies genomic variability. While ORF 4 was not detected by hybridization in B. japonica IKA2, hybridization of the ORF 4 probes with B. japonica HO14 DNA was observed. This could reflect differences in plasmid content, plasmid copy number, or ORF 4 sequences among B. japonica isolates.
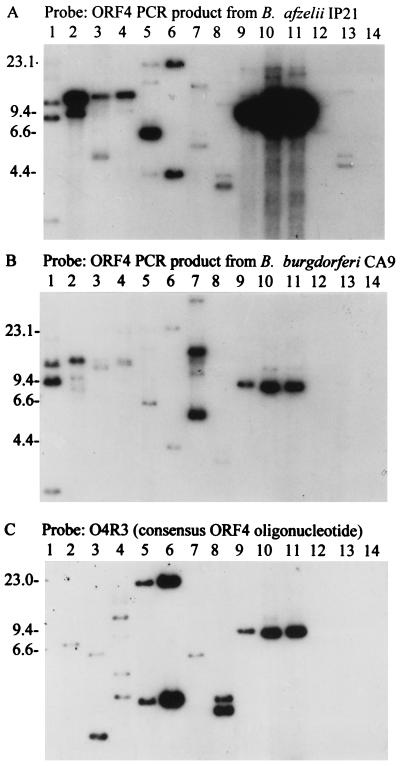
FIG. 3.
ORF 4 RFLP pattern analyses. The blots were probed with ORF 4 PCR probes obtained by amplification of ORF 4 from B. afzelii IP21 (A) or B. burgdorferi CA9 (B) or with the O4R3 oligonucleotide probe (C). The isolates analyzed were B. japonica HO14 (lane 1); B. burgdorferi LP7, CA9, and 297 (lanes 2 to 4, respectively); B. garinii FRG and N34, B. valaisiana VS116, and B. garinii VSBP (lanes 5 to 8, respectively); B. afzelii IP21, ECM1, and UMO1 (lanes 9 to 11, respectively); B. andersonii 19857 and 21038 (lanes 12 and 13, respectively); and B. japonica IKA2 (lane 14). Molecular size standards (in kilobases) are indicated on the left.
Zuckert and Meyer recently identified an ORF 4 homolog (ORF G) in B. burgdorferi B31 that exhibits 80% identity to ORF 4 of isolate IP21 (63). Alignment of these sequences allowed for the design of a consensus oligonucleotide (O4R3) that could be used to further assess the potential multicopy nature of ORF 4-related sequences. O4R3 hybridized strongly with multiple restriction fragments in several B. burgdorferi sensu lato isolates (Fig. 3C). In many isolates, the same fragments that bound the PCR probes also bound the oligonucleotide probes. Hybridizing fragments greater than 8.3 kb in size were detected in isolates 297, FRG, and N34. The detection of multiple hybridizing bands with the oligonucleotide probes clearly demonstrates that there is more than one copy of ORF 4-related sequences in some isolates. Furthermore, the sizes or sums of the sizes of the hybridizing restriction fragments detected indicate that some copies are carried on genomic components other than cp8.3. This is consistent with the detection of ORF 4-related sequences on plasmids larger than 8.3 kb in B. burgdorferi B31 (63).
Localization of the multiple copies of ORFs 1, 2, and 4 to specific genomic elements through Southern blot analysis of genomic DNA fractionated by 2D CHEF-PFGE.
To identify the genetic elements that carry the ORF 1-, 2-, and 4-related sequences, DNA fractionated by 2D CHEF-PFGE was hybridized with ORF-specific PCR-generated probes. 2D CHEF-PFGE allows for the differentiation of LPs and CPs and has been widely used in the study of Borrelia plasmids (1, 11, 14, 15, 27, 33, 34, 45, 46, 52). CPs migrate with retarded mobility in PFGE systems and upon electrophoresis in the second dimension lag behind the axis along which the LPs migrate. An exception are small CPs whose mobility is not significantly retarded upon electrophoresis in the second dimension and thus migrate along the same axis as LPs (this suggestion was confirmed through control experiments discussed below). DNA fractionated by this method is shown in Fig. 4A. A variety of LPs and CPs, but not the chromosome, hybridized with the ORF 1, 2, or 4 probes. Not all probes hybridized with the same plasmids, indicating that ORF 1-, 2-, and 4-related sequences are not always linked and that the presence of these sequences on plasmids other than cp8.3 is not in all cases due to a simple integration event of cp8.3. Consistent with this, Barbour et al. detected only ORF 1 homologs on a 17-kb LP of B. burgdorferi (5).
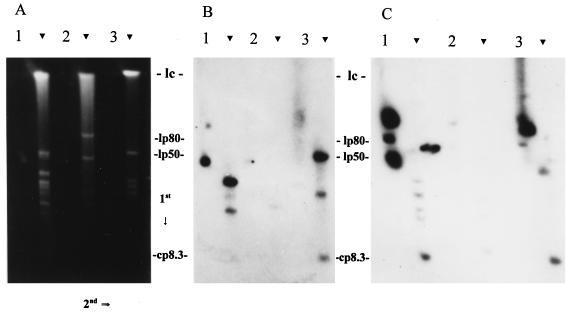
FIG. 4.
Determination of the size and conformation of the genomic elements that carry ORF 1- and ORF 4-related sequences. DNA was fractionated by 2D CHEF-PFGE as described in the text. The direction of electrophoresis in each dimension is indicated. Arrowheads indicate the locations of the linear plasmids. The gel shown in panel A was blotted and probed with an ORF 1 probe (B), while a similar gel was used in the ORF 4 analyses (C). Isolates analyzed were B. garinii FRG (lane 1), B. valaisiana VS116 (lane 2), and B. afzelii IP21 (lane 3). The migration positions of the approximately 980-kb linear chromosome (lc) (7), 50-kb ospAB-carrying LP (lp50) (8), 80-kb LP of isolate VS116 (lp80) (27), and cp8.3 are indicated. The migration positions of the CPs are not indicated but can be seen just to the left of the LPs of isolate FRG in panel A. It is important to note that lp80, the size of which was determined in a previous study, is carried only by VS116 (27). The signal to the right of the FRG LPs in panel C represents background.
The ORF 1 probe hybridized with several plasmids in isolates FRG and IP21 (Fig. 4B). Only weak hybridization with VS116 DNA was observed. In FRG, a CP hybridized with the probe. This plasmid is faintly visible in the ethidium bromide-stained gel just to the left of the 50-kb ospAB-carrying LP. Based on its migration characteristics, this plasmid is likely one or more of the 32-kb CPs that carry members of the UHB gene family (1, 11, 35). This was confirmed through Southern analysis by using the uhb(+) oligonucleotide (35) as a probe (data not shown). To ascertain if ORF 1 sequences are present on the ospC-carrying plasmid cp26, the blots were probed with an ospC-targeting oligonucleotide (32, 44) (data not shown). The probe hybridized with a CP slightly smaller than the one that bound the ORF 1 probe, indicating that ORF 1-related sequences are not present on cp26. Significant hybridization of the ORF 1 probe to a CP was observed only with isolate FRG. In the ethidium bromide-stained gels, two CPs are visible in isolate FRG, while these bands are not visible in VS116 and IP21. It is unclear if these isolates lack conserved 32-kb CPs or if they are carried by only a small percentage of the isolate population and thus were not detected by Southern hybridization.
The ORF 1 probe also hybridized with what appeared to be an LP of 8 to 10 kb in isolates IP21 and FRG (faint hybridization was observed with VS116). Linear plasmids of this size have not been observed in B. burgdorferi sensu lato isolates, and in light of this, we wanted to determine if this plasmid might be cp8.3. We speculated that due to the size of this plasmid, its migration in the second dimension might not be retarded to the same degree as that of larger Borrelia CPs. To test this, we electrophoresed under 2D CHEF-PFGE conditions the following: (i) a purified 6-kb control CP mixed with lambda DNA monocut molecular size standards (linear DNA), (ii) purified plasmid alone, (iii) lambda monocut molecular size standards alone, and (iv) Borrelia DNA. The 6-kb CP migrated along the linear axis of migration and exhibited no retarded mobility in the second dimension (data not shown). To verify that the plasmid had not been linearized, we also performed electrophoresis under constant field conditions. Under these conditions, the plasmid migrated with characteristics indicative of a supercoiled conformation (i.e., with an apparent size of 3.5 kb). These experiments demonstrate that the migration of small CPs are not significantly regarded in 2D CHEF-PFGE and suggest that the 8- to 10-kb plasmid observed along the linear plasmid axis is in fact cp8.3.
ORF 1-hybridizing LPs were also detected in some isolates. B. garinii FRG carries two hybridizing LPs of approximately 30 and 17 kb, while B. afzelii IP21 carries two hybridizing LPs of 50 and 25 kb. Weak hybridization with a 20-kb LP was observed with isolate VS116. This 20-kb LP was not visible in the stained gels; hence, the weak signal likely reflects a low copy number of the plasmid in the isolate population. The detection of ORF 1-related sequences on LPs is consistent with the detection of ORF 1 homologs on a 17-kb LP in B. burgdorferi B31 (5). However, the analyses presented here demonstrate that ORF 1-carrying LPs vary in size and are not present in all isolates.
Hybridization analyses of ORF 2 detected this ORF on cp32 in isolates FRG, VS116, and IP21. In VS116 and IP21, other hybridizing CPs that migrated close to cp32 were also detected (data not shown). Since these other CPs did not hybridize with the ORF 1 or UHB probes, it can be concluded that they are not conformational variants of cp32 and are distinct CPs. Weak hybridization with 50- and 20-kb LPs and with cp8.3 was observed with all three isolates. As with ORF 1, ORF 2-related sequences appear to be present on variably sized plasmids.
The PCR-generated ORF 4 probe hybridized with both CPs and LPs (Fig. 4C). Five hybridizing plasmids were detected in IP21, and four were detected in FRG. While hybridization with the 32-kb CPs of FRG was detected, this was not the case for isolates IP21 and VS116. In FRG, several LPs hybridized with the probe but only weakly. It is unclear if this represents specific hybridization, and in light of this, we will not focus on these plasmids in this discussion. In isolate IP21, the 8- to 10-kb plasmid that hybridized with the ORF 1 and 2 probes also hybridized with the ORF 4 probe, supporting the identification of this plasmid as cp8.3. Hybridization with a plasmid of equivalent size was observed in FRG as well. In FRG and IP21, the ORF 4 probe also hybridized with large CPs that migrated slower than the 32-kb CPs (Fig. 4C). Although these plasmids were not visible in stained gels, they were readily detected by Southern blotting. Size determinations based on 2D CHEF-PFGE alone are not sufficient for accurate size determination of CPs; however, based on its relative migration, we tentatively estimate this plasmid to be approximately 70 kb. CPs of this size have not been previously described in B. burgdorferi sensu lato isolates. Since these plasmids did not hybridize with the ospC, UHB, and ORF 1 and 2 probes, which hybridize with either cp26, cp32, or cp8.3, it can be concluded that they are not artifacts, altered conformations, or multimers of cp32, cp26, or cp8.3 but are distinct plasmids.
To determine if these plasmids are present in other isolates, one-dimensional CHEF-PFGE and Southern hybridization with the ORF 4 PCR probe were performed. Hybridizing plasmids that migrated to positions analogous to those of the large CPs of B. garinii FRG and B. afzelii IP21 were detected in B. burgdorferi LP7, CA9, and 297 and in B. afzelii ECM1 and UMO1 (data not shown). Other isolates listed in Table 1 do not carry plasmids with similar migration characteristics that hybridize with the ORF 4 probe. This does not preclude the possibility that they carry a similarly sized plasmid which lacks ORF 4.
Southern blot and spPCR analyses of IRA.
In light of the repeated nature of cp8.3-carried sequences, we wanted to determine if the IR elements of cp8.3 may have contributed to the multicopy state of these ORFs by promoting the integration of cp8.3 or portions thereof into other plasmids. To determine if related IR elements exist in other isolates that carry multiple copies of the ORFs, digested DNA was probed with the IRA-F1 oligonucleotide. B. burgdorferi LP7 and CA9, B. garinii FRG, B. valaisiana VS116, and B. afzelii IP21 and UMO1 were hybridization positive, and multiple bands were observed in some isolates (Fig. 5). As predicted by the restriction map of cp8.3, an 8.3-kb hybridizing fragment was detected in isolates LP7, IP21, and UMO1. Smaller fragments were detected in FRG (6.5 kb), VS116 (6.0 kb), and CA9 (5.6 kb), and larger fragments were detected in LP7 (10 kb), CA9 (10 kb), and VS116 (15 kb). The sizes of these larger restriction fragments indicate that they must be derived from genomic elements other than cp8.3. The smaller fragments could be derived from other plasmids or from cp8.3 variants which have different EcoRV RFLP patterns.
FIG. 5.
RFLP pattern analyses of cp8.3-related IR elements. DNA was digested to completion with EcoRV, fractionated in an 0.8% agarose gel, blotted, and probed with the IRA-F1 probe as described in the text. For lane identification, see the legend to Fig. 2.
To identify the genomic elements carrying the IR element, the IRA-F1 probe was hybridized with B. garinii FRG and B. valaisiana VS116 DNA fractionated by 2D CHEF-PFGE (data not shown). In both isolates, strong hybridization with cp32 was detected. A weaker signal was associated with two slightly smaller CPs in both isolates, although the precise sizes of these plasmids differed in each isolate. The IRA-F1 probe also hybridized weakly with LPs of 25 and 17 kb in FRG and VS116, respectively. While the IR element is distributed across species lines, it is not carried by all isolates, and like the ORFs, its location on specific genomic elements differs among isolates.
To determine if the IRA-F1 hybridizing sequences exist with IR orientation, spPCR analyses were performed with the IRA-F1 primer (Table 1). spPCR will yield product only if the primer binding sites exist with an IR orientation and are within an amplifiable range of each other. Amplicons of 3 kb were obtained from isolates CA9, N34, IP21, and UMO1, demonstrating an IR organization. This size is consistent with that predicted by the cp8.3 sequence of IP21. Isolates LP7 and VS116, which hybridized with the IRA-F1 probe, did not yield spPCR amplicons, indicating that multiple primer target sites in these isolates either are not IRs, are located too far apart, are variable in sequence such that PCR amplification is prevented, or are located on different plasmids.
PCR and DNA sequence analysis of the regions flanking IRA.
IR elements often flank mobile genetic elements, and as a consequence of translocation and integration, regions adjacent to IRs tend to be polymorphic. The IRA element of cp8.3 is flanked by ORFs 1 and 7, which have an opposite orientation to each other. To screen for polymorphisms around the borders of IRA, IRA and its flanking regions were amplified by using the O1R1 and O7R1 primers (Fig. 1). Amplicons of 700 bp were predicted and obtained from isolates FRG, N34, VS116, IP21, and UMO1 (Fig. 6A). However, the amplicon obtained from B. andersonii 21038 was only 300 bp in size. Sequence analyses of the amplicons from isolates 21038, IP21, and UMO1 were performed to identify the molecular basis for this truncated amplicon. The B. afzelii UMO1 amplicon carries a conserved IR element that differs from the IP21 sequence at only a few positions. The amplicon from isolate 21038 exhibits 67% nucleotide identity with a segment of the IP21 cp8.3 sequence extending from just upstream of ORF 7 through the majority of ORF 8 (with the exception of its 5′ end), while the other end of the amplicon exhibits identity with the 5′ end of ORF 1 (Fig. 6B). The IRA sequence and the start codons of ORFs 1 and 8 are absent from this amplicon. Hence, these copies of ORFs 1 and 8 can be considered to be pseudogenes. Relative to the IP21 cp8.3 sequence, there is a deletion of several hundred bases and a possible insertion of foreign sequence. The sequence analyses also explain why the IRA-F1 probe did not hybridize with B. andersonii 21038 DNA and provide suggestive evidence for previous recombination events that may have involved the IR element.
FIG. 6.
PCR and sequence analyses of IRA and its flanking regions. (A) Amplicons obtained with the O7R1 and O1R1 primers are presented. The O7R1-O1R1 primer pair was designed to amplify across IRA of cp8.3. For lane identification, see the legend to Fig. 2. (B) Selected amplicons were partially sequenced. Gaps introduced by alignment are indicated by dashes, conserved nucleotides are indicated by periods, and the sequence corresponding to IRA is indicated by boldface letters. The start codon of ORF 8 is underlined and indicated with italic letters.
Conclusions.
The goals of this study were to assess the distribution and molecular organization of the cp8.3 ORFs and IR elements among species of the B. burgdorferi sensu lato complex. RFLP pattern analyses of the ORFs revealed that their organization varies widely among isolates and is of greater complexity than previously appreciated. These ORFs (or closely related sequences) exist in a multicopy state in some isolates, with copies carried on both LPs and CPs. A significant body of evidence that demonstrates pronounced redundancy in the plasmid component of the Borrelia genome has now accumulated (5, 11, 21, 35, 39, 50, 52, 54, 62, 63). The presence of repeated sequences and IR elements on some Borrelia plasmids could provide a molecular basis for homologous recombination at the inter- and intraplasmid level. Recombination among plasmids could lead to hypervariability in plasmid composition and organization even among closely related isolates. Consistent with this, the organization and copy number of ORFs 1, 2, and 4 and the IR element differ among isolates and do not appear to be evolutionarily stable traits. It is important to note that not all plasmids of the Borrelia genome exhibit hypervariability. The ospC-carrying plasmid cp26 is conserved in size and is universal among isolates of the B. burgdorferi sensu lato complex (32, 55). This is not surprising since cp26 carries housekeeping genes such as those involved in purine biosynthesis (37). Hence, there may be selective pressure to maintain conservation of cp26, while variation in other plasmids can be tolerated.
In light of the multicopy nature of cp8.3 ORF-related sequences, we looked for evidence of genetic recombination in these sequences and sought to assess the possible contribution of the IR elements in these processes. IRA-related sequences were detected in some isolates on both LPs and CPs, and spPCR analyses confirmed their IR orientation. PCR and sequence analyses revealed that the regions adjacent to the IR elements of at least one isolate, B. andersonii 21038, are polymorphic, possibly indicating earlier recombinational activity. Interestingly, the first 39 nucleotides of the coding sequences of ORFs 8 and 4 are carried within the IR elements of cp8.3 (IRA and IRB, respectively) (13). Hence, transposition or deletion of the IR-flanked sequence of cp8.3 could conceivably regulate the expression of these ORFs. In fact, many isolates lack the IR elements and therefore may not carry the start codons for ORFs 4 and 8. Direct evidence for the absence of IRA and some of its flanking sequence was obtained for isolate 21038 through hybridization and DNA sequence analyses. The IRA flanking sequence that is absent in isolate 21038 also carries the start codon for ORF 1. Hence, this isolate lacks the start codon for both ORFs 1 and 8 and presumably for ORF 4 (as inferred from the absence of IRB), generating pseudogenes. However, since the IR elements are absent from many isolates that carry multicopy ORFs, it cannot be concluded that the IR elements are solely responsible for generating the multicopy state of cp8.3 ORFs.
Hybridization analyses, conducted to identify the genetic elements that carry cp8.3 ORF-related sequences, revealed that the ORFs are carried on variable LPs and CPs but not on the chromosome. In addition, ORF 4-related sequences were detected on previously uncharacterized CPs that are considerably larger than the largest known B. burgdorferi sensu lato CPs (i.e., cp32). Since these large CPs did not hybridize with ORF 1, ORF 2, or IRA probes, it can be concluded (i) that a complete copy of cp8.3 has not been integrated into these plasmids and (ii) that these plasmids are not multimers or altered conformations of cp32 or cp8.3. The origin, organization, and coding capacity of the CPs identified here require further analysis.
The role of the putative cp8.3 ORF-encoded gene products and homologs in the biology of the Lyme disease spirochetes remains unclear. While a function for these ORFs has yet to be determined, an important functional role seems likely in view of their wide distribution among natural populations and their multicopy state. Database searches have not proven helpful in identifying the possible functional role of these proteins, since they exhibit no homology with sequences or sequence motifs of known function. Preliminary analyses conducted in our laboratory have demonstrated that these ORFs are not transcriptionally expressed during in vitro cultivation, indicating that they do not play an essential role under these environmental conditions (26). Expression may require different environmental stimuli, perhaps those encountered in the tick or mammalian host. In conclusion, the data presented here demonstrate significant complexity and variability in the organization of cp8.3 ORF-related sequences. Although the ORFs are widely distributed among isolates, the organization of these sequence elements is not evolutionarily stable and appears to have been influenced by interplasmid recombination. Work in progress is focusing on assessing the transcriptional patterns of expression of these ORFs in different environments (i.e., in vitro, in the tick, and in the mammalian host). These studies will provide clues as to the possible biological role of the multicopy ORF 1-, 2-, and 4-related sequences in the biology of the B. burgdorferi sensu lato complex.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Jeffress Trust and the National Institutes of Health.
We thank Wolfram Zuckert, Jurg Meyer, Steve Porcella, Scott Samuels, and the Virginia Commonwealth University molecular pathogenesis group for helpful discussions and Todd Kitten for computer assistance.
REFERENCES
- 1.Akins D, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Individualization of two new genomic groups among American Borrelia burgdorferi sensu lato strains. FEMS Microbiol Lett. 1994;121:93–98. doi: 10.1111/j.1574-6968.1994.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G, Carter C J, Bundoc V, Hinnebusch J. The nucleotide sequence of a linear plasmid of Borrelia burgdorferi reveals similarities to those of circular plasmids of other prokaryotes. J Bacteriol. 1996;178:6635–6639. doi: 10.1128/jb.178.22.6635-6639.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour A G, Garon C F. The genes encoding the major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann N Y Acad Sci. 1988;539:144–153. doi: 10.1111/j.1749-6632.1988.tb31847.x. [DOI] [PubMed] [Google Scholar]
- 7.Baril C, Richaud C, Baranton G, Saint Girons I. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989;140:507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- 8.Bergström S, Bundoc V G, Barbour A G. Molecular analysis of the linear plasmid encoded major surface proteins, OspA and OspB, of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Casjens S, DeLang M, Ley III H L, Rosa P, Huang W M. Linear chromosome of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J Bacteriol. 1995;177:2769–2780. doi: 10.1128/jb.177.10.2769-2780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn J J, Buchstein S R, Butler L-L, Fisenne S, Polin D S, Lade B N, Luft B J. Complete nucleotide sequence of a circular plasmid from the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1994;176:2706–2717. doi: 10.1128/jb.176.9.2706-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferdows M S, Barbour A G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci USA. 1989;86:5969–5973. [Google Scholar]
- 15.Ferdows M S, Serwer P, Griess G A, Norris S J, Barbour A G. Conversion of a linear to a circular plasmid in the relapsing fever agent Borrelia hermsii. J Bacteriol. 1996;178:793–800. doi: 10.1128/jb.178.3.793-800.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleche A L, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 17.Fukunaga M, Hamase A, Okada K, Inoue H, Tsuruta Y, Miyamoto K, Nakao M. Characterization of spirochetes isolated from ticks (Ixodes tanukin, Ixodes turdus, and Ixodes columnae) and comparison of the sequences with those of Borrelia burgdorferi sensu lato strains. Appl Environ Microbiol. 1996;62:2338–2344. doi: 10.1128/aem.62.7.2338-2344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukunaga M, Hamase A, Okada K, Nakao M. Borrelia tanuki sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga M, Okada K, Nakao M, Konishi T, Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int J Syst Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch J, Barbour A G. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991;173:7233–7239. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinnebusch J, Bergstrom S, Barbour A G. Cloning and sequence analysis of linear plasmid telomeres of the bacterium Borrelia burgdorferi. Mol Microbiol. 1990;4:811–820. doi: 10.1111/j.1365-2958.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyde F W, Johnson R C. Characterization of a circular plasmid from Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1988;26:2203–2205. doi: 10.1128/jcm.26.10.2203-2205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jauris-Heipke S, Liegl G, Preac-Mursic V, Röbler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 25.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marconi, R. T. Unpublished data.
- 27.Marconi R T, Casjens S, Munderloh U G, Samuels D S. Analysis of linear plasmid dimers in Borrelia burgdorferi sensu lato isolates: implications concerning the potential mechanism of linear plasmid replication. J Bacteriol. 1996;178:3357–3361. doi: 10.1128/jb.178.11.3357-3361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marconi R T, Garon C F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol. 1992;138:533–536. doi: 10.1099/00221287-138-3-533. [DOI] [PubMed] [Google Scholar]
- 29.Marconi R T, Garon C F. Phylogenetic analysis of the genus Borrelia: a comparison of North American and European isolates of B. burgdorferi. J Bacteriol. 1992;174:241–244. doi: 10.1128/jb.174.1.241-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi R T, Konkel M E, Garon C F. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect Immun. 1993;61:2611–2617. doi: 10.1128/iai.61.6.2611-2617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marconi R T, Samuels D S, Garon C F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marconi R T, Samuels D S, Schwan T G, Garon C F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993;31:2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marconi R T, Sung S Y, Hughes C A N, Carlyon J A. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1996;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marconi R T, Wigboldus J, Weissbach H, Brot N. Transcriptional start site and MetR binding sites on the Escherichia coli metH gene. Biochem Biophys Res Commun. 1991;175:1057–1063. doi: 10.1016/0006-291x(91)91672-y. [DOI] [PubMed] [Google Scholar]
- 37.Margolis N, Hogan D, Tilly K, Rosa P. Plasmid location of Borrelia purine biosynthesis gene homologs. J Bacteriol. 1994;176:6427–6432. doi: 10.1128/jb.176.21.6427-6432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porcella S F, Popova T G, Akins D R, Li M, Radolf J R, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:733–742. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 41.Postic D, Belfazia J, Isogai E, Saint Girons I, Grimont P A D, Baranton G. A new genomic species in Borrelia burgdorferi sensu lato isolated from Japanese ticks. Res Microbiol. 1993;144:467–473. doi: 10.1016/0923-2508(93)90054-6. [DOI] [PubMed] [Google Scholar]
- 42.Postic D, Edlinger C, Richaud C, Grimont F, Dufresne Y, Perolat P, Baranton G, Grimont P A D. Two genomic species in Borrelia burgdorferi. Res Microbiol. 1990;141:465–475. doi: 10.1016/0923-2508(90)90072-x. [DOI] [PubMed] [Google Scholar]
- 43.Rosa P A, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 44.Sadziene A, Wilske B, Ferdows M S, Barbour A G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuels D S, Marconi R T, Garon C F. Variation in the size of the ospA-containing linear plasmid, but not the linear chromosome, among the three Borrelia species associated with Lyme disease. J Gen Microbiol. 1993;139:2445–2449. doi: 10.1099/00221287-139-10-2445. [DOI] [PubMed] [Google Scholar]
- 46.Samuels D S, Marconi R T, Huang W M, Garon C F. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J Bacteriol. 1994;176:3072–3075. doi: 10.1128/jb.176.10.3072-3075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan T G, Schrumpf M E, Karstens R H, Clover J R, Wong J, Daugherty M, Struthers M, Rosa P A. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993;31:3096–3108. doi: 10.1128/jcm.31.12.3096-3108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson W J, Garon C F, Schwan T G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 51.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 52.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sung, S.-Y., C. LaVoie, J. A. Carlyon, and R. T. Marconi. Evidence for extensive recombination among ospE homolog members of the UHB gene family in isolates of the Borrelia burgdorferi sensu lato complex. Submitted for publication.
- 55.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 56.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallich R, Helmes C, Schaible U E, Lobet Y, Moter S E, Kramer M D, Simon M M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992;60:4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, van Dam A P, Le Flecha A, Postic D, Peter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 59.Welsh J, Pretzman C, Postic D, Saint Girons I, Baranton G, McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992;42:370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y, Johnson R C. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J Clin Microbiol. 1995;33:2679–2685. doi: 10.1128/jcm.33.10.2679-2685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuckert, W. R., R. T. Marconi, J. A. Carlyon, and J. Meyer. 1997. Unpublished data.
- 63.Zuckert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]