Abstract
Neuroendocrine prostate cancer (NEPC) is a lethal subtype of prostate cancer. NEPC arises de novo only rarely; the disease predominantly develops from adenocarcinoma in response to drug-induced androgen receptor signalling inhibition, although the mechanisms behind this transdifferentiation are a subject of debate. The survival of patients with NEPC is poor, and few effective treatment options are available. To improve clinical outcomes, understanding of the biology and molecular mechanisms regulating NEPC development is crucial. Various NEPC molecular drivers make temporal contributions during NEPC development, and despite the limited treatment options available, several novel targeted therapeutics are currently under research.
Prostate cancer has the second highest incidence of all malignancies in men worldwide1. Of prostate cancers diagnosed, 90–95% are adenocarcinoma with a luminal phenotype characterized by strong androgen receptor (AR) and PSA expression2. Prostate cancer requires AR-mediated signalling for tumour growth; therefore, androgen deprivation therapy (ADT) is the first-line treatment for advanced prostate cancer3,4. However, prostate cancer cells can eventually adapt to androgen deprivation and relapse by restoring AR signalling even in a low androgen production environment, a status referred to as castration-resistant prostate cancer (CRPC)5,6. As most CRPCs remain AR-dependent5, potent AR pathway inhibitors (ARPIs), such as abiraterone, enzalutamide, apalutamide and darolutamide, have been developed. These therapies have been demonstrated to improve survival over placebo7-11 in patients with CRPC. However, AR-independent mechanisms in prostate cancer enable the development of resistance to ARPIs and continued tumour growth12,13. A subset of ARPI-resistant CRPC, commonly referred to as neuroendocrine prostate cancer (NEPC)5,14, is characterized by low or absent AR expression, independence of AR signalling and gain of a neuroendocrine phenotype.
De novo NEPC is rare, accounting for less than 2% of all prostate cancers at the time of diagnosis15,16; however, treatment-induced NEPC occurs in 10–17% of patients with CRPC by evolving from adenocarcinoma in response to ADT and/or APRI treatment13,17,18. Frequent biallelic deletion and/or mutation of the tumour suppressor genes TP53, RB1 and PTEN are observed in de novo NEPC, similar to the alterations found in treatment-induced NEPC19. De novo NEPC also closely resembles treatment-induced NEPC at an RNA level19. However, whether de novo NEPC and treatment-induced NEPC are the same disease, and whether they both derive from adenocarcinoma, even in the absence of AR pathway inhibition, is unclear.
A variety of terms have been used to describe NEPC12,16,20,21. In 2014, clinical and molecular data from both de novo and treatment-emergent disease led the Prostate Cancer Foundation to propose a revised pathological consensus for defining NEPC, covering a wide spectrum of neuroendocrine differentiation in prostate cancer ranging from mixed adenocarcinomas with neuroendocrine features to pure small-cell NEPC20. Histologically, this classification includes: prostate adenocarcinoma with neuroendocrine differentiation, adenocarcinoma with Paneth cell neuroendocrine differentiation, carcinoid tumour, small-cell carcinoma, large-cell neuroendocrine carcinoma and mixed neuroendocrine carcinoma and acinar adenocarcinoma. Immunohistochemically, NEPC cancer cells are characterized by the expression of neuroendocrine markers such as synaptophysin (SYP), neural cell adhesion molecule 1 (CD56), and chromogranin A (CHGA) and the absence of AR and PSA secretion22,23. The poorly differentiated small-cell carcinoma is the most aggressive subtype of NEPC with a similar histology, disease progression and treatment response to small-cell lung cancer (SCLC) and other small-cell carcinomas20,22. Small-cell carcinoma of the prostate has the poorest outcome, with a median survival of less than 1 year14,24.
Clinically, the definition of NEPC overlaps with anaplastic prostate carcinoma and aggressive variant prostate cancer (AVPC)12,16,21. CRPC that fulfils at least one of seven defined clinical features (such as extensive visceral metastases, presence of neuroendocrine markers on histology or in serum and short duration of response to ADT) is considered anaplastic prostate carcinoma21. AVPC includes both anaplastic prostate cancer and NEPC, and represents a broader range of AR-independent CRPCs than the term NEPC12. Another subtype of AR-independent CRPC is double-negative prostate cancer (DNPC). Similar to NEPC, DNPC is characterized as having low or absent AR expression and growth is independent of AR signalling, but these tumours lack the expression of neuroendocrine makers13. Lineage plasticity is considered to be associated with loss of AR signalling dependence and the acquisition of alternative lineage programmes in both DNPC and NEPC25. However, whether NEPC and DNPC share the same developmental mechanisms, and whether DNPC is a precursor of NEPC, is unclear.
The molecular pathways that regulate NEPC development are unclear, although a number of genomic, epigenetic and transcriptional alterations are reported to be involved in this process. However, the timing, interconnection and feedback mechanisms of these drivers of NEPC development, together with their relationships with other oncogenic pathways and suppression of AR expression, remain to be fully elucidated25. The lack of serial sampling during the development of NEPC in patients owing to the difficulty in predicting which patients will develop NEPC, and problems obtaining serial biopsy samples at multiple time points between initiation of ADT and the diagnosis of relapsed NEPC, as well as a lack of patient-derived preclinical models that recapitulate disease progression, limits the volume of data available to address how NEPC develops25. Thus, in 2019, a National Cancer Institute workshop focusing on AR-independent prostate cancer, the ‘establishment of preclinical models that recapitulate biology of the disease and the recognized phenotypes’ and ‘determining the temporal contribution and cooperation of emerging drivers’ were highlighted as the two major areas that need to be addressed in the AR-independent prostate cancer field25.
In this Review, we summarize the hypotheses of the origin of NEPC in the context of the reported major molecular alterations; mainly focusing on pure small-cell NEPC according to the Prostate Cancer Foundation classification. We discuss the current knowledge of the cell origin of NEPC, reported molecular alterations in NEPC, longitudinal changes in these molecular alterations and their potential temporal contributions to NEPC development, and highlight therapeutics for NEPC that are available or undergoing preclinical development. Finally, we discuss the major questions that can be addressed by longitudinal studies of NEPC development and their potential contribution to improving future patient care.
The origin of NEPC
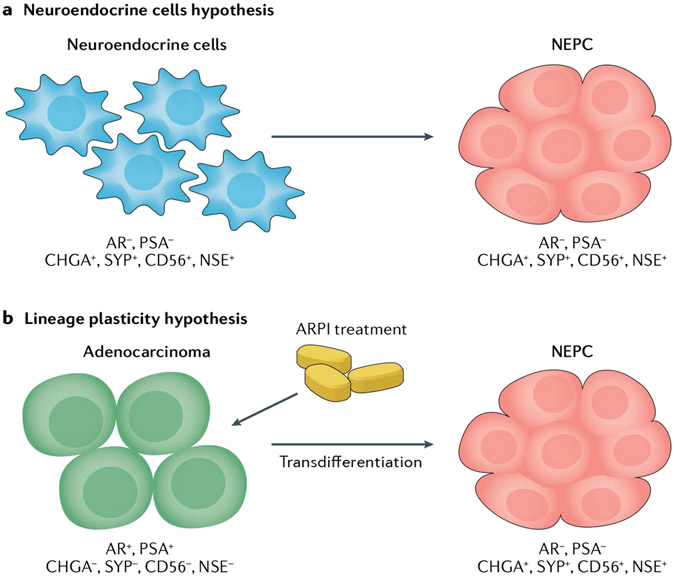
The origin of NEPC is a controversial topic that has been debated over the past few decades. Two prevailing hypotheses are under investigation (FIG. 1): the neuroendocrine cells hypothesis and the lineage plasticity hypothesis.
Fig. 1 ∣. Hypotheses of the origin of neuroendocrine prostate cancer.
a ∣ Neuroendocrine prostate cancer (NEPC) might develop from neuroendocrine cells via neoplastic transformation, and maintain certain neuroendocrine cell properties including the expression of neuroendocrine markers such as Chromogranin A (CHGA), Synaptophysin (SYP) and neuron-specific enolase (NSE), as well as the lack of androgen receptor (AR) and PSA secretion. b ∣ The lineage plasticity hypothesis suggests that AR+PSA+ prostate adenocarcinoma cells, which do not express neuroendocrine markers, undergo transdifferentiation following AR pathway inhibitor (ARPI) treatment and develop into AR−PSA− neuroendocrine marker+ NEPC cells.
The neuroendocrine cells hypothesis
Neuroendocrine cells are normally scattered among the more abundant basal and luminal epithelial cells in benign prostate tissue, representing a minor cell population comprising 1% of the entire epithelial cell population26. These cells produce and secrete potent neuro-hormones such as neural growth factor, bombesin (otherwise known as gastrin-releasing peptide), serotonin, calcitonin and parathyroid hormone-related peptide, which regulate the growth and homeostasis of the adjacent epithelial cells via paracrine signalling27,28. Neuroendocrine cells and NEPC cells share some common features, including the expression of neuroendocrine markers, such as CHGA, SYP and gamma-enolase (ENO2). Normal prostatic neuroendocrine cells have been proposed as the origin of non-proliferative neuroendocrine cells in prostate adenocarcinoma29,30.
Studies in a genetically engineered mouse model have suggested the transformation of normal prostatic neuroendocrine cells as the origin of NEPC31. In this model, the mouse cryptdin-2 (Cr2) gene promoter was used to induce expression of the simian virus 40 T antigen (SV40 T-Ag) in a subset of neuroendocrine cells in the prostate lobes. At 8 weeks of age, the proliferative SV40 T-Ag-positive cells observed in this model invariably expressed two neuroendocrine markers, SYP and CHGA. As animals aged, NEPC developed and metastasized to regional lymph nodes. The NEPC tumour co-expressed SV40 T-Ag and neuroendocrine markers and was resistant to host castration31. This model indicates that the prostatic neuroendocrine cell population can undergo malignant transformation. Although this model could indicate the possibility of a neuroendocrine cell origin of de novo NEPC, more evidence is in favour of the transdifferentiation of NEPC directly from adenocarcinoma cells32.
The lineage plasticity hypothesis
De novo NEPC is rare, but NEPC can be observed in 10–17% of patients with prostate adenocarcinoma who have been treated with hormonal therapies13,17,18. A growing body of evidence supports the hypothesis that prostatic adenocarcinoma cells can lose their adenocarcinoma features and gain a neuroendocrine phenotype during long-term ADT, indicating a process driven by cellular lineage plasticity.
Evidence of transdifferentiation from human prostate cancer studies.
This model is supported by genomic analyses of human prostate biopsy and surgically excised samples23,33-35. For example, the frequency of gene fusions between ERG and TMPRSS2 in NEPC is around 50%, which is similar to the frequency observed in prostatic adenocarcinomas, but not in normal neuroendocrine cells36,37. Moreover, in adenocarcinoma and NEPC mixed tumours, a concordance of TMPRSS2–ERG fusion23 and other genomic alterations such as RB1 loss and TP53 loss or mutation38,39 were observed in adenocarcinoma and adjacent neuroendocrine foci. Allelic imbalance analyses of eight radical prostatectomy specimens also showed that NEPC cells share identical allelic profiles with adenocarcinoma cells but not with non-cancerous neuroendocrine cells40. Furthermore, temporal allele-specific analyses of serial biopsy tissue samples taken from a patient with prostatic adenocarcinoma during the course of disease progression on abiraterone treatment demonstrated that the genomic profiles were similar between the same patient’s CRPC and NEPC, suggesting divergent clonal evolution of adenocarcinoma towards either an AR-driven or an AR-independent state during ARPI treatment41.
A single-cell RNA sequencing study of clinical CRPC biopsy samples with small-cell carcinoma morphology showed that the neuroendocrine cells in cancer tissues exhibited a luminal epithelial phenotype rather than a basal phenotype42. In two tumours that contain both neuroendocrine cells and non-neuroendocrine epithelial cells, intra-tumoural RNA velocity analysis suggested that the neuroendocrine cells arose from adenocarcinoma cells. Inferred copy number variation analysis on the basis of the average expression of 101 genes showed that the neuroendocrine cells and luminal cells in the same patient’s cancer tissues shared a similar copy number variation pattern, indicating a common clonal origin of NEPC and adenocarcinoma42. These findings further support the hypothesis that NEPC originates from an adenocarcinoma precursor.
Evidence of transdifferentiation from cell models.
Observations in preclinical models also support the transdifferentiation of NEPC from adenocarcinoma. LNCaP, an AR+, PSA+ androgen-sensitive human prostate adenocarcinoma cell line43, is widely used for studies of neuroendocrine phenotype induction. The expression of neuroendocrine markers such as CHGA and SYP can be induced in LNCaP cells by treatment with cyclic AMP, interleukins, various growth factors, or ADT13,35-46. In an in vivo study, increased numbers of neuroendocrine marker-positive cells were observed to be scattered within LNCaP xenografts implanted into castrated mice compared with the xenografts in intact male mice44. However, these neuroendocrine marker-positive cells were non-proliferative; thus, this model cannot fully represent the aggressiveness of human NEPC. To overcome this limitation, two cell models, 42DENZR and 42FENZR, were developed from LNCaP xenograft tumours that relapsed after treatment with enzalutamide. Both models exhibit downregulation of AR signalling, upregulation of neuroendocrine marker expression, and increased proliferative abilities45. These LNCaP and LNCaP-derived cell models partially demonstrate that adenocarcinoma cells could undergo neuroendocrine transdifferentiation in response to androgen ablation. However, the cells do not recapitulate the full features of terminal NEPC in patients, such as complete loss of AR signalling and the highly proliferative phenotype44.
Evidence of transdifferentiation from genetically modified mouse models.
In the transgenic adenocarcinoma of the mouse prostate (TRAMP) model, SV40-T-Ag expression is driven by the rat probasin promoter specifically in the mouse prostate and spontaneously induces neoplastic epithelial transformation46-48.
By the age of 12 weeks, most TRAMP mice develop prostate adenocarcinomas. The tumours show variable responses to host castration, with approximately 80% of mice who are castrated at 12 weeks of age developing poorly differentiated carcinomas with neuroendocrine features by 24 weeks of age47,49. The NEPC cells found in this model have variable to no AR expression and all express the neuroendocrine marker SYP with a high proliferative index as determined by Ki67 (REF.49), recapitulating the features observed in human NEPC. The absence of SYP + NEPC cells in early disease suggests that the emergence of the neuroendocrine features is a late event during disease development49. Thus, the model indicates an adenocarcinoma to neuroendocrine transdifferentiation event related to prostate cancer progression, although the theory that NEPC originates from bipotential stem cells that are capable of expressing both epithelial (E-cadherin) and neuroendocrine (SYP) markers, rather than directly from epithelial cells, is also possible50. However, all prostate cancers of TRAMP mice will progress to NEPC even without host castration49, and, therefore, the model might not mimic the treatment-induced NEPC development seen in patients.
Probasin promoter-driven Pten and Rb1 double knockout (DKO) in luminal epithelial cells of PBCre4:Ptenf/f:Rb1f/f mice induce prostate adenocarcinoma with heterogeneous expression of AR. Surgical castration of these mice extended survival from 38 to 48 weeks compared with intact mice, suggesting that the prostate adenocarcinoma induced in this model is ADT sensitive51. Further development of Pten, Rb1 and Trp53 triple-knockout (TKO) mice (PBCre4:Ptenf/f:Rb1f/f:Trp53f/f) showed an aggressive phenotype with metastases in lung, liver and bone51. This TKO tumour is ADT resistant, and exhibits histopathological and molecular features of NEPC, including patchy expression of the neuroendocrine marker SYP and decreased AR signalling compared with the DKO tumour51. These findings suggest that the genetic alterations can enhance luminal cell lineage plasticity and spontaneously induce NEPC development from prostate luminal cells in vivo, albeit in the absence of ADT51.
In another mouse model, Pten and Trp53 are deleted in Nkx3.1+ prostate luminal cells to generate NPp53 (Nkx3.1CreERT2/+; Ptenfl/fl; Trp53fl/fl) mice52. Following castration, NPp53 mice developed CRPC that retained features of adenocarcinoma. NPp53 CRPC did not respond to treatment with abiraterone (an inhibitor of cytochrome P450). Tumours in a subset of these mice showed accelerated growth and displayed histological and molecular features of NEPC, such as a small-cell phenotype, increased expression of SYP and reduced expression of AR compared with regions of adenocarcinoma in the vehicle-treated mice52. In exceptional non-responding tumours, SYP+/AR− NEPC cells comprised the bulk of tumour cells in some regions and were highly proliferative, as indicated by Ki67 staining52. Furthermore, lineage tracing in NPp53 mice was performed with a R26R-yellow fluorescent protein (YFP) reporter allele expressed in luminal epithelial cells of the adult prostate. Co-expression of YFP in neuroendocrine marker-positive tumour cells that arose after castration strongly supports that these NEPC cells originate from the luminal adenocarcinoma cells52.
Evidence of transdifferentiation from patient-derived xenograft models.
The findings of studies using the LTL331/LTL331R patient-derived xenograft (PDX) model strongly support the lineage plasticity hypothesis53. LTL331 is a tissue line derived from a prostatectomy sample of ADT-naive prostate adenocarcinoma. Tumour cells in LTL331 express AR and PSA and have a typical adenocarcinoma transcriptomic profile53. The PDX model responds initially to host castration with a dramatic reduction in both tumour volume and PSA levels. However, castration-resistant relapsed tumour growth (LTL331R) is reproducibly observed several months post-castration53. Tumour cells in LTL331R lack AR and PSA expression and uniformly express a range of neuroendocrine markers, including SYP, CHGA, chromogranin B and CD56. The transcriptomic profile of LTL331R tumour cells is very similar to that of NEPC cells from patients, with an increased neuroendocrine score46,54,55. Additionally, LTL331R retains its highly proliferative, neuroendocrine phenotype and androgen-independent growth when regrafted into intact, testosterone-supplemented, or castrated hosts, suggesting that LTL331R arises as a result of a stable and irreversible lineage transition53.
LTL331 and LTL331R have different transcriptomic profiles but extremely similar genomic profiles53. Moreover, histopathological studies and single-cell sequencing analyses have found no evidence of pre-existing neuroendocrine cells in the original LTL331 PDX tumour (Yuzhuo W. unpublished data). These data indicate that ADT induces an adaptive response in adenocarcinoma cells, leading to neuroendocrine lineage transition, rather than clonal selection of an existing population of NEPC cells.
Overall, findings from preclinical models and clinical patients’ samples provide strong evidence that NEPC arises by transdifferentiation of prostatic adenocarcinoma. However, divergent clonal evolution could also be involved in NEPC development from adenocarcinoma under the selective pressure of AR inhibitors41. Clonal evolution and cell lineage plasticity are not necessarily mutually exclusive as both mechanisms could contribute to NEPC development41. The detailed roles of clonal evolution and cell lineage reprogramming during disease progression remain to be explored.
Molecular mechanisms underlying NEPC
Over the past decade, numerous studies have unveiled the molecular mechanisms underlying NEPC development. These mechanisms include genomic, epigenetic, transcriptional and other molecular pathways.
Genomic alterations
Several genomic alterations are enriched in NEPC. Whole-exome sequencing of surgically excised and biopsy specimens of metastatic prostate cancer identified enrichment of co-occurring RB1 loss and TP53 mutation or deletion in NEPC compared with adenocarcinoma18,41,56. Inactivation of Rb1 and Trp53 in mouse prostate luminal epithelium drives the development of a highly metastatic, ADT-resistant prostate carcinoma with neuroendocrine differentiation in vivo57. Rb1 depletion also facilitates lineage plasticity and metastasis of prostate adenocarcinoma in Pten-null mice, with additional Trp53 loss driving ADT resistance51. Furthermore, the functional loss of RB1 and TP53 in LNCaP-AR cells (a strain engineered to have increased expression of AR58) drives prostate cancer progression via lineage plasticity with the gain of prostate basal cell and neuroendocrine features and AR-indifferent status59. However, combined deletion of RB1 and TP53 in the parental LNCaP cell line triggered resistance to ADT and enzalutamide treatment, but did not induce cell lineage alterations60, suggesting that neuroendocrine differentiation is not an obligatory consequence of inactivating TP53 and RB1 and that different molecular contexts might lead to different results. N-Myc proto-oncogene protein (N-myc) (encoded by the MYCN gene) is a neural development-related transcription factor that binds directly to the promoters of NSE, SYP and AR genes in MYCN-overexpressing LNCaP cells23. MYCN overexpression together with AKT1 activation drives the transformation of prostate basal epithelial cells to adenocarcinoma and NEPC54. In addition, overexpression of Mycn in a Pten+/− mouse model of prostate cancer was demonstrated to drive the development of aggressive prostate carcinomas with a variety of morphologies, including adenocarcinoma, poorly differentiated carcinoma with divergent differentiations, and NEPC61. These studies establish causal relationships between MYCN and neuroendocrine differentiation. Aurora kinase A (encoded by AURKA) is involved in mitosis, asymmetric division, cilia dynamics of normal cells and can also promote cancer development62. Aurora kinase A can bind to and stabilize n-myc, and this interaction can be disrupted by the Aurora kinase A inhibitor alisertib63,64. The precise function and mechanism of AURKA itself in driving neuroendocrine differentiation remains elusive. In a clinical cohort including 169 patients with primary adenocarcinoma and 37 patients with NEPC, immunohistochemistry (IHC) staining showed an increase in aurora kinase A expression in 12% of adenocarcinoma specimens and 76% of NEPC specimens23. RNA sequencing also demonstrated that overexpression and gene amplification of both MYCN and AURKA occurs in 40% of NEPC samples compared with only 5% of adenocarcinomas23. In more than 90% of AURKA and MYCN amplification cases, amplification of AURKA or MYCN was concurrent23. Functionally, overexpression of either AURKA or MYCN in a benign human prostate cell line (RWPE-1) induced expression of the neuroendocrine markers NSE and SYP23. In vivo, human NEPC H660 cell xenograft and PDX models are sensitive to the pan aurora kinase inhibitor, PHA-739358. By contrast, no sensitivity to PHA-739358 was demonstrated in adenocarcinoma LNCaP xenografts23. These findings suggest that AURKA and MYCN might be involved in neuroendocrine differentiation and NEPC development.
The increased frequency of loss of RB1, TP53 mutation or deletion, and MYCN amplification in NEPC and functional studies support their roles in NEPC development. Apart from these alterations, few recurrent genomic alterations have been reported to be enriched in NEPC compared with adenocarcinoma, suggesting that non-genomic dysregulation could be involved in neuroendocrine transdifferentiation.
Epigenetic regulators
Neuroendocrine transdifferentiation is a dynamic process that arises as a result of androgen deprivation. However, the distinct cell lineage phenotype and transcriptome landscape change between NEPC and adenocarcinoma cannot be entirely explained by the limited recurrent genomic alterations. Epigenetic mechanisms can regulate tissue-specific gene expression patterns and, in turn, establish and maintain cell identity by chromatin modification and chromatin remodelling65. Thus, a mechanism that is not genetic but rather epigenetic is also considered to drive NEPC development. A number of epigenetic regulators have been reported to be key drivers of NEPC.
Polycomb group proteins.
Polycomb group (PcG) proteins are important epigenetic regulators that influence cell growth and differentiation in a wide range of cancer types66,67. PcG proteins contain two core complexes: polycomb repressive complexes 1 and 2 (PRC1 and PRC2)68. Upregulation of multiple PcG genes such as enhancer of zeste homologue 2 (EZH2) and CBX2 is a common feature of NEPC compared with adenocarcinoma across multiple cell lines, mouse models and clinical samples23,61,69.
EZH2 is the most frequently studied of the PcG genes. This gene encodes a catalytic subunit of PRC2 that tri-methylates histone H3 at Lysine 27 (H3K27me3) using its C-terminal SET (Su(var)3–9, enhancer-of-zeste and trithorax) domain to promote transcriptional silencing70. Increased expression of EZH2 was first reported in clinical NEPC samples compared with adenocarcinoma23 and has been further validated in NEPC mouse models51,59, PDX models and clinical cohorts26,36,49,53,65,69. Additionally, in preclinical cell-line and genetically engineered mouse models of NEPC, inhibiting EZH2 can reverse neuroendocrine transdifferentiation by restoring AR expression and regaining sensitivity to enzalutamide treatment51,61,71. The precise mechanisms underlying EZH2-regulated neuroendocrine differentiation remain unclear. However, studies in N-myc-driven NEPC mouse models have demonstrated that EZH2 directly interacts with N-myc to inhibit the AR signalling axis and drive NEPC development61. How other PcG genes function and cooperate in NEPC is not yet known.
Heterochromatin protein 1α.
Heterochromatin protein 1α (HP1α, encoded by CBX5) recognizes and binds to histone H3 tails that are dimethylated or trimethylated at Lysine 9 (H3K9me2 and H3K9me3)72. HP1α is prominently associated with constitutive heterochromatin and mediates concomitant gene silencing73. A longitudinal analysis of the LTL331/LTL331R PDX model showed that HP1α expression is increased within weeks following castration and is stably maintained in terminal NEPC. This trend in HP1α gene expression is different from those of other known NEPC driver genes (for example, EZH2), which are only upregulated in terminal NEPC55. The upregulation of HP1α RNA and protein expression has also been verified in clinical NEPC samples, although no apparent increase in HP1α expression has been demonstrated in adenocarcinoma compared with benign human prostate tissue55.
Depletion of HP1α inhibits proliferation and induces apoptosis in NEPC cells in vitro and in vivo55. Moreover, ectopic expression of HP1α promotes neuroendocrine differentiation in adenocarcinoma cells subjected to ADT55. Mechanistically, HP1α enriches H3K9me3 at the promoters of AR and REST genes, thereby repressing their expression55. These data indicate that HP1α is an early driver of NEPC and a novel therapeutic target. Functional studies using in vivo models and a deeper understanding of the underlying molecular mechanisms will pave the way for the development of drugs that target HP1α. Upregulation of other heterochromatin-related genes has also been observed in NEPC models and clinical samples55. The contributions of these genes and their interactions during NEPC development require further investigation.
Protein DEK.
Protein DEK (DEK) is a DNA topology modulator74 that interacts with other epigenetic modulators to recruit histones, histone modulators and chromatin remodelling factors to DNA75,76. Elevated DEK expression is observed in NEPC PDX models and clinical samples. Upregulation of DEK mRNA and protein expression is more commonly observed in NEPC than in human benign prostate tissues and adenocarcinomas77. Increased DEK expression is an independent prognostic factor that was inversely associated with disease-free survival in 163 hormone therapy-naive patients with prostate cancer. Elevated DEK expression has a relative risk of 6.91 for relapse-free survival (95% CI 1.33–35.96, P = 0.022)77. Depletion of DEK inhibits the proliferation and motility of the PC-3 prostate cancer cell line77. Although these data indicate that DEK plays a role in NEPC, its function in chromatin remodelling and interaction with other epigenetic regulators during NEPC development are still largely unknown.
SWItch/Sucrose Non-Fermentable complex.
The SWItch/Sucrose Non-Fermentable (SWI/SNF) complex is a multi-subunit chromatin-remodelling complex that uses energy from ATP hydrolysis to alter the composition of nucleosomes78. The complex regulates gene expression by enabling DNA binding proteins and the transcriptional machinery to access DNA79. Exome and genome-wide sequencing studies have demonstrated that more than 20% of human cancers bear genomic alterations to at least one SWI/SNF subunit gene80, suggesting a tumour suppressor function. However, increasing evidence from various cell lines and animal models supports a tumour-promoting function of SWI/SNF in some types of tumours, such as synovial sarcoma, leukaemia and neuroblastoma81-84.
The deregulation of SWI/SNF subunits has been demonstrated in NEPC by using large patient datasets, patient-derived organoids and cancer cell lines85. SMARCA4 (also known as BRG1), a SWI/SNF subunit, was significantly upregulated in clinical NEPC samples compared with adenocarcinoma CRPC at both the mRNA and protein levels (P = 0.015)85. In a cohort of 203 patients with localized ADT treatment-naive prostate cancer, high SMARCA4 protein expression was associated with a significantly shorter overall survival (hazard ratio (HR) = 2.17, 95% CI 1.07–4.42, P=0.028) than in patients with low SMARCA4 expression. Furthermore, knockdown of SMARCA4 reduced proliferation of LNCaP cells and the LNCaP-derived androgen-independent cell line C4-2 (REF.85). These data suggest that SMARCA4 overexpression is associated with aggressive prostate cancer. In addition, immunoprecipitation followed by mass spectrometry in human NEPC H660 and adenocarcinoma LNCaP-AR cell lines showed that the SWI/SNF complex subunit SMARCC1 (BAF155) immunoprecipitated with different lineage-specific factors in NEPC compared with prostate adenocarcinoma85. These data suggest that SWI/SNF interact with distinct partners in NEPC and prostatic adenocarcinoma and have a role in facilitating lineage transition during NEPC development.
Long non-coding RNAs.
In addition to protein-coding genes, some non-coding RNAs have emerged as crucial epigenetic players in multiple facets of human pathophysiology86. Long non-coding RNAs (lncRNAs) are transcripts longer than 200 bp that fold into 3D structures that perform a variety of cellular functions including the regulation of gene expression and translation, and are involved in the initiation and progression of various cancers including prostate cancer86,87.
Aberrant expression of several lncRNAs has been correlated with prostate cancer progression88. Analysis of lncRNA profiles of the LTL331/LTL331R model identified 821 lncRNAs that were expressed in NEPC. Moreover, a 122-lncRNA signature can distinguish patients’ NEPC from prostate adenocarcinoma in a robust manner89. The lncRNA myocardial infarction associated transcript (MIAT) is specifically upregulated in the LTL331R NEPC PDX tumour, and its expression is restricted to the nucleus of NEPC cells90. MIAT expression correlates positively with CBX2 expression and negatively with RB1 expression90.
The lncRNA HOX transcript antisense RNA (HOTAIR) is another important driver of progression from androgen-sensitive prostate cancer to CRPC91. A 2018 study reported that upregulation of HOTAIR induces the expression of neuroendocrine marker tubulin III in LNCaP cells and identified HOTAIR as a new target suppressed by RE1-silencing transcription factor (REST), a master repressor of neuron-specific genes in CWR22Rv1 cells92. However, a comparison between NEPC and CRPC adenocarcinoma samples in PDX models and a clinical dataset (CbioPortal, ‘Trento-Cornell-Broad 2016’) showed that no significant difference in HOTAIR expression was observed, although REST was consistently downregulated in NEPC92. Thus, the function and regulation of HOTAIR needs to be studied further.
The lncRNA p21 is also reported to be highly expressed in NEPC. Enzalutamide treatment could increase the expression of p21 and induce neuroendocrine differentiation at least, in part, via altering AR binding to different androgen response elements, which switches EZH2 function from histone methyltransferase to non-histone93.
Collectively, the genomic, transcriptomic and functional findings support that epigenetic reprogramming drives the emergence and maintenance of NEPC. These findings provide a foundation for targeting epigenetic mechanisms to delay or reverse NEPC development.
Transcription factors
The right combination of transcription factors has been demonstrated to induce cell plasticity and reprogramme terminally differentiated cells into pluripotent stem cells94,95. The process of cell lineage specification is also governed by the interaction of different transcription factors96,97. In NEPC, de-regulation of transcription factors that enhance or repress neuroendocrine lineage phenotype has been reported.
Paternally expressed 10.
Paternally expressed 10 (PEG10) is a placental gene that is normally repressed by AR signalling in prostatic adenocarcinoma98. Upon treatment with ARPI, expression of PEG10 is de-repressed and becomes regulated by the AR and transcription factor E2F–RB1 pathways distinctively at the early and late stages of NEPC development98. PEG10 expression is substantially elevated in biopsy and surgically excised metastatic human NEPC and PDX models compared with adenocarcinoma98.
PEG10 protein promotes cell-cycle progression and also promotes cell invasion via a downstream signalling cascade involving Snail and transforming growth factor-β (TGF-β) in PC-3 cells that lack TP53 expression98. These findings highlight PEG10 as a downstream functional effector of RB1 and/or TP53 loss in NEPC. RB1 and/or TP53 loss is a common feature of NEPC and is not readily targetable; thus, targeting PEG10 and other similar downstream effectors could provide a potential alternative therapeutic strategy for NEPC treatment99. The precise function and mechanisms of PEG10 involvement in neuroendocrine lineage transition remains to be explored.
RE1-silencing transcription factor.
Downregulation of REST is another important molecular feature of NEPC100,101. REST was initially identified as a master repressor of neuron-specific genes in mouse neuronal progenitor and non-neuronal cells102. By recruiting co-repressors (for example, REST corepressor and histone deacetylases) to the RE1 site103, REST suppresses neuronal gene expression and, therefore, prevents neural differentiation of non-neuronal cells. Expression of REST is extremely low in neuronal cells and stem cells, but is otherwise universally expressed in non-neuronal tissues. Notably, REST is highly expressed in prostate adenocarcinoma but is substantially downregulated in NEPC100,104.
Deletion of REST in prostate adenocarcinoma cell lines can induce the expression of neuroendocrine markers100,101. In addition, REST can suppress the expression of cell-cycle-related genes such as AURKA32,101. Treatment with an ARPI induces the upregulation of serine/arginine repetitive matrix protein 4 (SRRM4) (an RNA splicer), which in turn alternatively splices REST into a loss-of-function REST4 isoform lacking a transcriptional repressor domain87,89-91. However, the mechanisms leading to the silenced expression of REST in NEPC have not been identified.
Transcription factor SOX2.
SOX2 is a key embryonic stem cell gene encoding sex-determining region Y-box 2, a transcription factor that has important roles in inducing and maintaining pluripotency as well as in balancing neuronal progenitor cell proliferation and differentiation105. This transcription factor can be involved in prostate cancer progression owing to loss of AR-mediated repression during ADT106. In NEPC, upregulation of SOX2 is observed in mouse models and clinical patient samples compared with adenocarcinoma45,51,107. RB1 and/or TP53 functional loss induces the expression of SOX2 in LNCaP-AR cells, but enzalutamide treatment does not affect the expression of SOX2 (REF.59). SOX2 overexpression or knockdown of RB1 and/or TP53 in LNCaP-AR cells increased the expression of certain basal cell markers (CK5, CK14 and TP63) and neuroendocrine markers (SYP, CHGA and NSE) compared with control LNCaP-AR cells, and increased resistance to enzalutamide59. However, in parental LNCaP cells, expression of SOX2 or other neuroendocrine markers was not induced when RB1 and/or TP53 were knocked down in parental LNCaP cells60, suggesting that different molecular contexts could lead to different results. The specific mechanism of regulation between RB1, TP53 and SOX2 in the context of NEPC is not well defined, although the mechanism in fibroblasts could serve as a reference. In fibroblasts, TP53 regulates the miR-34 family of microRNA precursors, which mediate the degradation of SOX2 mRNA108. RB1 can interact with E2F in fibroblasts and be recruited to the promoter region of SOX2, giving it a repressive mark109. This mechanism suggests a link between RB1 genetic alterations and the activation of SOX2-dependent pathways.
Brain-specific homeobox/POU domain protein 2.
Brain-specific homeobox/POU domain protein 2 (BRN2) is known to promote neuronal differentiation and is encoded by the POU3F2 gene110. BRN2 was identified as a key driver of NEPC development by comparing the transcriptomic profiles of the LNCaP-derived PSA cell lines 42DENZR and 42FENZR with that of the AR-driven 16DCRPC cell line. BRN2 is highly expressed in 42DENZR and 42Fenzr compared with 16DCRPC45. The increased expression of BRN2 was also observed in NEPC PDX LTL331R compared with parental adenocarcinoma PDX LTL331. Clinical NEPC samples also showed upregulated BRN2 (mRNA and protein) expression compared with CRPC adenocarcinoma samples25,49,99. Deletion or stable knockdown of BRN2 in 16DCRPC cells prevented enzalutamide-induced upregulation of NEPC markers, suggesting its role in the regulation of the neuroendocrine phenotype. In addition, gain and loss of function of BRN2 studied in multiple cell lines showed that its function in neuroendocrine transdifferentiation is, at least in part, caused by BRN2-mediated regulation of SOX2 expression. Furthermore, BRN2 expression and reporter activity were directly repressed by the AR in adenocarcinoma cell lines, whereas knockdown of BRN2 induces a marked reduction of NE markers in NEPC cells, which links BRN2 to ARPI treatment-induced NEPC development45. Whether BRN2 is universally upregulated in patients receiving ARPIs, and why only a portion of these patients develop NEPC, is still unclear.
Forkhead box family.
Forkhead box A1 (FOXA1) is the founding member of the FOXA gene family. FOXA1 binds to highly condensed (or closed) chromatin via a domain similar to that of linker histones111,112, and its C-terminal domain renders these closed genomic regions more accessible to other transcription factors such as the oestrogen receptor, the progesterone receptor and the AR113-115. FOXA1 has an important role in the development and differentiation of prostate epithelial cells116 and its expression is downregulated in NEPC. FOXA1 knockdown can induce morphological changes in LNCaP cells, conferring neuroendocrine cell phenotypes such as multiple neurite extensions and expression of the neuroendocrine marker ENO2 (REF.117). However, ~11% of patients with primary or metastatic prostate adenocarcinoma show FOXA1 mutations. Mutations in two hotspots in the forkhead domain, Wing2 and R219, account for more than 50% of all mutations118. R219 mutations block luminal differentiation and activate the neuroendocrine transcriptional programme in mouse prostate organoids, indicating the involvement of FOXA1 in lineage plasticity118. Increased expression of another member of the FOXA gene family, FOXA2, has also been observed in NEPC cell lines, PDXs and clinical small-cell neuroendocrine carcinoma tissues119. The function of FOXA2 in the underlying mechanisms driving NEPC is unknown.
One cut domain family member 2.
One cut domain family member 2 (ONECUT2; encoded by ONECUT2) is a transcription factor involved in the control of cell differentiation in endoderm-derived tissues and in nervous system development120-124. ONECUT2 has been identified as a candidate master regulator of lethal metastatic CRPC and NEPC by bioinformatic analysis of clinical prostate cancer transcriptome datasets125,126. ONECUT2 expression is substantially higher in clinical NEPC samples than in benign prostate, primary adenocarcinoma and adenocarcinoma CRPC (Spearman’s rank correlation coefficient 0.54, P = 7.96e-35)126. As REST represses the promotor of ONECUT2, loss of function of REST in NEPC leads to gradual de-repression of this gene125. ONECUT2 inhibits the expression of FOXA1 and activates PEG10, contributing to the emergence of neuroendocrine phenotypes and the proliferation of NEPC125. Treatment with CSRM617, an inhibitor of ONECUT2, inhibits cell proliferation and expression of PEG10 in 22Rv1 xenograft tumours (derived from a prostate cancer cell line with moderate expression of ONECUT2)125. However, the effect of CSRM617 on NEPC has not yet been evaluated.
ONECUT2 can also specifically activate mothers against decapentaplegic homologue 3 (SMAD3), which regulates hypoxia signalling and hypoxia-inducible factor 1α chromatin binding126. Hypoxia correlates with prostate cancer progression and induction of neuroendocrine differentiation127,128; thus, targeting ONECUT2-dependent tumour hypoxia signalling might be an effective therapeutic strategy. Consistent with this hypothesis, a hypoxia-activated prodrug, TH-302, inhibited the tumour growth of PC-3 xenograft and NEPC PDX models126. Preclinical studies of CSRM617 and TH-302 in a wider range of NEPC and pan-neuroendocrine cancer settings could potentially set the precedent for early-phase clinical trials of these agents.
Other regulators
In addition to the genomic alterations, epigenetic regulators and transcription factors, some genes directly or indirectly involved in cell lineage plasticity, tumour proliferation and invasion have also been reported in NEPC.
Serine/arginine repetitive matrix protein 4.
Serine/arginine repetitive matrix protein 4 (encoded by SRRM4) is an RNA splicing factor involved in alternative splicing events129. This protein regulates neuronal cell differentiation by promoting neural-specific gene exon inclusion129. SRRM4 is highly upregulated in 50% of NEPC clinical samples but in only 3% of prostatic adenocarcinoma23,100,104,130. SRRM4 has been suggested as a driver of NEPC progression131.
Combined with ARPI treatment, overexpression of SRRM4 alone induces the expression of neuroendocrine markers in a range of cell lines, including prostate adenocarcinoma, prostate stromal and uterine smooth muscle. Loss of RB1 and/or TP53 function can exacerbate this effect in LNCaP cells131. The mechanism of SMMR4-induced neuroendocrine phenotype partially involves an increase in SRRM4-targeted splice variants, especially the generation of inactive REST4 isoform of a mater neural repressor REST and de-repression of downstream neuroendocrine marker genes131. Similarly, chromatin modification-related protein MYST/Esa1-associated factor 6-1 (MEAF6-1) is a splice variant of chromatin modification-related protein MEAF6 and is also highly expressed in NEPC as a result of increased SRRM4 activity132. Instead of driving neuroendocrine transdifferentiation, MEAF6-1 upregulation stimulates the proliferation and invasion abilities of LNCaP and PC-3 cells132.
Delta-like protein 3.
Delta-like protein 3 (DLL3) is an inhibitory ligand of the Notch signalling pathway that is primarily localized in the Golgi apparatus of neuronal cells133. Increased expression of DLL3 was shown to be more common in NEPC (36 of 47 clinical samples (76.6%)) than in CRPC adenocarcinomas (7 of 56 clinical samples (12.5%)), localized prostate cancer (1 of 194 clinical samples (0.5%)), or benign prostate disorders (0 of 103 clinical samples). Samples with RB1 genomic loss showed substantially higher expression of DLL3 compared with the samples without RB1 loss134. Similarly, increased expression of DLL3 has been reported in SCLC samples135, and RB1 loss in SCLC correlates with DLL3 positivity as well136. Moreover, expression of DLL3 correlated with neuroendocrine marker expression (SYP: r = 0.5316, P = 0.0208; CHGA: r = 0.5667, P = 0.0128). Compared with DLL3-negative cases, the expression of DLL3 was also associated with worse overall survival, calculated from the time of prostate cancer diagnosis (35 months versus 81.5 months, HR 2.22, 95% CI 1.04–4.74, P = 0.015) and survival from the time of metastasis (11.8 months versus 71.6 months, HR, 1.91, 95% CI 0.92–3.96, P = 0.032)134.
Treatment with a single dose of rocalpituzumab tesirine, a DLL3-targeted antibody drug conjugate, resulted in a complete response in DLL3+ NEPC xenografts but had no effect on DLL3− xenografts. Moreover, a patient with DLL3-expressing metastasis who was treated with rocalpituzumab tesirine during a phase I clinical trial exhibited shrinkage of target nodal metastases as observed on CT134, indicating that DLL3 is a promising therapeutic target in NEPC. Further work is needed to identify the major Notch receptor that mediates DLL3 signalling and elucidate its functional role in NEPC development.
CXC-chemokine receptor 2.
CXC-chemokine receptor 2 (CXCR2) is a receptor of IL-8 that is expressed by prostatic neuroendocrine cells and might contribute to keeping these cells in a quiescent state137,138. Immunofluorescence analysis of tissue microarrays showed that CXCR2 is a cell surface marker of neuroendocrine cells in prostate adenocarcinoma and NEPC139. CXCR2+ neuroendocrine cells isolated from adenocarcinoma prostatectomy specimens demonstrate a NEPC gene expression signature that is similar to that reported in NEPC139. In addition, CXCR2 was found to promote the neuroendocrine phenotype and contribute to ARPI resistance when overexpressed in AR+ LNCaP cells139. CXCR2 also stimulated the secretion of proangiogenic factors, thereby promoting angiogenesis and the proliferation and survival of neighbouring adenocarcinoma cells139. However, the function of CXCR2 in fully developed NEPC is still not clear.
Protein kinase C iota type.
Protein kinase C iota type (PKC λ/ι) (encoded by PRKCI) belongs to the PKC family, a group of serine/threonine kinases that encompasses approximately 2% of the human kinome140. PKCλ/ι, together with PKC zeta type, constitute the atypical PKC subgroup141. PRKCI expression is downregulated in human NEPC compared with adenocarcinoma CRPC samples, and correlates negatively with neuroendocrine marker expression142. Loss of PKCλ/ι is sufficient to promote neuroendocrine differentiation in prostate cancer cells, accelerate prostate cancer progression and promote gain of NEPC features, such as loss of nuclear AR and increased CHGA expression in Pten-null mouse prostate epithelium142. PKCλ/ι deficiency also enhances the serine, glycine, one-carbon pathway via the cytosolic arginine sensor for mTORC1 subunit 1–cyclic AMP-dependent transcription factor ATF-4–D-3-phosphoglycerate dehydrogenase (mTORC1–ATF4–PHGDH) axis to fuel DNA methylation, whereas inhibition of DNA methyltransferase (DNMT) activity blocks neuroendocrine differentiation and tumour growth142. The rational design of therapeutic tools based on the loss of PKCλ/ι warrants further study.
Longitudinal analysis of mechanisms
Many genomic, epigenetic and other molecular changes have important roles in NEPC development (BOX 1. However, when these changes emerge and how they contribute temporally to drive treatment-emergent NEPC remains an important but challenging question25. Better understanding of the timing and cooperation between emergent drivers will lead to improved understanding of NEPC development and more timely administration of targeted therapies.
Box 1. Key molecular events observed during neuroendocrine prostate cancer development.
Genetic features
RB1 loss
TP53 loss
PTEN loss
MYCN amplification
Downregulation of transcription factors
RE1-silencing transcription factor
Forkhead box A1
Upregulated transcription factors
Transcription factor SOX2
Paternally expressed 10
Brain-specific homeobox/POU domain protein 2
One cut domain family member 2
Upregulation of epigenetic regulators
Polycomb group proteins (for example, enhancer of zeste homologue 2)
Heterochromatin protein 1α
Protein DEK
Upregulation of other regulators
Delta-like protein 3
Aurora kinase A
Protein kinase C iota type
Serine/arginine repetitive matrix protein 4
The analysis of serial tumour samples from five patients with prostate cancer as their disease progressed from adenocarcinoma to treatment-emergent NEPC constitutes the only reported clinical data derived from matched samples obtained at different time points. However, only genomic changes were reported41. A similar longitudinal study including transcriptomic data is required to provide more insights into the temporal changes during the development of treatment-emergent NEPC.
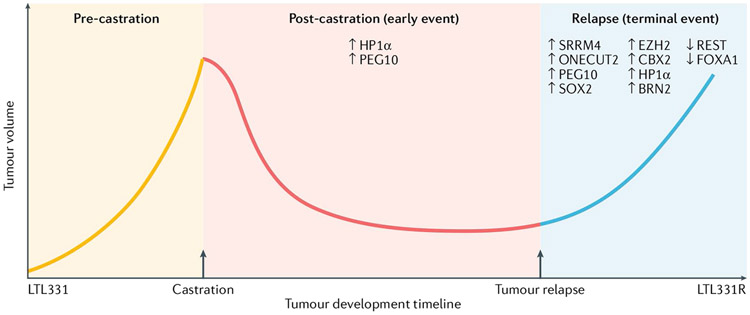
The LTL331/LTL331R model is the only available clinically relevant PDX model for which both genomic and transcriptomic data can be obtained throughout the transdifferentiation process53,98. Longitudinal analyses of the genomic and transcriptomic profiles of the LTL331/LTL331R model provide a unique opportunity to determine the temporal contribution and co-operation of emerging NEPC drivers53,98. Accordingly, we mapped the previously reviewed NEPC molecular features longitudinally based on RNA-seq data from this model98 (FIG. 2. The various molecular changes can be classified as either early (transition-stage) events or late (terminal) events (that is, events that are only observed when NEPC has fully developed). Upregulation of HP1α and PEG10 were observed in the early phases of treatment-emergent NEPC development and also in terminal NEPC55,98. This early upregulation of HP1α and PEG10 might have a key role in maintaining cell survival during ADT and in regulating epigenetic and transcriptional landscape changes, leading to cell lineage plasticity55,98. In future, early prognostic prediction and/or patient risk stratification could be based on these early upregulation events, which could also potentially be therapeutically targeted to delay or even prevent the emergence of NEPC.
Fig. 2 ∣. Timing of the emergence of molecular events in the LTL331/LTL331R patient-derived xenograft model of neuroendocrine prostate cancer53,98.
The LTL331 patient-derived xenograft model is derived from androgen deprivation therapy-naive prostate adenocarcinoma. After castration, the tumour eventually relapses and develops into LTL331R (androgen receptor (AR)−PSA− neuroendocrine marker+ neuroendocrine prostate cancer (NEPC)). The key NEPC-specific molecular events can be categorized as early or terminal events based on when they are detected at the RNA expression level during NEPC development. The RNA expression values of post-castration samples at 8 and 12 weeks are averaged as the ‘early’ time point. An upregulation or downregulation in expression of over twofold between this early time point and the parental LTL331 tumour is considered an important early alteration if the change is also consistently observed in the terminal LTL331R sample. Expression changes that are only observed in the LTL331R sample are considered ‘terminal’ events.
Most other reported molecular alterations were only observed in the terminal LTL331R NEPC PDX tumour in which HP1α and PEG10 are also upregulated. The molecular alterations found in fully developed NEPC could contribute to establishing the neuroendocrine phenotype and tumour aggressiveness at a later stage of NEPC development in this model. Improved understanding of the role of various molecular alterations at different stages of NEPC development will improve the diagnosis of NEPC and lead to the development of novel targeted therapies to delay NEPC development or treat NEPC in the future.
Therapeutic options for NEPC
Despite progress in the understanding of NEPC development, treatment options remain limited. The first-line treatment for both de novo and treatment-induced NEPC is platinum-based chemotherapy, reflecting the strategies employed in treating other neuroendocrine small-cell carcinomas, for example, SCLC. Advances in NEPC research have led to a number of additional potential therapies that are undergoing investigation in clinical trials or are in preclinical development.
Chemotherapy
For patients diagnosed with de novo and treatment-induced NEPC, a platinum-based chemotherapy regimen, such as a combination of cisplatin and etoposide, is recommended143,144. NEPC, especially poorly differentiated small-cell NEPC, shares similar histology, disease progression and treatment response with SCLC and other small-cell carcinomas20,22; thus, strategies for treating NEPC are mainly based on SCLC chemotherapeutic experience with these agents12,21,145. Cisplatin crosslinks with the purine bases on DNA and etoposide targets DNA topoisomerase II activities. Both mechanisms lead to DNA damage and subsequently induce apoptosis in cancer cells146,147. Their adverse effects include renal and cardiovascular toxicity, nausea and vomiting146,148. Considering the highly proliferative nature of NEPC, such combination therapy could provide synergistic effects and delay the development of drug resistance. The objective response rate for patients treated with this regimen is more than 60%, and studies have demonstrated an overall survival of ~10 months145,149 (TABLE 1. For patients with tumours that exhibit some clinical features of NEPC (so-called anaplastic CRPC), a less toxic carboplatin and docetaxel regimen is usually applied, which is efficacious114,116, although the response rate and overall survival varies between studies owing to the heterogeneity of this patient population21,150,151 (TABLE 1).
Table 1 ∣.
Neuroendocrine prostate cancer-related clinical trials using platinum-based chemotherapy
| Regimen | Indication | n | RR | PFS in months (range) |
OS in months (range) |
Ref. |
|---|---|---|---|---|---|---|
| Cisplatin + etoposide +/− doxorubicin | mSCPC | 13 | 69% | NR | 9.4 (1–25) | 128 |
| Cisplatin + etoposide +/− doxorubicin | SCPC (89% metastatic) | 36 | 61% | 5.8 (4.1–6.9) | 10.5 (7.5–14.3) | 127 |
| Cisplatin + docetaxel | CRPCa (51% metastatic) | 41 | 41% | 4 (2–10) | 12 (1–38) | 130 |
| Carboplatin + etoposide | Anaplastic mCRPCb | 60 | 8.9% | 2.9 (1.7–3.5) | 9.6 (8.7–12.7) | 129 |
| First line: carboplatin + docetaxel; second line: cisplatin + etoposide | Anaplastic mCRPCc | 113; 74 | 34%; 30% | 5.1 (4.2–6.0); 3.0 (1.6–3.5) | 16 (13.6–19) | 13 |
| Cabazitaxel alone versus cabazitaxel + carboplatin | mCRPCd | 79; 81 | 59%; 54% | 4.5 (3.5–5.7); 7.3 (5.5–8.2) | 17.3 (13.8–21.9); 18.5(16.7–21.9) | 131 |
CRPC, castration-resistant prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; mSCPC, metastatic small-cell prostate cancer; n, sample size; NR, not reported; OS, overall survival; PFS, progression-free survival; RR, response rate. aPatients with CRPC with serum neuroendocrine markers. b75% of patients had elevated neuroendocrine marker. cIncludes 29 cases of SCPC. dIncludes 6 cases of SCPC.
A regimen of carboplatin plus cabazitaxel was assessed in a phase II clinical trial on 160 patients with metastatic CRPC, including 6 patients with small-cell prostate cancer. This combination therapy improved patients’ median progression-free survival from 4.5 months (95% CI 3.5–5.7) to 7.3 months (95% CI 5.5–8.2, HR 0.69, 95% CI 0.50–0.95, P = 0.018) over cabazitaxel alone (TABLE 1. Unfortunately, conclusions could not be drawn as to whether the combined treatment demonstrated survival benefits for patients with small-cell prostate cancer, owing to the limited number of cases in this study152.
In general, the initial response of NEPC to chemotherapy is considerable. However, its limitations are obvious: high and short response duration owing to acquired drug resistance21. Thus, novel potent therapeutic strategies for treating NEPC are urgently needed.
Novel therapeutics
Multiple genomic alterations and epigenetic or transcriptional regulators contribute to NEPC development. Based on this emerging knowledge, novel treatment strategies targeting the drivers of NEPC are being developed. Alisertib, an Aurora kinase A inhibitor that inhibits the interaction between N-myc and Aurora kinase A, has been tested in a phase II clinical trial. In this trial, 60 patients with metastatic prostate cancer and at least one of the key eligibility criteria (small-cell neuroendocrine morphology, ≥50% neuroendocrine marker expression, new liver metastases without PSA progression and elevated serum neuroendocrine markers) were enrolled and treated with alisertib. Radiographic progression-free survival at 6 months (the primary end point) was 13.4% in all patients. Although the study failed to meet its primary end point (6-month radiographic progression-free survival <15%), exceptional responders were identified with molecular alterations potentially contributing to response. AURKA amplification was associated with improved overall survival (P = 0.05) but no difference in progression-free survival (P = 0.4) on alisertib. Molecular features suggesting N-myc overactivity were observed in responders, although MYCN amplification was not associated with outcomes. These data suggest that a subset of patients with NEPC who have molecular characteristics suggestive of N-myc and/or Aurora kinase A activation will benefit from alisertib treatment153.
Owing to the strong resemblance between NEPC and SCLC154, agents that are effective in treating SCLC are also being evaluated for NEPC. SCLCs harbour a high tumour mutation burden, which suggests potential targets for immunotherapy155. First-line combination of atezolizumab, an immune-checkpoint PDL1 inhibitor, with chemotherapy, led to longer overall survival and progression-free survival than chemotherapy alone in patients with SCLC, and has been approved by the FDA156. In prostate cancer, immunohistochemical staining and analysis of RNA-seq data of clinical samples show a higher PDL1 expression in NEPC than CRPC samples, indicating that patients with NEPC might have a better response to immunotherapy than patients with adenocarcinoma CRPC157,158. The efficacy of the PDL1 inhibitor avelumab in NEPC has been evaluated in a phase II clinical trial. In this single-arm trial, 15 patients with NEPC or AVPC received avelumab treatment. Median radiographic progression-free survival was 1.8 months (95% CI 1.6–2.0 months), and median time on therapy was 56 days (range 28–356 days). Median overall survival was 7.4 months (95% CI 2.8–12.5 months). Two grade 3 adverse events (abdominal pain due to hepatic disease progression versus immune hepatitis and pericarditis) and one grade 4 (immune hepatitis) adverse event were attributed to avelumab, with no grade 5 adverse events159. Interestingly, one patient with a complete response was found to carry a high tumour mutational burden owing to MSH2 alteration, suggesting that patients with a high mutational burden will derive substantial benefit from such immunotherapy.
The efficacy of rocalpituzumab tesirine, an agent targeting DLL3, has been evaluated in patients with refractory or advanced SCLC. However, in a single-arm phase II clinical trial, the drug only showed a 12.4% and a 14.3% objective response rate in all (339 patients) and DLL3-high (238 patients) patients, respectively. Median overall survival was 5.6 months in all patients and 5.7 months in DLL3-high patients, suggesting modest clinical activity of this drug in SCLC160. A phase III trial evaluated the efficacy of rocalpituzumab tesirine and topotecan, a topoisomerase-I inhibitor, in SCLC and showed poorer overall survival of the rocalpituzumab tesirine-treated group (6.3 months) compared with the topotecan-treated group (8.6 months)161. Another phase I clinical trial that investigated the efficacy of rocalpituzumab tesirine in DLL3-expressing neuroendocrine tumours, including NEPC, also failed to meet the primary end point owing to a low response rate162.
Despite the failure of rocalpituzumab tesirine, DLL3 is still regarded as a promising therapeutic target and novel DLL3-targeting antibody-drug conjugates are being developed. AMG 757, an IgG-like T cell-engaging bispecific antibody that redirects T cells to lyse DLL3-expressing cells, and rova-IR700, an anti-DLL3 antibody conjugated to an IR700 photosensitizer, have shown high potency and sensitivity in preclinical models of SCLC163-165. The safety and tolerability of AMG 757 will be evaluated in NEPC in a phase I trial as well (NCT04702737).
Other known genetic and epigenetic drivers of NEPC could also be promising therapeutic targets. For example, inhibitors of EZH2, one of the most strongly upregulated epigenetic regulators, have demonstrated marked anti-tumour effects in NEPC cells in preclinical studies, but have not yet been tested in the clinical environment61.
Future perspectives
The understanding of NEPC has considerably improved over the past decade, and various molecular alterations involved in NEPC development have been identified. However, how neuroendocrine transdifferentiation occurs and how emerging drivers temporally contribute and cooperate during the development of treatment-emergent NEPC is still unclear. The ability to delineate the contributions of molecular changes to the NEPC developmental process is restricted by the extremely limited availability of time-course profiling data describing adenocarcinoma-to-NEPC transdifferentiation, owing to a lack of transcriptional data generated longitudinally from serial samples derived from patients with treatment-emergent NEPC. Serial sampling of tumours in individual patients by needle biopsies, and the collection of circulating tumour cells from pre-treatment, post-ARPI, and follow-up therapy stages, combined with DNA and RNA sequencing, will help to determine how NEPC develops.
The availability of patient-derived preclinical models that recapitulate the entire trans-differentiation process of treatment-emergent NEPC development is also very limited. Other NEPC PDX models and patient-derived organoids are available for NEPC research166,167. However, to our knowledge, the LTL331/LTL331R model is the only clinically relevant PDX model that spontaneously develops from adenocarcinoma to NEPC after ADT, accurately mimicking the clinical progression of the donor patient53. This model enables longitudinal analysis of the development of treatment-emergent NEPC from adenocarcinoma and provides a valuable tool for tracing temporal molecular changes during this process. However, the difficulty of gene manipulation in PDX models in vivo makes them challenging to use in gene function and mechanism studies directly. These limitations can be at least partially overcome by developments in conditionally reprogrammed cells and organoid culture methodologies in vitro. For example, gene manipulation can be performed on these conditionally reprogrammed cells and organoids in vitro, and cells or organoids can be grafted back to animals to re-establish xenografts for functional evaluation166,168,169. Comprehensive and systematic analysis of the genomic, epigenetic, transcriptomic and proteomic changes in this model during the transdifferentiation process will be extremely important in the future. Furthermore, the establishment of additional PDX and organoid models of human origin that recapitulate treatment-emergent NEPC development after exposure to ARPIs will give a broader representation of the complex molecular landscape and provide very valuable tools for longitudinal studies. The increasing availability of longitudinal data will be helpful to address some unanswered but fundamental questions in the treatment-emergent NEPC field, for example, when and how AR signalling is completely shut down, what early events are the key determinants of the neuroendocrine phenotype during neuroendocrine differentiation and whether these determinants can be used as prognostic markers and/or targets for early intervention.
Loss or alterations of RB1 and/or TP53 are the most commonly observed genomic alterations in NEPC and are thought to contribute to cellular plasticity and promote the development of treatment-emergent NEPC18,41,51,56. However, 35% of tumours deficient in RB1 and/or TP53 can be classified as AR-active adenocarcinomas without a neuroendocrine phenotype60, and most tumours harbouring functional loss of RB1 and TP53 show no neuroendocrine transformation in biopsy samples17. Thus, whether loss of RB1 and/or TP53 in adenocarcinoma is necessary and sufficient for treatment-emergent NEPC development, and how these alterations coordinate with ARPI treatments and other emerging genomic alterations, still needs to be explored. Comparative longitudinal analysis of prostate adenocarcinoma models or patient tumours with RB1 and/or TP53 loss but different post-ARPI treatment disease progression will provide more insights into what additional factors are involved.
Alterations in some transcription factors and epigenetic regulators are reportedly involved in treatment-emergent NEPC development, although the precise transcriptional regulation of cell lineage changes during this process is largely unknown. Considering the complexity of epigenetic regulation and its important role in facilitating cellular plasticity, further comprehensive analyses need to be performed to demonstrate the epigenetic landscape change during the development of NEPC. Longitudinal analyses will help to distinguish the key epigenetic changes that occur early after the initiation of ARPI treatment from those that are mainly observed in terminal NEPC, and integrated analysis with transcriptomic and proteomic data will provide a clearer picture of the regulatory machinery during development of treatment-emergent NEPC.
Neuroendocrine transdifferentiation is regarded as a crucial mechanism of therapeutic resistance in prostate cancer, but the presence of DNPC lacking both AR and neuroendocrine markers in patients with ARPI-resistant CRPC suggests that multiple cell lineage plasticity options are possible13. Whether DNPC and treatment-emergent NEPC share similar driving mechanisms and treatment vulnerabilities is unclear; thus, further studies are needed to identify the key regulators in determining different post-treatment cell-fate changes. Longitudinal and comparative multi-omic analyses of treatment-emergent NEPC and DNPC could further clarify the cellular behaviour during early transition periods (for example, how and when AR is silenced during disease progression, and whether DNPC is an indispensable intermediate stage of NEPC transdifferentiation). These studies will lead to the identification of shared and differential mechanisms between DNPC and treatment-emergent NEPC, and demonstrate whether targeting these key drivers could, to some degree, reverse lineage plasticity and resensitize prostate cancers to ARPI. Such information will provide important clinical criteria for therapy selection.
Neuroendocrine transdifferentiation is not restricted to prostate cancer. Other malignancies also demonstrate lineage plasticity and acquire resistance to targeted therapies. For example, lung adenocarcinoma can acquire resistance to epidermal growth factor receptor-targeted therapies via SCLC development170. New findings in NEPC might, therefore, provide insights and therapeutic options for other treatment-emergent small-cell neuroendocrine tumours. In addition to the neuroendocrine phenotype, such tumours have pathological features that differ from those of their parental adenocarcinoma, such as more aggressive tumour growth and preferential metastatic sites (that is, visceral metastases)170. Little is known about the biological advantages that neuroendocrine phenotypes contribute to disease aggressiveness. Although some factors that promote tumour metastasis, (for example, EZH2) are reportedly involved in treatment-emergent NEPC development23, this link cannot completely explain why visceral metastases are common in patients with treatment-emergent NEPC. The contributions of the tumour microenvironment and cancer–stromal interactions to this development and progression are rarely addressed. Longitudinal analysis of treatment-emergent NEPC development by single-cell sequencing can illustrate the transcriptomic changes in different cell types and improve our understanding of their interaction and cooperation.
Increasing evidence suggests that prostate cancer can progress via various potential routes. More attention should be paid to patients with advanced metastatic CRPC, especially those who receive long-term AR pathway inhibition and/or develop aggressive variants of prostate cancer as these patients have an increased likelihood of developing AR CRPC including NEPC13,17,18. Because of the differences in clinical features and treatment strategy between NEPC and adenocarcinoma CRPC, the National Comprehensive Cancer Network guidelines were updated to include consideration of metastatic biopsy in any patient with CRPC to look for NEPC transformation171. In view of this, longitudinal monitoring of disease development and early detection and/or diagnosis of NEPC will be important for guiding patient counselling and clinical treatment decisions. Repeat metastatic or liquid biopsies will be helpful for monitoring cell plasticity and lineage change as histological and molecular examinations of serially collected clinical samples can provide insights into disease progression and guide timely modifications of treatment strategies.
Finally, cytotoxic chemotherapy is the only treatment currently available for NEPC21. However, its benefits are short-lived. Patients with metastatic prostate cancer (including NEPC) who have mutations in DNA damage repair (DDR)-related genes such as BRCA2 have higher response rates to carboplatin-based chemotherapy172; thus, the genetic status of crucial DDR genes could serve as good markers for precision medicine in NEPC. More clinical studies will demonstrate which subsets of patients with NEPC and DDR gene defects will benefit from various treatments, such as PARP inhibitors or immunotherapies. Further studies on the mechanisms underlying treatment response driven by different DDR defects can also offer insights for optimizing therapeutic strategies and improving disease management in subsets of patients with NEPC. In addition, a number of genes have been identified to have a role in driving NEPC development. The inhibition of these candidates has shown NEPC tumour growth suppression in in vivo models. For example, inhibition of HP1α, PEG10, SRRM4, SOX2 and BRN2 can suppress neuroendocrine transdifferentiation and proliferation45,55,59,87,98,102. The success of the development of inhibitors targeting these candidates will provide more potential options for NEPC treatment. The identification of early, upstream drivers of treatment-emergent NEPC will also establish a foundation for early intervention and/or prevention of the disease and provide novel therapeutic targets for improved disease management.
Conclusions
The incidence of treatment-emergent NEPC has greatly increased over the past decade owing to the development of more potent ARPIs for the treatment of prostate cancer. Lineage plasticity is regarded as an important mechanism of treatment-emergent NEPC development. Concomitant inactivation of TP53 and RB1, and alterations of epigenetic regulators, transcription factors and various cellular molecules have been identified as molecular drivers of this phenomenon. Longitudinal analyses of these molecular alterations can divide them into early and terminal events, suggesting different roles during different phases of adenocarcinoma-to-NEPC transdifferentiation. In the future, more systematic multi-omic analyses of clinical or preclinical time-series samples during NEPC development will lead to the identification of early events and upstream driver signals. This analysis of events will provide essential insights for elucidating their temporal contributions to the process. We hope that this knowledge will translate into more effective and precise therapeutic strategies for the prevention and management of this currently incurable disease.
Key points.
Neuroendocrine prostate cancer (NEPC) is an aggressive variant form that is characterized by low or absent androgen receptor (AR) expression, gain of the neuroendocrine phenotype and is not responsive to therapies targeting AR signalling.
De novo NEPC accounts for less than 2% of all prostate cancers, but treatment-induced NEPC occurs in 10–17% of patients with castration-resistant prostate cancer by evolving from adenocarcinoma, probably as a result of a transdifferentiation process.
Molecular mechanisms underlying NEPC development include genomic alterations, abnormal regulation of epigenetic regulators, transcription factors and other molecular pathways. The temporal contribution and co-operation of NEPC drivers during adenocarcinoma to NEPC transdifferentiation is largely unknown; thus, longitudinal study of serial patient samples and preclinical models that recapitulate the entire disease progression is warranted.
Longitudinal analyses of the only clinically relevant patient-derived xenograft model with serial genomic and transcriptomic data available throughout the adenocarcinoma-to-NEPC transdifferentiation process (LTL331/331 R) could group NEPC-driving molecular alterations into early and terminal events, suggesting their roles during different phases of NEPC development.
Platinum-based chemotherapy is the only treatment currently available for NEPC. Advances in NEPC research have led to new potential therapies that are undergoing investigation in clinical trials or in preclinical development.
Acknowledgements
The authors thank all members of the Living Tumor Laboratory (www.livingtumorlab.com) for helpful discussions. This research was supported in part by the Canadian Institutes of Health Research (#141635, #144159, #153081, #173338, Yuzhuo W.), Terry Fox Research Institute (#1062, Yuzhuo W.), Mitacs Accelerate Program (#IT10125, #IT06414, #IT12387, IT14958, Yuzhuo W., NCI grant (P50CA097186) pilot project award (Yuzhuo W.), Prostate Cancer Foundation BC (X.C.), and China Scholar Council award (Yu W.).
Glossary
- Lineage plasticity
The ability of a cell to convert from one cell type to another, which could refer to the potential of a fully differentiated cell to de-differentiate and then re-differentiate into a different cell lineage status
- Simian virus 40 T antigens
(SV40 T-Ag). Dominant-acting oncoproteins encoded by the polyomavirus SV40, which are capable of inducing malignant transformation of a variety of cell types
- Allelic imbalance
A phenomenon in which the two alleles of a given gene are expressed at different levels in a given cell
- Genomic profiles
Identification of genomic alterations in a particular cell or tissue type
- Divergent clonal evolution
A tumour evolution model, in which different tumour clones share some genetic alterations inherited from a common ancestor cell, but also display unique alterations that are acquired during early evolutionary divergence
- Probasin promoter
The promoter of rat probasin, an androgen-regulated protein, which commonly uses prostate-specific promoters to drive targeting expression of genes of interest to the prostate epithelium
- Transcriptomic profiles
Identification and quantification of RNA transcripts expressed in a particular cell or tissue type
- AR-indifferent status
Sustained growth of prostate tumour cells that is not driven by, or dependent on, canonical AR signalling
- Notch signalling pathway
A highly conserved signalling pathway that occurs through direct interaction between Notch, the receptor, and the Jagged or Delta family of ligands to trigger proteolytic cleavage to release Notch intracellular fragment that functions to regulate transcription
- Human kinome
The complete set of protein kinases expressed in human cells
RELATED LINKS
Living tumor lab: https://www.livingtumorlab.com
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Sung H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Humphrey PA Histological variants of prostatic carcinoma and their significance. Histopathology 60, 59–74 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Huggins C & Hodges CV Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin 22, 232–240 (1972). [DOI] [PubMed] [Google Scholar]
- 4.Gillessen S. et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann. Oncol 26, 1589–604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson PA, Arora VK & Sawyers CL Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CD et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med 10, 33–39 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Beer TM et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med 371, 424–433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan CJ et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med 368, 138–148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fizazi K. et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N. Engl. J. Med 383, 1040–1049 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Beltran H. et al. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res 20, 2846–2850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluemn EG et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 32, 474–489.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HT et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis — a systematic review and pooled analysis. J. Clin. Oncol 32, 3383–3390 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Zaffuto E. et al. Contemporary incidence and cancer control outcomes of primary neuroendocrine prostate cancer: a SEER database analysis. Clin. Genitourin. Cancer 15, e793–e800 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Vlachostergios PJ, Puca L & Beltran H Emerging variants of castration-resistant prostate cancer. Curr. Oncol. Rep 19, 32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal R. et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J. Clin. Oncol 36, 2492–2503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abida W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chedgy EC et al. Biallelic tumour suppressor loss and DNA repair defects in de novo small-cell prostate carcinoma. J. Pathol 246, 244–253 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Epstein JI et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol 38, 756–767 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aparicio AM et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res 19, 3621–3630 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W & Epstein JI Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am. J. Surg. Pathol 32, 65–71 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Beltran H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 1, 487–495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conteduca V. et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 121, 7–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltran H. et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res 25, 6916–6924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler W & Huang J Neuroendocrine cells of the prostate: histology, biological functions, and molecular mechanisms. Precis. Clin. Med 4, 25–34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YH, Zhang YQ & Huang JT Neuroendocrine cells of prostate cancer: biologic functions and molecular mechanisms. Asian J. Androl 21, 291–295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelivianski S. et al. Multipathways for transdifferentiation of human prostate cancer cells into neuroendocrine-like phenotype. Biochim. Biophys. Acta 1539, 28–43 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate Suppl. 8, 18–22 (1998). [PubMed] [Google Scholar]
- 30.Bonkhoff H, Stein U & Remberger K Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma are postmitotic cells. Hum. Pathol 26, 167–170 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Garabedian EM, Humphrey PA & Gordon JI A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc. Natl Acad. Sci. USA 95, 15382–15387 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry S & Beltran H The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol 4, 60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B. et al. Characterization of ETS gene aberrations in select histologic variants of prostate carcinoma. Mod. Pathol 22, 1176–1185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotan TL et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod. Pathol 24, 820–828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson SR et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod. Pathol 24, 1120–1127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demichelis F. et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26, 4596–4599 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Rajput AB et al. Frequency of the TMPRSS2:ERG gene fusion is increased in moderate to poorly differentiated prostate cancers. J. Clin. Pathol 60, 1238–1243 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan HL et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res 20, 890–903 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansel DE et al. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate 69, 603–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauer CG, Roemer A & Grobholz R Genetic analysis of neuroendocrine tumor cells in prostatic carcinoma. Prostate 66, 227–234 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Beltran H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med 22, 298–305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong B. et al. Single-cell analysis supports a luminal-neuroendocrine transdifferentiation in human prostate cancer. Commun. Biol 3, 778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horoszewicz JS et al. LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 (1983). [PubMed] [Google Scholar]
- 44.Burchardt T. et al. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J. Urol 162, 1800–1805 (1999). [PubMed] [Google Scholar]
- 45.Bishop JL et al. The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 7, 54–71 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Greenberg NM et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol. Endocrinol 8, 230–239 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Gingrich JR et al. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 57, 4687–4691 (1997). [PubMed] [Google Scholar]
- 48.Greenberg NM et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA 92, 3439–3443 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan-Lefko PJ et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 55, 219–237 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Chiaverotti T. et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am. J. Pathol 172, 236–246 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ku SY et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou M. et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 7, 736–749 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin D. et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 74, 1272–1283 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Lee JK et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell 29, 536–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ci X. et al. Heterochromatin protein 1alpha mediates development and aggressiveness of neuroendocrine prostate cancer. Cancer Res. 78, 2691–2704 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Aparicio AM et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin. Cancer Res 22, 1520–1530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z. et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 66, 7889–7898 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Tran C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mu P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nyquist MD et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 31, 107669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dardenne E. et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30, 563–577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan M. et al. Aurora-a kinase: a potent oncogene and target for cancer therapy. Med. Res. Rev 36, 1036–1079 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Otto T. et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15, 67–78 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Brockmann M. et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell 24, 75–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet 3, 662–673 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Richly H, Aloia L & Di Croce L Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis. 2, e204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sauvageau M & Sauvageau G Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 7, 299–313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Croce L & Helin K Transcriptional regulation by Polycomb group proteins. Nat. Struct. Mol. Biol 20, 1147–1155 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Clermont PL et al. Polycomb-mediated silencing in neuroendocrine prostate cancer. Clin. Epigenetics 7, 40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim KH & Roberts CW Targeting EZH2 in cancer. Nat. Med 22, 128–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleb B. et al. Differentially methylated genes and androgen receptor re-expression in small cell prostate carcinomas. Epigenetics 11, 184–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nielsen AL et al. Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7, 729–739 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Nielsen AL et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18, 6385–6395 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kraemer D, Wozniak RW, Blobel G & Radu A The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc. Natl Acad. Sci. USA 91, 1519–1523 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cleary J. et al. p300/CBP-associated factor drives DEK into interchromatin granule clusters. J. Biol. Chem 280, 31760–31767 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R & Grosveld G Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci 115, 3319–3330 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Lin D. et al. Identification of DEK as a potential therapeutic target for neuroendocrine prostate cancer. Oncotarget 6, 1806–1820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mittal P. & Roberts CWM The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat. Rev. Clin. Oncol 17, 435–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts CW & Orkin SH The SWI/SNF complex– chromatin and cancer. Nat. Rev. Cancer 4, 133–142 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Kadoch C & Crabtree GR Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci. Adv 1, e1500447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kadoch C & Crabtree GR Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell 153, 71–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buscarlet M. et al. Essential role of BRG, the ATPase subunit of BAF chromatin remodeling complexes, in leukemia maintenance. Blood 123, 1720–1728 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jubierre L. et al. BRG1/SMARCA4 is essential for neuroblastoma cell viability through modulation of cell death and survival pathways. Oncogene 35, 5179–5190 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Laurette P. et al. Chromatin remodellers Brg1 and Bptf are required for normal gene expression and progression of oncogenic Braf-driven mouse melanoma. Cell Death Differ. 27, 29–43 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cyrta J. et al. Role of specialized composition of SWI/SNF complexes in prostate cancer lineage plasticity. Nat. Commun 11, 5549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wapinski O & Chang HY Long noncoding RNAs and human disease. Trends Cell Biol. 21, 354–361 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Statello L, Guo CJ, Chen LL & Huarte M Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol 22, 96–118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh AL, Tuzova AV, Bolton EM, Lynch TH & Perry AS Long noncoding RNAs and prostate carcinogenesis: the missing ‘linc’? Trends Mol. Med 20, 428–436 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Ramnarine VR et al. The long noncoding RNA landscape of neuroendocrine prostate cancer and its clinical implications. Gigascience 7, giy050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crea F. et al. The role of epigenetics and long noncoding RNA MIAT in neuroendocrine prostate cancer. Epigenomics 8, 721–731 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Zhang A. et al. LncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep. 13, 209–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mather RL, Wang Y & Crea F Is HOTAIR really involved in neuroendocrine prostate cancer differentiation? Epigenomics 10, 1259–1261 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Luo J. et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun 10, 2571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Orkin SH Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. Genet 1, 57–64 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Panman L. et al. Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell 8, 663–675 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Akamatsu S. et al. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. 12, 922–936 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Akamatsu S, Inoue T, Ogawa O & Gleave ME Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int. J. Urol 25, 345–351 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Lapuk AV et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J. Pathol 227, 286–297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Svensson C. et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 42, 999–1015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schoenherr CJ & Anderson DJ The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267, 1360–1363 (1995). [DOI] [PubMed] [Google Scholar]
- 103.Ballas N, Grunseich C, Lu DD, Speh JC & Mandel G REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Zhang X. et al. SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin. Cancer Res 21, 4698–4708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarkar A & Hochedlinger K The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12, 15–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kregel S. et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS ONE 8, e53701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russo MV et al. SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget 7, 12372–12385 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi YJ et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol 13, 1353–1360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kareta MS et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell 16, 39–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dominguez MH, Ayoub AE & Rakic P POU-III transcription factors (Brn1, Brn2, and Oct6) influence neurogenesis, molecular identity, and migratory destination of upper-layer cells of the cerebral cortex. Cereb Cortex 23, 2632–2643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cirillo LA et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002). [DOI] [PubMed] [Google Scholar]
- 112.Iwafuchi-Doi M. et al. The pioneer transcription factor foxa maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol. Cell 62, 79–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carroll JS et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet 38, 1289–1297 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Clarke CL & Graham JD Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS ONE 7, e35859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao N. et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol. Endocrinol 17, 1484–1507 (2003). [DOI] [PubMed] [Google Scholar]
- 116.Mirosevich J, Gao N & Matusik RJ Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate 62, 339–352 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Kim J. et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adams EJ et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park JW, Lee JK, Witte ON & Huang J FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Mod. Pathol 30, 1262–1272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roy A. et al. Onecut transcription factors act upstream of Isl1 to regulate spinal motoneuron diversification. Development 139, 3109–3119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clotman F. et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 19, 1849–1854 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Margagliotti S. et al. The Onecut transcription factors HNF-6/OC-1 and OC-2 regulate early liver expansion by controlling hepatoblast migration. Dev. Biol 311, 579–589 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Espana A & Clotman F Onecut factors control development of the Locus Coeruleus and of the mesencephalic trigeminal nucleus. Mol. Cell Neurosci 50, 93–102 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Espana A & Clotman F Onecut transcription factors are required for the second phase of development of the A13 dopaminergic nucleus in the mouse. J. Comp. Neurol 520, 1424–1441 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Rotinen M. et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat. Med 24, 1887–1898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo H. et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun 10, 278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Milosevic M. et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin. Cancer Res 18, 2108–2114 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Qi J. et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 18, 23–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calarco JA et al. Regulation of vertebrate nervous system alternative splicing and development by an SR-related protein. Cell 138, 898–910 (2009). [DOI] [PubMed] [Google Scholar]
- 130.Taylor BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y. et al. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur. Urol 71, 68–78 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Lee AR et al. Alternative RNA splicing of the MEAF6 gene facilitates neuroendocrine prostate cancer progression. Oncotarget 8, 27966–27975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chapman G, Sparrow DB, Kremmer E & Dunwoodie SL Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum. Mol. Genet 20, 905–916 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Puca L. et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl. Med 11, eaav0891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saunders LR et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med 7, 302ra136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brcic L. et al. Comparison of four DLL3 antibodies performance in high grade neuroendocrine lung tumor samples and cell cultures. Diagn. Pathol 14, 47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen H. et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocr. Relat. Cancer 19, 321–331 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang J. et al. Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer. Am. J. Pathol 166, 1807–1815 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y. et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci. Transl. Med 11, eaax0428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanks SK & Hunter T Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9, 576–596 (1995). [PubMed] [Google Scholar]
- 141.Moscat J & Diaz-Meco MT The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 1, 399–403 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reina-Campos M. et al. Increased serine and one-carbon pathway metabolism by PKClambda/iota deficiency promotes neuroendocrine prostate cancer. Cancer Cell 35, 385–400 e389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tagawa ST Neuroendocrine prostate cancer after hormonal therapy: knowing is half the battle. J. Clin. Oncol 32, 3360–3364 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Tritschler S, Erdelkamp R, Stief C & Hentrich M Neuroendocrine prostate cancer. Urologe A 56, 1475–1484 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Amato RJ et al. Chemotherapy for small cell carcinoma of prostatic origin. J. Urol 147, 935–937 (1992). [DOI] [PubMed] [Google Scholar]
- 146.Dasari S & Tchounwou PB Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol 740, 364–378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Montecucco A, Zanetta F & Biamonti G Molecular mechanisms of etoposide. EXCLI J. 14, 95–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang HM, Moudgil R, Scarabelli T, Okwuosa TM & Yeh ETH Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J. Am. Coll. Cardiol 70, 2536–2551 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Papandreou CN et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol 20, 3072–3080 (2002). [DOI] [PubMed] [Google Scholar]
- 150.Flechon A. et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann. Oncol 22, 2476–2481 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Culine S. et al. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J. Urol 178, 844–848 discussion 848 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Corn PG et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol. 20, 1432–1443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beltran H. et al. A phase II trial of the aurora kinase a inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin. Cancer Res 25, 43–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rickman DS, Beltran H, Demichelis F & Rubin MA Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med 23, 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Alexandrov LB et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Horn L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med 379, 2220–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Vicier C, Xie W, Hamid A, Evan C & Sweeney C Impact of new systemic therapies on outcomes of patients with non-metastatic castration resistant prostate cancer (nmCRPC). J. Clin. Oncol 37, 244–244 (2019). [Google Scholar]
- 158.Nappi L. et al. Immunogenomic landscape of neuroendocrine small cell prostate cancer. J. Clin. Oncol 37, 217–217 (2019). [Google Scholar]
- 159.Landon Carter Brown SH et al. Efficacy of the PD-L1 inhibitor avelumab in neuroendocrine or aggressive variant prostate cancer: results from a phase II, single-arm study. J. Clin. Oncol 39, 1 (2021).32986527 [Google Scholar]
- 160.Morgensztern D. et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II trinity study. Clin. Cancer Res 25, 6958–6966 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blackhall F. et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J. Thorac. Oncol (2021). [DOI] [PubMed] [Google Scholar]
- 162.Mansfield AS et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3-expressing, advanced solid tumors. J. Clin. Oncol 38, 3552–3552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Isobe Y. et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine 52, 102632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giffin MJ et al. AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small cell lung cancer. Clin. Cancer Res (2020). [DOI] [PubMed] [Google Scholar]
- 165.Hipp S. et al. A bispecific DLL3/CD3 IgG-Like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin. Cancer Res 26, 5258–5268 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Puca L. et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun 9, 2404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Navone NM et al. Movember GAP1 PDX project: an international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models. Prostate 78, 1262–1282 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Ci X. et al. Conditionally reprogrammed cells from patient-derived xenograft to model neuroendocrine prostate cancer development. Cells 9, 1398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gleave AM, Ci X, Lin D & Wang Y A synopsis of prostate organoid methodologies, applications, and limitations. Prostate 80, 518–526 (2020). [DOI] [PubMed] [Google Scholar]
- 170.Sequist LV et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med 3, 75ra26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohler JL et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc Netw 17, 479–505 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Pomerantz MM et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer 123, 3532–3539 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]