Abstract
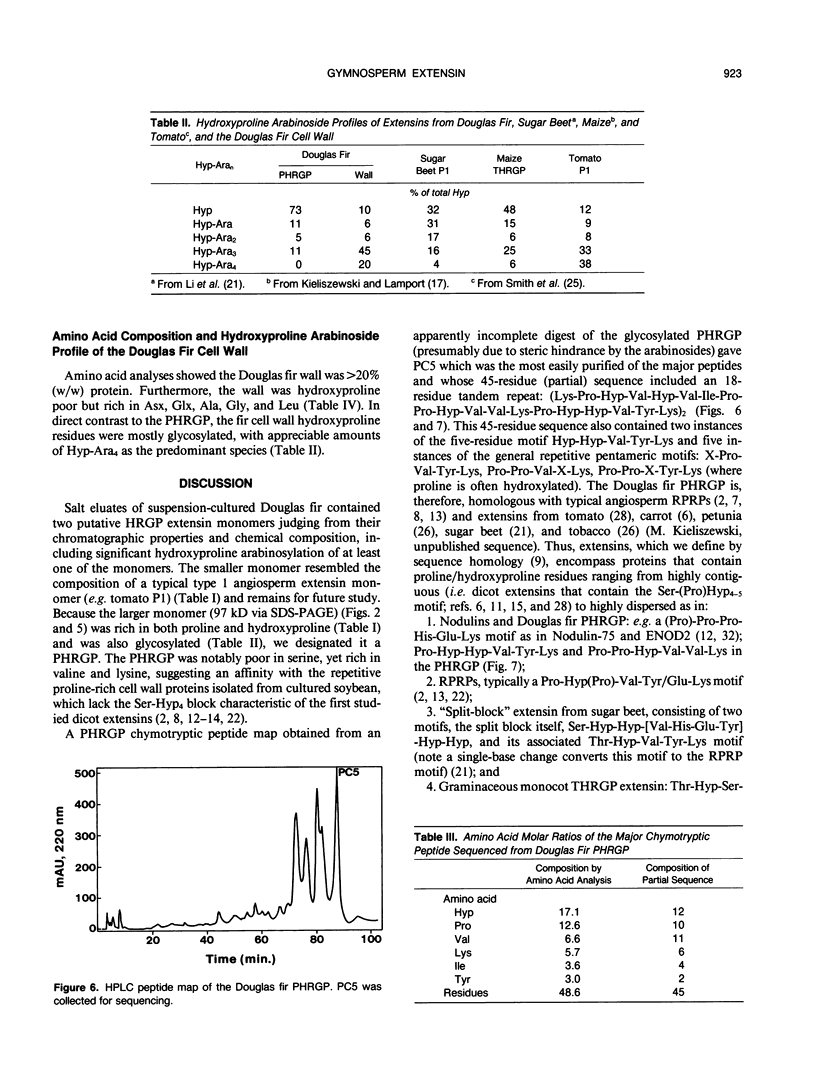
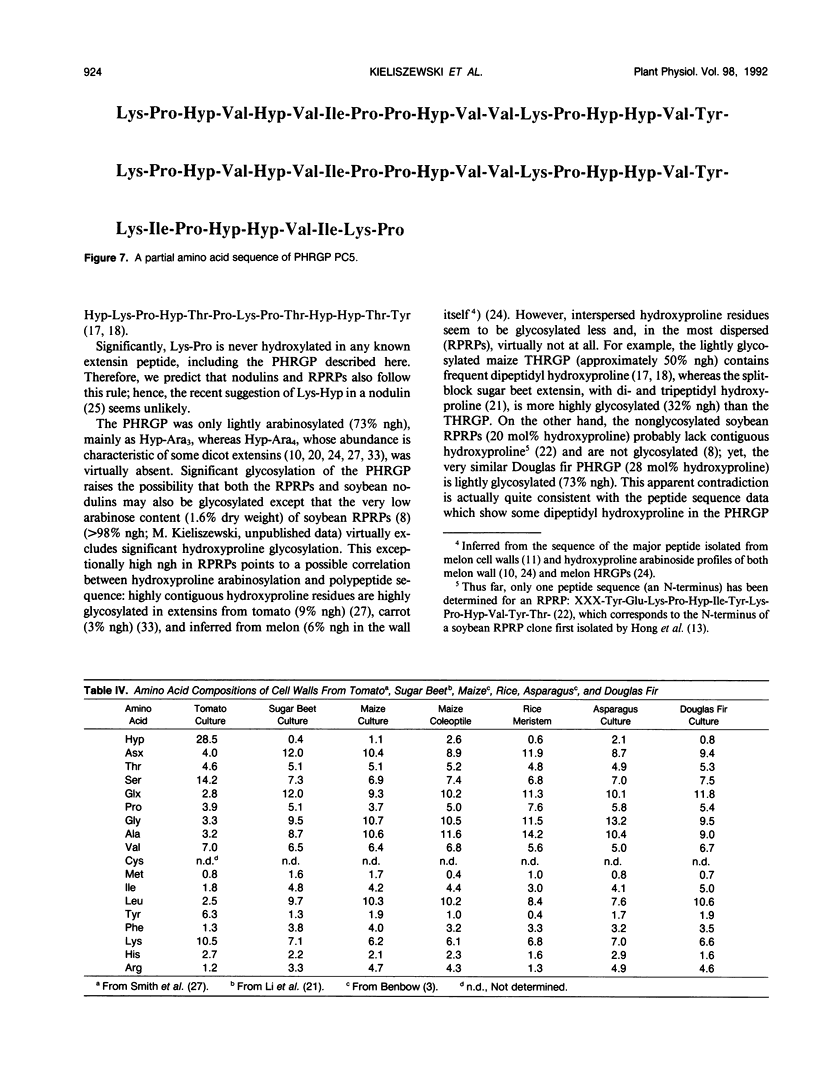
Intact cell elution of suspension cultures derived from Douglas fir, Pseudotsuga menziesii (Mirbel) Franco, yielded two extensin monomers, the first hydroxyproline-rich glycoproteins (HRGPs) to be isolated from a gymnosperm. These HRGPs resolved on Superose-6 gel filtration. The smaller monomer was compositionally similar to angiosperm extensins like tomato P1. The larger monomer had a simple composition reminiscent of repetitive proline-rich proteins (RPRPs) from soybean cell walls and contained proline, hydroxyproline, and sugar; hence designated a proline-hydroxyproline-rich glycoprotein (PHRGP). The simple composition of the PHRGP implied a periodic structure which was confirmed by the simple chymotryptic map and 45-residue partial sequence of the major proline-hydroxyproline-rich glycoprotein chymotryptide 5: Lys-Pro-Hyp-Val-Hyp-Val-Ile-Pro-Pro-Hyp-Val-Val-Lys-Pro-Hyp-Hyp-Val- Tyr-Lys-Pro-Hyp-Val-Hyp-Val-Ile-Pro-Pro-Hyp-Val-Val-Lys-Pro-Hyp-Hyp- Val-Tyr-Lys-Ile-Pro-Pro(Hyp)-Val-Ile-Lys-Pro. Proline-hydroxyproline-rich glycoprotein chymotryptide 5 contained an 18-residue tandem repeat devoid of tetra(hydroxy)-proline or serine; it also contained two instances of the five-residue motif Hyp-Hyp-Val-Tyr-Lys and five of the general Pro-Pro-X-X-Lys motif, thereby establishing its homology with typical angiosperm RPRPs and extensins from tomato, petunia, carrot, tobacco, sugar beet, and Phaseolus. Unlike the nonglycosylated soybean RPRP, the highly purified Douglas fir PHRGP was lightly glycosylated, confirmed by a quantitative hydroxyproline glycoside profile, indicating that extensins can range from highly glycosylated hydroxyproline to little or no glycosylated hydroxyproline. Comparison of extensin sequence data strongly indicates that a major determinant of hydroxyproline glycosylation specificity is hydroxyproline contiguity: extensins with tetrahydroxyproline blocks are very highly arabinosylated (>90% hydroxyproline glycosylated), tri- and dihydroxyproline are less so, and single hydroxyproline residues perhaps not at all. Despite high yields of extensins eluted from intact cells, the Douglas fir cell wall itself was hydroxyproline poor yet remarkably rich in protein (>20%), again emphasizing the existence of other structural cell wall proteins that are neither HRGPs nor glycine-rich proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. Invertebrate collagens. Science. 1978 Nov 10;202(4368):591–598. doi: 10.1126/science.212833. [DOI] [PubMed] [Google Scholar]
- Averyhart-Fullard V., Datta K., Marcus A. A hydroxyproline-rich protein in the soybean cell wall. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1082–1085. doi: 10.1073/pnas.85.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K., Marcus A. Nucleotide sequence of a gene encoding soybean repetitive proline-rich protein 3. Plant Mol Biol. 1990 Feb;14(2):285–286. doi: 10.1007/BF00018570. [DOI] [PubMed] [Google Scholar]
- Datta K., Schmidt A., Marcus A. Characterization of two soybean repetitive proline-rich proteins and a cognate cDNA from germinated axes. Plant Cell. 1989 Sep;1(9):945–952. doi: 10.1105/tpc.1.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H. J., Nap J. P., Gloudemans T., Stiekema W., Van Dam H., Govers F., Louwerse J., Van Kammen A., Bisseling T. Characterization of cDNA for nodulin-75 of soybean: A gene product involved in early stages of root nodule development. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4495–4499. doi: 10.1073/pnas.84.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Characterization and sequence analysis of a developmentally regulated putative cell wall protein gene isolated from soybean. J Biol Chem. 1987 Jun 15;262(17):8367–8376. [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Developmentally regulated expression of soybean proline-rich cell wall protein genes. Plant Cell. 1989 Sep;1(9):937–943. doi: 10.1105/tpc.1.9.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B., Lamb C. J. Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev. 1989 Oct;3(10):1639–1646. doi: 10.1101/gad.3.10.1639. [DOI] [PubMed] [Google Scholar]
- Keller B., Sauer N., Lamb C. J. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988 Dec 1;7(12):3625–3633. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M. J., Leykam J. F., Lamport D. T. Structure of the Threonine-Rich Extensin from Zea mays. Plant Physiol. 1990 Feb;92(2):316–326. doi: 10.1104/pp.92.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M., Lamport D. T. Purification and Partial Characterization of a Hydroxyproline-Rich Glycoprotein in a Graminaceous Monocot, Zea mays. Plant Physiol. 1987 Nov;85(3):823–827. doi: 10.1104/pp.85.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Li X. B., Kieliszewski M., Lamport D. T. A chenopod extensin lacks repetitive tetrahydroxyproline blocks. Plant Physiol. 1990 Feb;92(2):327–333. doi: 10.1104/pp.92.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. T., Vodkin L. O. A soybean cell wall protein is affected by seed color genotype. Plant Cell. 1991 Jun;3(6):561–571. doi: 10.1105/tpc.3.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazau D., Rumeau D., Esquerre-Tugaye M. T. Two different families of hydroxyproline-rich glycoproteins in melon callus: biochemical and immunochemical studies. Plant Physiol. 1988 Feb;86(2):540–546. doi: 10.1104/pp.86.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B., Van De Wiel C., Zalensky A., Horvath B., Spaink H., Van Eck H., Zwartkruis F., Wolters A. M., Gloudemans T., Van Kammen A. The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell. 1990 Jan 26;60(2):281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Woessner J. P., Goodenough U. W. Molecular characterization of a zygote wall protein: an extensin-like molecule in Chlamydomonas reinhardtii. Plant Cell. 1989 Sep;1(9):901–911. doi: 10.1105/tpc.1.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Varner J. E. Reinforced Polyproline II Conformation in a Hydroxyproline-Rich Cell Wall Glycoprotein from Carrot Root. Plant Physiol. 1984 Feb;74(2):247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wiel C., Scheres B., Franssen H., van Lierop M. J., van Lammeren A., van Kammen A., Bisseling T. The early nodulin transcript ENOD2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J. 1990 Jan;9(1):1–7. doi: 10.1002/j.1460-2075.1990.tb08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]