Abstract
In order to access the role of the Porphyromonas gingivalis Arg-gingipain proteases in the virulence of this organism, a mutant defective in the rgpA gene was constructed in strain 381. This mutant, MT10, displayed only 40% of the Arg-specific cysteine protease activity of the wild-type strain. In addition, MT10, as well as the recently characterized protease mutant G-102, which is defective in the rgpB gene, displayed reduced self-aggregation, hemagglutination, and the ability to bind to immobilized type I collagen compared to levels of the wild-type parent. However, unlike mutant G-102, the rgpA mutant displayed increased binding to epithelial cells relative to that of the parental organism. Mutant MT10 also did not express detectable levels of the FimA protein as assessed by both Western and Northern blotting or fimbriae visible by electron microscopy of the cells. Furthermore, the ability of MT10 to degrade rat tail collagen fibers when it was cultured at 37°C was markedly attenuated compared to that of strain 381. These results suggest that Arg-gingipain A may play a significant role in the pathogenicity of P. gingivalis by altering the colonization and toxic properties of the organism.
It is now well documented that gram-negative anaerobic bacteria are associated with human periodontitis (24). Among these microorganisms, Porphyromonas gingivalis appears to be a major factor in this disease. A variety of potential virulence factors have been described for this organism, including proteases, endotoxins, collagenases, fatty acids, and fimbriae (9). The use of a monospecific protease mutant of P. gingivalis in the mouse model system (7) has also provided in vivo evidence of an important role for proteases in virulence. These enzymes may be involved in pathogenicity by directly degrading host tissues, activating host proenzymes, altering the host immune response, and exposing host cell cryptotopes, as well as altering blood clotting (5, 9, 29). In addition, more recent evidence indicating that protease mutations attenuate P. gingivalis colonization of host oral tissue either directly or indirectly has been obtained (27). However, the molecular basis for these alterations has not yet been documented.
Since all bacteria, including P. gingivalis, express a variety of distinct proteases, it is important to identify the specific proteases which may be involved in virulence. It is now clear that these organisms express both arginine- and lysine-specific proteases in addition to other unrelated proteolytic enzymes (21). Several laboratories have characterized genes (rgpA, rgp-1, prtH, prpR1, and cpgR) from different strains of P. gingivalis which appear to code for the major Arg-specific cysteine protease Arg-gingipain (RgpA protease will be used to designate this protease in this communication). In addition, multiple forms of the enzyme can be elaborated by these organisms (22). Moreover, a distinct homologous form of this gene, rgp-2 or rgpB, which lacks sequences coding for the adhesion domain has been identified (15, 21, 22). Likewise, genes for a Lys-specific cysteine protease, Kgp, have also been characterized (16, 20) and appear to be identical to the prtP gene recently proposed to encode the Lys- and Arg-specific enzyme porphypain (1). In addition, two other protease genes, prtT (17) and tpr (3), with no significant homologies to the rgp and kgp-related genes were also identified in P. gingivalis, although the role of each of these enzymes in the virulence of this organism remains to be determined.
The recent construction of a protease mutant, G-102, of strain 381 in our laboratory (27) has revealed that the mutation decreased the ability of the mutant to bind to epithelial cells and gram-positive bacteria, as well as extracellular matrix proteins. These defects appeared to be related to decreased expression of the fimA gene, which codes for the fimbrillin subunit of the fimbriae of these organisms. However, it is not clear how the protease defect resulted in reduced expression of the fimA gene. More recent evidence in our laboratory (27a) has also indicated that the colonization-defective protease mutant G-102 contains a defect in the rgpB gene and not in the rgpA gene as originally proposed (31). Therefore, we have further examined the role of proteases in colonization by constructing a specific rgpA mutant in order to differentiate between the roles of the two genes in the virulence of P. gingivalis. Our results indicate that the rgpA mutation has a more dramatic effect on some P. gingivalis interactions with host tissue than does the rgpB mutation in mutant G-102. In addition, these results also indicate that the RgpA enzyme can play a significant role in the degradation of collagen under host environmental conditions.
MATERIALS AND METHODS
Bacterial strains.
P. gingivalis 381 was maintained anaerobically on blood agar plates containing tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) supplemented with 10% sheep blood, hemin (5 μg/ml), and menadione (1.0 μg/ml) as previously described (27).
Construction of mutant MT10.
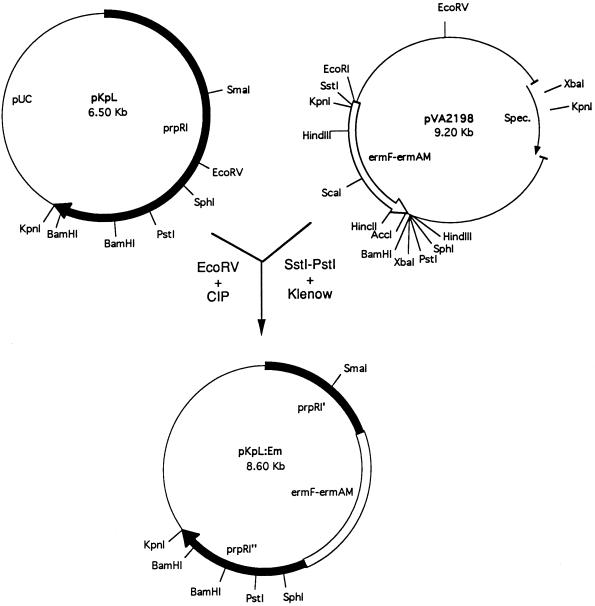
Plasmid pKpL containing the 5′ end of the prpR1 gene from strain W50 (4) was kindly provided by M. Curtis (London Hospital Medical College, London, United Kingdom). Plasmid pVA2198 (7) containing an ermF-ermAM cassette was obtained from F. Macrina (Medical College of Virginia, Richmond, Va.). Each plasmid was maintained in Escherichia coli JM109 in the presence of 50 μg of ampicillin per ml (for pKpL) or 50 μg of spectinomycin per ml (for pVA2198). A blunt-ended 2.1-kb DNA fragment was isolated from pVA2198 by SstI and PstI cleavage followed by overhang removal with T4 DNA polymerase and ligated to pKpL linearized with EcoRV. The resulting plasmid was next linearized with EcoRI and used to electroporate strain 381. Electroporation was carried out by a modification of the procedure of Smith et al. (25). P. gingivalis 381 competent cells were prepared by suspending early-log-phase cells in electroporation buffer (10% glycerol, 1.0 mM MgCl2). The cells together with linearized plasmid pKpL:Em were pulsed with a Bio-Rad (Hercules, Calif.) gene pulser at 2.5 kV, added to TSB supplemented with hemin and menadione, and incubated anaerobically for 16 h. The cell cultures were next plated onto TSB agar plates containing erythromycin (5 μg/ml) and incubated anaerobically at 37°C for 7 to 10 days.
Complementation of the MT10 mutation.
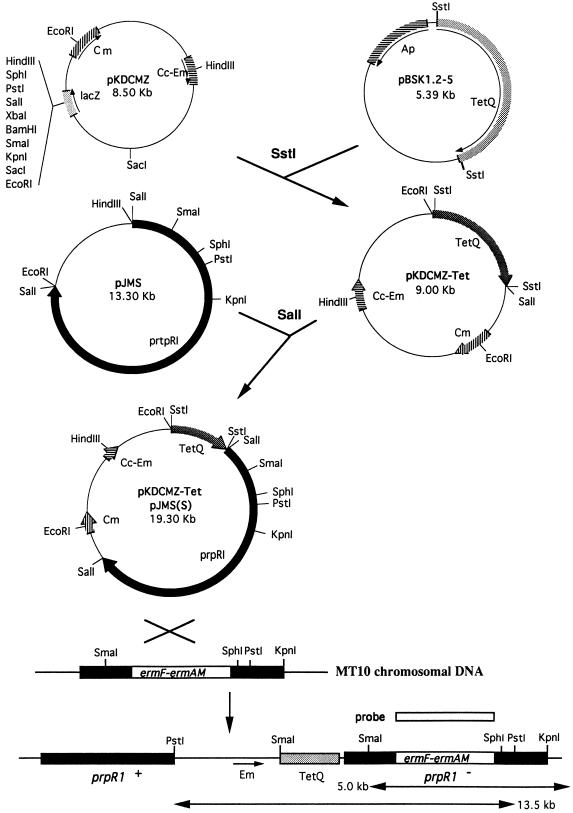
Plasmid pBSK1.2-5 (13) containing the tetracycline resistance gene [tetA(Q)] derived from Bacteroides fragilis was kindly provided by G. Lepine (University of Toronto, Toronto, Canada). Plasmid pJMS containing the intact prpR1 gene was obtained from M. Curtis. Both plasmids were maintained in E. coli JM109 in the presence of ampicillin (50 μg/ml). Plasmid pKDCMZ (14) and E. coli S17.1 (11) were used for suicide integration into P. gingivalis 381 (see Fig. 6 for the construction strategy). Briefly, a 2.4-kb SstI fragment containing the tetA gene was isolated from pBSK1.2-5 and ligated to pKDCMZ, yielding plasmid pKDSMZ-Tet. A 10.3-kb SalI fragment containing the intact prpR1 gene was then isolated from pJMS and ligated to pKDCMZ-Tet, resulting in plasmid pKDCMZ-Tet/pJMS(S). E. coli S17.1 (pKDCMZ-Tet/pJMS) and P. gingivalis MT10 were grown to late log phase (A600 = 0.8 to 1.0) for conjugation. Two milliliters of each culture was mixed, and the suspension was harvested by centrifugation at 9,600 × g for 5 min. The cell pellet was then suspended in 0.2 ml of TSB, spotted onto a TSB blood agar plate, and incubated aerobically at 37°C for 4 h followed by anaerobic incubation for 48 h at 37°C. The cells were then harvested by scraping and suspended in 1.0 ml of TSB. The suspension was plated onto TSB blood agar plates containing erythromycin (10 μg/ml), tetracycline (10 μg/ml), and gentamicin (100 μg/ml) and incubated anaerobically at 37°C for 7 to 10 days. The resulting colonies were picked and suspended in TSB plus erythromycin plus tetracycline plus gentamicin and grown anaerobically for characterization.
FIG. 6.
Strategy for complementation of mutant MT10. A 2.2-kb SstI fragment from the tetA(Q) gene from pBSK1.2-5 and a 10.5-kb SalI fragment containing the intact prpR1 gene from plasmid pJMS were isolated and ligated into pKDCMZ. Following conjugation, the predicted integration of the resulting plasmid, pKDCMZ-Tet/pJMS(S), into MT10 is indicated at the bottom. Cm, chloramphenicol; Cc-Em, clindamycin-erythromycin; Ap, ampicillin.
Southern blot analysis.
Chromosomal DNA from P. gingivalis strains was prepared with a Puregene isolation kit by following the supplier’s (Gentra System, Inc., Minneapolis, Minn.) protocol. DNA was digested with the restriction enzymes indicated below and loaded onto 0.8% agarose gels, and the DNA fragments were transferred to nylon membranes (Amersham Corp., Arlington Heights, Ill.) after alkaline treatment (25). The labeling of the probes, hybridization, and detection by an enhanced chemiluminescence system were performed as recommended by the supplier (Amersham).
Autoaggregation of P. gingivalis.
P. gingivalis 381 and its mutants were initially cultured to the early stationary phase. The cells were then washed twice with phosphate-buffered saline (PBS) and suspended to a density at an A600 of 1.0. Each suspension was monitored for autoaggregation (decrease in absorbance as the cells clumped) as previously described (27). All assays were carried out at least three times, and representative data are shown in the figures.
Binding of P. gingivalis to immobilized type I collagen.
Acid-soluble rat tail type I collagen was purchased from Sigma Chemical Co. (St. Louis, Mo.). Binding was determined as previously described (27) following the attachment of the collagen to microtiter plates. Briefly, the cells were incubated with the immobilized collagen, the wells containing the incubation mixtures were washed with PBS-Tween 20, the attached cells were stained with crystal violet, and the cells were quantitated by spectrophotometry.
Binding of P. gingivalis to epithelial cells.
The interaction of the bacteria with human oral epithelial KB cells was carried out as previously described (27). Briefly, the P. gingivalis cells (late log phase, 107 CFU/ml) were added to 5 × 105 human oral epithelial KB cells growing as confluent monolayers in tissue culture plates. After incubation for 2 h at 37°C, the washed cells were lysed with distilled water and the attached bacteria were quantitated by counting of viable cells on Trypticase soy agar plates. All assays were carried out in triplicate, and the results presented are the average percentages of input bacteria which attached.
Expression of fimA mRNA in P. gingivalis.
Total RNA was isolated from P. gingivalis cells grown to mid-log phase as recently described (27). Equal amounts of RNA (20 μg) were loaded onto 1.0% agarose–2.2 M formaldehyde gels, electrophoresed in MOPS (morpholinepropanesulfonic acid) buffer, and transferred to Hybond N membranes (Amersham) following capillary transfer. The BamHI-NcoI fragment from pEfim (23) containing the intact fimA gene was used as the probe. Detection of mRNA was carried out with the enhanced chemiluminescence detection system (Amersham) as described by the supplier.
Degradation of intact rat tail collagen fibers.
Collagen fibers (18) were aseptically dissected from the tail tendons of sacrificed newborn Sprague-Dawley rats (institutional protocol no. ORB22062Y), cut into small fragments (5- to 10-mm lengths), and incubated with P. gingivalis cells anaerobically for 7 to 10 days at 37°C. The fibers were visually observed daily for disintegration. At 7 days (prior to complete dissolution in the presence of P. gingivalis 381), the collagen fibers were carefully removed from culture with forceps and suspended in 10 mM acetic acid buffer, pH 7.5. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed under reducing conditions with a Protean II electrophoresis system (Bio-Rad Laboratories) as described previously (12). Each sample was loaded onto 10% polyacrylamide gels, and the resulting gels were stained with Coomassie brilliant blue or subjected to protein transfer onto Immobilon P membranes (Millipore Corp., Bedford, Mass.). The membranes were then analyzed by Western blotting (28) with anti-type I collagen serum (Sigma) at a 1:2,000 dilution.
Electron microscopy.
Bacterial cells from an 18-h anaerobic culture were collected by centrifugation (10,000 × g for 1 min), washed, and suspended (5 × 108 cells/ml) in PBS. Ten microliters of cell suspension was applied to a copper grid coated with a thin Formvar film and air dried. The samples were negatively stained with 2% (wt/vol) uranyl acetate for 1 min, air dried, and photographed with a Hitachi H-600 electron microscope operating at 75 kV.
RESULTS
Construction of a rgpA mutant.
Inactivation of the P. gingivalis 381 rgpA gene was accomplished following electroporation with an Erm cassette (7) inserted into the gene after homologous recombination (Fig. 1). The Erm cassette was introduced into the EcoRV site which is present within the region coding for the mature form of the protease (19). Southern blot analysis confirmed that the cassette was inserted into a single site on the strain 381 chromosome within the rgpA gene, yielding mutant MT10 (data not shown). It is important that this analysis can readily differentiate between insertions in the rgpA gene and those in the homologous rgpB gene, since the SmaI fragment hybridizing with the rgpA probe is much larger for rgpB (approximately 9 kb) than rgpA (3 kb) (15, 22).
FIG. 1.
Construction of mutant MT10. A 2.0-kb ermF-ermAM cassette isolated from pVA2198 following cleavage of the plasmid with SstI and PstI and treatment with the Klenow fragment was ligated into the EcoRV site of plasmid pKpL. The resulting plasmid, pKpL:Em, was linearized with EcoRI and electroporated into P. gingivalis 381. CIP, calf intestine phosphatase; Spec., spectinomycin.
Colonization-related properties of the MT10 mutant.
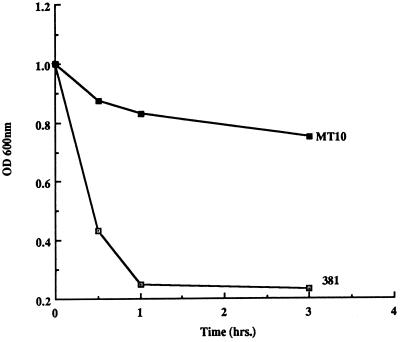
The rgpA mutation affected the growth of mutant MT10, since this mutant grew more slowly than strain 381 in the complex TSB medium when levels of absorbance of the cultures were monitored (data not shown). Furthermore, cell extracts of mutant MT10 expressed approximately 40% of the specific activity of cysteine protease (BAPNA [N-α-benzoyl-dl-arginine-p-nitroanilide] hydrolysis [27]) relative to that of parental strain 381. It is of interest that the mutation in the rgpB gene in mutant G-102 resulted in a level of activity approximately 60% of that of the parent (27). Similarly, the autoaggregation of mutant MT10 was markedly attenuated relative to that of strain 381 (Fig. 2) and also appeared to be weaker than that of mutant G-102 (27).
FIG. 2.
Autoaggregation of strains 381 and MT10. Strains 381 and MT10 were suspended in PBS buffer (A600 = 1.0), and levels of aggregation were measured by the decrease in A600. OD 600nm, optical density at 600 nm.
Both genetic and biochemical approaches (4, 15, 20) have indicated a close relationship between the hemagglutination and protease activities of P. gingivalis strains. Peptide sequences associated with hemagglutinating activity constitute domains within the RgpA protease (19), and mutants defective in protease activity exhibit reduced hemagglutinating activities (15, 31). Therefore, it was not surprising that the hemagglutination titer of mutant MT10, 1:2, was much lower than that of strain 381, 1:32 (data not shown). This reduction in hemagglutination activity with sheep erythrocytes was also significantly greater than the level previously observed for mutant G-102 (31).
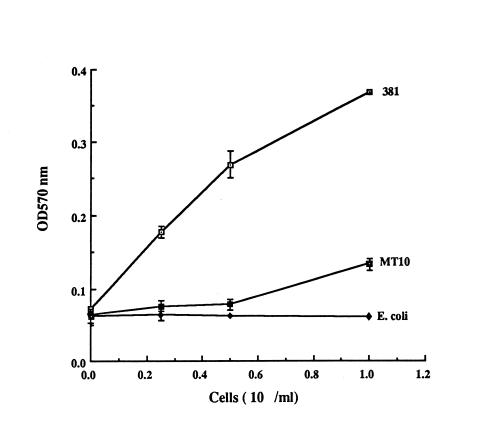
Since mutant G-102 was also defective in binding to extracellular matrix proteins (27), it was of interest to examine MT10 in this regard. Inactivation of the rgpA gene also resulted in a greater decrease in the ability of mutant MT10 to bind to immobilized collagen (Fig. 3). This alteration also appeared to be stronger than that exhibited by mutant G-102 (27). In addition, the ability of mutant MT10 to aggregate oral streptococci such as Streptococcus gordonii was also attenuated relative to that of parental strain 381 by the spectrophotometric assay (reference 27 and data not shown).
FIG. 3.
Adherence of strains 381 and MT10 to immobilized type I collagen. P. gingivalis 381 and MT10 were incubated with precoated type I collagen (5 μg per well) in microtiter plates, and binding was detected as described in the text. E. coli JM109 was used as a negative control. I bars reflect average standard errors of the means (symbols) of duplicate samples. OD570 nm, optical density at 570 nm.
Since P. gingivalis cells may colonize the gingival margin by attaching to gingival epithelial cells (6), it was of interest to compare the binding of mutant MT10 with that of the strain 381 parent. Surprisingly, there was a twofold increase in the binding of mutant MT10 to cultured epithelial cells compared to that of the parent (Table 1). By contrast, mutant G-102 bound less than the parent to the same cell line (27). It was also of interest that pretreatment of P. gingivalis 381 cells with the cysteine protease inhibitor TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone) markedly increased its attachment severalfold, but similar treatment of MT10 cells led to only a twofold increase. Furthermore, the fimA mutant DPG3, which does not bind to epithelial cells (27), displays a significant attachment when it is treated with TLCK.
TABLE 1.
Attachment of P. gingivalis strains to epithelial cells
| Strain | Treatment | Mean % attachment ± SDa |
|---|---|---|
| 381 | None | 7.9 ± 2.8 |
| 0.5 mM TLCKb | 71.6 ± 25.2 | |
| MT10 | None | 19.9 ± 3.1 |
| 0.5 mM TLCK | 38.6 ± 13.9 | |
| DPG3 | None | 0.5 ± 0.3 |
| 0.5 mM TLCK | 16.7 ± 0.6 |
Attachment relative to that of the initial inoculum.
Bacteria were pretreated with TLCK for 15 min at room temperature prior to being washed and added to the epithelial cells.
Expression of fimbriae by mutant MT10.
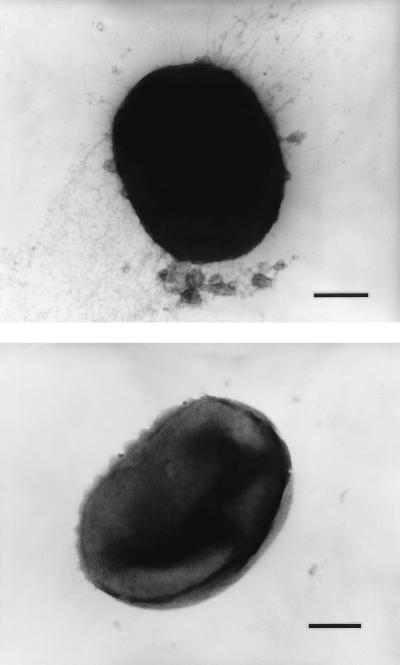
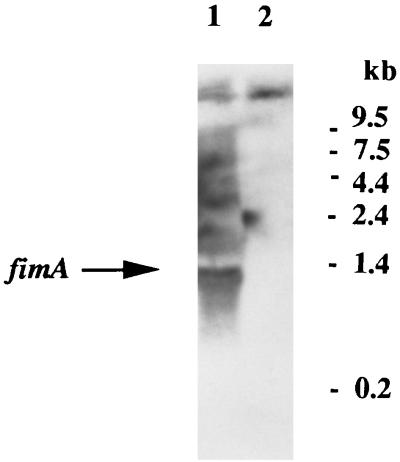
Recent results (27) have suggested that the colonization defects in mutant G-102 might be explained by a reduction in the expression of fimbriae. Interestingly, a direct analysis of fimbrial expression by mutant MT10 by electron microscopy revealed that the mutant was completely devoid of the fimbrial structures characteristic of strain 381 (Fig. 4). This finding was confirmed by the lack of staining of the mutant after treatment with immunogold-labeled antibody (data not shown). Furthermore, this lack of fimbriae was confirmed by Western blot analysis with anti-FimA sera, which revealed the marked reduction in the expression of the fimbrial subunit protein FimA relative to that in strain 381 (see Fig. 7). Faint FimA protein bands could be detected for mutant MT10 when large amounts of cells were analyzed (data not shown). This defect apparently occurred at the transcriptional level, since Northern blot analysis (Fig. 5) indicated the presence of a 1.4-kb mRNA for the fimA gene in strain 381 but the corresponding hybridizing band for mutant MT10 could not be detected. Thus, the absence of visible fimbriae in mutant MT10 may be correlated with the repression of fimA expression. However, it is also possible that increased degradation of the fimA mRNA explains such results.
FIG. 4.
Electron microscopic determination of fimbrial expression in strains 381 and MT10. Transmission electron micrographs of strains 381 (top) and MT10 (bottom) show the absence of visible fimbriae in mutant MT10. Bars, 0.2 μm.
FIG. 7.
Western blot analysis of FimA expression in the MT10 complemented mutants. Sonic extracts of each strain (35 μg) were resolved on SDS–10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes for analysis with anti-FimA serum. Lanes: 1, P. gingivalis W50; 2, MT10-C1; 3, MT10-C2; 4, MT10-C3; 5, MT10; 6, 381. The FimA protein is indicated by the arrowhead. Molecular weight markers (in thousands) are at the right.
FIG. 5.
Northern blot analysis of fimA mRNA expression in strains 381 and MT10. Equal amounts of RNA (20 μg per lane) from strains 381 (lane 1) and MT10 (lane 2) were analyzed. The position of the fimA mRNA (1.35 kb) is indicated by the arrow. The probe used was a 994-bp fragment containing the fimA gene. Molecular size markers are depicted on the right.
Complementation of the rgpA mutation.
In order to determine if the colonization defects exhibited by mutant MT10 directly resulted from the inactivation of the rgpA gene, complementation of the protease defect was carried out. Since the rgpA gene from strain 381 was not available, a strategy was devised to introduce the rgpA homolog from strain W50 (4) into the chromosome of MT10 (Fig. 6), thus restoring cysteine protease activity. Following conjugation, the resulting purified colonies were demonstrated to exhibit 84% of strain 381’s cysteine protease-specific activities. In addition, Southern blot analysis confirmed the predicted integration of plasmid pKDCMZ-Tet-pJMS into the MT10 chromosome (data not shown).
Western blot analysis (Fig. 7) of three of the complemented isolates, MT10-C1, -C2, and -C3, confirmed that introduction of the rgpA homolog from strain W50 into the MT10 chromosome resulted in expression of FimA protein at levels equivalent to that of strain 381. Strain W50, which does not express visible fimbriae, demonstrated no detectable protein bands which reacted with anti-FimA sera. Therefore, the presence of the RgpA protease may be correlated with the expression of the FimA protein. In addition, PCR analysis of the region immediately upstream of the fimA gene for strains 381 and MT10 revealed no detectable difference in size, suggesting no major alterations in this regulatory region (data not shown). Furthermore, the complemented mutants also expressed parental colonization properties (autoaggregation and hemagglutination). Taken together, these results suggest that the defects exhibited by MT10 resulted from the loss of RgpA protease activity and not from a secondary spontaneous mutation affecting the expression of the fimA gene.
Role of the RgpA protease in collagen degradation.
Several laboratories have reported that the P. gingivalis RgpA protease is capable of degrading type I collagen (2, 10) and may therefore play a role in the breakdown of this tooth-supportive protein during periodontitis. However, concerns regarding the lack of proper controls utilized in these studies have brought such conclusions into question (21). In order to address this issue, we have examined the role of the RgpA protease in type I collagen degradation with mutant MT10 under in vivo conditions (37°C) using intact collagen fibrils from freshly extracted rat tail tendons (a model system which might mimic the human homolog). When strains 381 and MT10 were cultured together with the collagen fibrils for approximately 10 days, the fibrils were completely digested by strain 381 but remained essentially intact when they were incubated with mutant MT10. When the fibrils were removed from parallel cultures at shorter intervals, it could be demonstrated that strain 381 degraded the type I collagen subunits much more rapidly than did mutant MT10 (Fig. 8). However, incubation of the collagen fibrils with the purified RgpA protease alone (obtained from M. Curtis, London Medical College) did not result in significant visible solubilization. These results suggested that the RgpA protease plays a role in type I collagen degradation by P. gingivalis, although it remains to be determined whether this is a direct effect.
FIG. 8.
Analysis of partially degraded rat tail collagen fibers. Coomassie brilliant blue-stained (a) and Western blot analysis of (b) partially degraded rat tail collagen fibers. Intact β, α1, and α2 collagen subunits are evident in the control (lanes 1, no cells), as indicated by the arrows. The fibers were incubated with P. gingivalis 381 (lanes 2) or MT10 (lanes 3) for 7 days at 37°C. Each sample was isolated from the cultures, boiled with SDS–β-mercaptoethanol sample buffer, and run on SDS–10% polyacrylamide gels (20 μg of protein per sample). The gels were stained with Coomassie brilliant blue or subjected to Western blot analysis with anti-type I collagen sera.
DISCUSSION
The ability of organisms to respond to environmental changes is an important survival mechanism. The induction of proteins which aid in the scavenging of nutrients under conditions of nutrient deprivation may be important in this regard. Since P. gingivalis appears to depend upon protein degradation and not fermentation for its primary energy requirements (9), it is very likely that proteases have pleiotropic effects on the physiology of this organism. Recent results (15, 27) have indicated that the reduction in cysteine protease activity in P. gingivalis following site-specific mutagenesis affects not only the growth of the organism but also the expression of cell surface structures such as fimbriae and vesicles. However, the molecular basis for such alterations has not yet been determined.
Our results with mutant MT10 suggest that the RgpA protease not only is required for normal growth of P. gingivalis but also is involved in maintaining many of the potential colonization properties of this organism. Thus, mutant MT10 is severely retarded in its ability to autoaggregate, interact with matrix proteins such as type I collagen, bind to gram-positive streptococci, and hemagglutinate sheep erythrocytes. The last property may be important in enabling P. gingivalis to bind and lyse erythrocytes to obtain essential hemin (9). Likewise, reduced interaction with gram-positive bacteria also suggests potential attenuation of P. gingivalis binding to preformed dental plaque. These properties are much more attenuated relative to those of the RgpB protease mutant G-102 (27). Therefore, the loss of cysteine protease activities in the two mutants can be correlated with reduction in colonization properties. Since the reduction in expression of the FimA protein at the transcription level also parallels these changes, a role for fimbriae in these colonization properties is suggested. These results are consistent with several observations from different laboratories (5, 27, 30) implicating fimbriae in the interaction of P. gingivalis with other bacteria and eucaryotic cells. Nevertheless, a specific effect of the proteases in these properties cannot be formally excluded at this time.
A recent study (15) using an RgpA mutant of P. gingivalis ATCC 33277 revealed that this mutant still possessed visible fimbriae and that only the rgpA-rgpB double mutant of this strain did not express fimbriae. However, visible fimbriae could not be detected in the strain 381 rgpA mutant MT10. It should be noted that the rgpA mutant of strain ATCC 33277 exhibited only 24% of the Arg-specific wild-type cysteine protease activity but that mutant MT10 displayed approximately 40% of the parental activity in crude cell extracts. The rgpA mutant of strain ATCC 33277 exhibited hemagglutination titers similar to those of the parental strain (15), while mutant MT10 was markedly attenuated in interacting with sheep erythrocytes. However, it is not clear why the rgpA mutations in the two strains yield distinct phenotypes. One possibility is that protease expression is regulated differentially. Preliminary results from this laboratory suggest that the expression of other proteases in P. gingivalis is influenced by the rgpA mutation (27a). In addition, since different strategies were used to construct the mutants (single versus double crossover integration of the inactivated gene fragments), differential levels of expression of the relevant genes may have occurred in the two mutant strains. Nevertheless, these studies with two different strains do suggest that protease defects in P. gingivalis result in attenuated expression of the major fimbriae, which ultimately affects the colonization properties of these organisms. In addition, the proteases may alter colonization independently of affecting fimbriae.
One major discrepancy in the correlation between reduction in protease activity and colonization of the two strain 381 mutants G-102 and MT10 was observed. The rgpB mutant showed decreased binding to epithelial cells (27), while the rgpA mutant showed increased binding relative to that of the 381 parental strain. Since fimA mutants are also attenuated in binding to epithelial cells (8, 27), it appears to be difficult to reconcile such differences. One possible explanation is that P. gingivalis cells also bind to epithelial cells by a fimbria-independent mechanism which is affected by protease activity. Accordingly, mutant MT10 may bind to epithelial cells exclusively by such a mechanism. This is consistent with the results demonstrating that the addition of the cysteine protease inhibitor TLCK markedly increased the ability of strain 381, and to a lesser extent that of mutant MT10, to bind to epithelial cells (Table 1). Recent results have also demonstrated a similar effect with strain ATCC 33277 (5a). Therefore, reduction of P. gingivalis protease activity resulting from either mutation (rgpA) or TLCK treatment may activate fibril-independent binding of the bacteria to epithelial cells. The very strong attachment exhibited by the wild-type 381 strain treated with TLCK may represent a synergistic effect of the expression of both the fibril-dependent and -independent binding mechanisms.
Previous results have indicated that the RgpA protease is capable of degrading type I collagen (2, 10) and therefore it may play a direct role in collagen degradation of periodontally inflamed tissue. However, such a conclusion has been called into question (21) in part because of the apparent contamination of commercial type I collagen preparations with gelatin. In addition, our own results have indicated that trypsin is able to degrade commercial type I collagen preparations (27a). However, the present results suggest that the RgpA protease does play a role in the degradation of collagen fibrils. With a system involving P. gingivalis growing cultures and intact rat tail collagen fibers, which more closely mimic the in vivo environment, it was demonstrated that the rgpA mutant, unlike the parental 381 strain, was not able to solubilize the fibers. Nevertheless, these results do not unequivocally demonstrate that the RgpA protease is directly responsible for cleavage of the collagen molecules, since this effect may be indirect (the protease may alter the collagen fibers such that another enzyme actually degrades the collagen molecules). Furthermore, purified RgpA did not dissolve the fibers under the assay conditions used. However, the soluble purified enzyme, unlike the cell-associated enzyme (data not shown), is not stable under the relatively long-term incubation conditions required for solubilization (10 days). Clearly, additional confirmation of the role of the RgpA protease in collagen degradation is required, but the present results do support a role for the protease in collagen dissolution.
The properties of mutant MT10 further confirm the recent suggestions of researchers examining mutant G-102 (27) that the cysteine proteases influence the colonization properties of P. gingivalis. For most of these properties, attenuated levels of colonization are paralleled by levels of protease activity. However, this parallelism is not the case for bacteria binding to epithelial cells and suggests that multiple factors of the bacterium are involved in such interactions. The molecular basis for these relationships will require further investigation into the regulatory mechanisms involved in the expression of fimbriae and the other cell surface molecules involved in colonization.
ACKNOWLEDGMENTS
We gratefully acknowledge the provision of reagents to us by M. Curtis and A. Sharma as well as the assistance of M.-I. Cho in isolating the collagen fibers.
This investigation was supported in part by National Institutes of Health grants DE08293 (H.K.) and DE10510 (M.D.).
REFERENCES
- 1.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz M S. Analysis of the prtP gene encoding porphypain, a cysteine protease of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedi G S, Williams T. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J Biol Chem. 1994;269:599–606. [PubMed] [Google Scholar]
- 3.Bourgeau G, Lapointe H, Peloquin P, Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992;60:3186–3192. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth W, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivalis which is present in multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler C W, Kalmer J R, Genco C A. Pathogenic strategies of the oral anaerobe Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 5a.Duncan, M. Unpublished results.
- 6.Duncan M J, Nakao S, Skobe Z, Xie H. Interaction of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada N, Watanabe K, Sasakawa C, Yoshikawa M, Yoshimura F, Umemoto T. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect Immun. 1994;62:1696–1704. doi: 10.1128/iai.62.5.1696-1704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Yoneda M, Okamoto K, Maeda K, Yamamoto K. Purification and characterization of a novel arginine-specific cysteine protease (Argingipain) involved in the pathogenesis of periodontal disease from the culture supernatant of Porphyromonas gingivalis. J Biol Chem. 1994;269:21371–21378. [PubMed] [Google Scholar]
- 11.Karunakaran T, Madden T, Kuramitsu H. Isolation and characterization of a hemin-regulated gene, hemR, from Porphyromonas gingivalis. J Bacteriol. 1997;179:1898–1908. doi: 10.1128/jb.179.6.1898-1908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lépine G, Lacroix J-M, Walker C B, Progulske-Fox A. Sequencing of a tet(Q) gene isolated from Bacteroides fragilis 1126. Antimicrob Agents Chemother. 1993;37:2037–2041. doi: 10.1128/aac.37.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994;176:1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine-specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 17.Otogoto J-I, Kuramitsu H K. Isolation and characterization of the Porphyromonas gingivalis prtT gene, coding for protease activity. Infect Immun. 1993;61:117–123. doi: 10.1128/iai.61.1.117-123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry D A, Craig A S. Quantitative electron microscope observation of the collagen fibrils in rat-tail tendons. Biopolymers. 1977;16:1015–1031. doi: 10.1002/bip.1977.360160506. [DOI] [PubMed] [Google Scholar]
- 19.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 20.Pavloff N, Pemberton P A, Potempa J, Chen W-C A, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 21.Potempa J, Pavloff N, Travis J. Porphyromonas gingivalis: a proteinase/gene accounting audit. Trends Microbiol. 1995;3:430–434. doi: 10.1016/s0966-842x(00)88996-9. [DOI] [PubMed] [Google Scholar]
- 22.Rangarajan M, Aduse-Opoku J, Slaney J M, Young K A, Curtis M A. The prpR1 and prpR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes. Mol Microbiol. 1997;23:955–965. doi: 10.1046/j.1365-2958.1997.2831647.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Sojar H T, Lee J-Y, Genco R J. Expression of a functional Porphyromonas gingivalis fimbrillin polypeptide in Escherichia coli: purification, physicochemical and immunochemical characterization, and binding characteristics. Infect Immun. 1993;61:3570–3573. doi: 10.1128/iai.61.8.3570-3573.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slots J, Genco R J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal diseases: virulence factors in colonization, survival and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 25.Smith C J, Parker A, Rogers M B. Plasmid transformation of Bacteroides spp. by electroporation. Plasmid. 1990;24:100–109. doi: 10.1016/0147-619x(90)90012-2. [DOI] [PubMed] [Google Scholar]
- 26.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 27.Tokuda M, Duncan M, Cho M-I, Kuramitsu H K. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Tokuda, M., and H. Kuramitsu. Unpublished results.
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travis J, Potempa J, Maeda H. Are bacterial proteinases pathogenic factors? Trends Microbiol. 1995;3:405–407. doi: 10.1016/s0966-842x(00)88988-x. [DOI] [PubMed] [Google Scholar]
- 30.Xie H, Cai S, Lamont R J. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 1997;65:2265–2271. doi: 10.1128/iai.65.6.2265-2271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneda M, Kuramitsu H K. Genetic evidence for the relationship of Porphyromonas gingivalis cysteine protease and hemagglutinin activities. Oral Microbiol Immunol. 1996;11:129–134. doi: 10.1111/j.1399-302x.1996.tb00347.x. [DOI] [PubMed] [Google Scholar]