Abstract
RNA has gained great interest for use in biomedical and therapeutic applications. This is due in part to RNA’s ability to perform multiple functions, including the regulation of endogenously expressed genes. However, the ability of RNA based drugs to distinguish target diseased cells from healthy tissue remains challenging. Here we present methods for the production of a recently developed conditional RNA switch that releases a Dicer substrate RNA in response to interaction with a specific RNA biomarker.
Keywords: RNA, Toehold, Nanotechnology, Nanomedicine, RNase H, RNA switch, Conditional RNA
1. Introduction
Nucleic acids have become one of the preferred materials for construction of objects and scaffolds at the nanoscale [1, 2]. RNA in particular has gained significant interest in the fields of nanomedicine and nanotechnology as a material for fabrication of nanoconstructs for therapeutic application due to several characteristics inherent to this biopolymer. Natural RNAs possess an assortment of recurrent structural motifs composed of classic and noncanonical base pair interactions [3, 4] providing a repository of building blocks that can be used to define the geometry, positioning, and assembly of monomeric strands within a rationally designed RNA construct [5-9]. In addition, the wide array of known functions performed by different RNA elements offers the potential to encode diverse functionalities within an RNA nanoconstruct. Such functions can include cell-specific targeting, knockdown of target gene expression, or organization of cellular machinery [10-12].
Critical to the design and function of RNA nanoconstructs is the specificity provided by Watson–Crick base pairing interactions (WC bps). From a structural standpoint, the specificity of WC bps provides a means to program the formation of helical regions which act as the scaffolding backbone that ultimately defines construct geometry [13, 14]. From a function standpoint, specific base pairing allows RNA to convey information in the form of the nucleotide sequence. This ability to store, search for, and recognize specific sequence information is central to many RNA-based regulatory functions, such as RNA interference (RNAi) and the CRISPR-Cas system, and RNA-based nanoconstructs that regulate gene expression have been designed utilizing such functions [11, 15].
However, the ability of RNA to store and read information at the level of its nucleotide sequence can also be used to regulate the function of the RNA nanoconstruct itself. Single-stranded nucleic acid toeholds can be used to produce conditional structural changes in response to the presence of a cognate “trigger” sequence that is complementary to the toehold region. Such a method can be used to conditionally knock down target gene expression if the induced conformational change either generates or liberates an RNA element capable of inducing an RNAi response [16, 17].
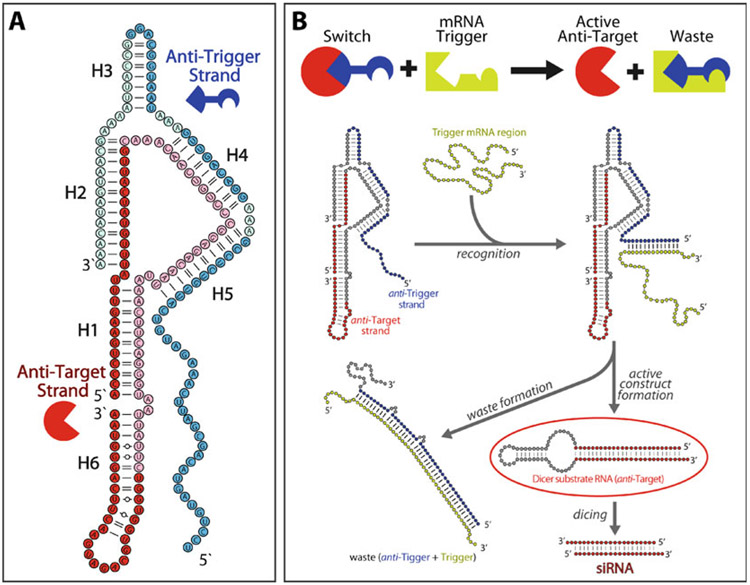
We have recently developed a two-stranded RNA switch (Fig. 1) that is able to knock down target gene expression in response to the presence of a specific mRNA biomarker and verified its function by gel electrophoresis and in a cultured human breast cancer cell line [18]. The switch is assembled from two individual RNA strands, named the “anti-trigger” and “anti-target” strands. On its own, the anti-target strand is designed to fold into an shRNA-like structure, which is able to be processed by Dicer and results in knockdown of a target gene. However, assembly of the anti-target with the anti-trigger strand sequesters the anti-target into a more thermodynamically favorable, intermolecular assembly and prevents formation of the active shRNA-like fold. The anti-trigger strand contains a single-stranded toehold within the assembly, which is used to perform the diagnostic function of the switch. Upon recognition of the biomarker trigger RNA through its single-stranded toehold, the anti-trigger strand unzips from the anti-target strand and results in the disassembly of the switch. This allows the anti-target strand to undergo a conformational rearrangement to form its active shRNA-like structure. As the switch design contains no overlap between the sequence regions that encode the siRNA component and the region complementary to the RNA trigger, virtually any combination of trigger and target pairs can be incorporated within the switch design. Additionally, as the 3’ end of the anti-trigger sequence contains no design constraints, it allows for further potential functionalization. Conjugation to a targeting aptamer or attachment to a multivalent RNA scaffold could be utilized to create a more robust, conditional drug delivery vehicle [19].
Fig. 1.
Design of a two-stranded RNA switch. (a) The two-stranded switch is assembled from an anti-trigger strand (blue) and an anti-target strand (red). The majority of the anti-trigger strand (dark blue nodes) is designed to be complementary to the trigger RNA sequence, while the 5′ and 3′ end regions of the anti-target strand (dark red nodes) encode siRNA sense and antisense sequences, respectively. (b) The RNA switch is initially in an inert conformation. Interaction of the anti-trigger’s single-stranded toehold with a trigger RNA induces a conformational change that releases the anti-target strand as an active shRNA-like Dicer substrate. Adapted and reprinted with permission from [18], copyright 2016 American Chemical Society
In the protocols that follow we describe detailed methods for the synthesis, assembly, purification, and functional validation of the two-stranded RNA switch designed to silence eGFP expression in response to the presence of connective tissue growth factor mRNA (CTGF/CCN2). It is possible to incorporate other target/trigger pairs within the switch design, and a brief discussion of sequence design is included in Subheading 3.1. In vitro transcription and PAGE purification of transcripts are discussed in Subheadings 3.2 and 3.3, respectively. As the two strands, and particularly the anti-target strand, require specific 5′ end sequences that deviate from the typical purine-rich 5′ sequence preferred by T7 RNA polymerase [20], in Subheading 3.4 we provide a method for the removal of a 5′ end leader sequence by addition of RNase H [18, 21]. Assembly and native purification of the RNA switch are described in Subheading 3.5 and 3.6, respectively. Methods for functional validation by nondenaturing PAGE and in cultured human cells are described in Subheading 3.7 and 3.8, respectively.
2. Materials and Equipment
All buffers and solutions should be made with ultrapure 18.2 MΩ water. The stock buffers and solutions should be filtered using a 0.22-μm filter. Ensure that reagents and enzymes used are RNase- and DNase-free. Any solutions and experiments prepared for cell culture studies should be made with water that is certified RNase-, DNase-, and endotoxin-free.
2.1. In Vitro Transcription Components
DNA templates encoding desired RNA sequences (see Note 1).
Transcription buffer, final concentrations (1×): 5 mM MgCl2, 2 mM spermidine, 40 mM Tris pH 7.5 (see Note 2). Typically prepared as a 1 mL stock solution at 10× concentration.
NTP mixture: 25 mM each ATP, GTP, CTP, UTP.
MnCl2, 5.0 mM solution.
Inorganic pyrophosphatase (IPP), 0.1 U/μL solution.
Dithiothreitol (DTT), 100 mM solution.
T7 RNA polymerase enzyme.
DNase 1 enzyme.
2.2. RNA Strand Purification Components
Urea, 8 M.
EDTA, 0.5 MpH 8.0.
Urea blue loading dye: 6 M urea, 20 mM EDTA, 10% (v/v) glycerol, 0.005% (w/v) xylene cyanol, 0.005% (w/v) bromophenol blue. Typically prepared in a 25 mL volume.
Tris–borate–EDTA (TBE) buffer, final concentrations (1×): 89 mM Tris, 89 mM boric acid, 2 mM EDTA, pH 8.3. Prepare 1 L as a 10× stock solution.
Denaturing PAGE gel: 8% acrylamide–bis-acrylamide (19:1), 1× TBE, 5.5 M urea; 1.6 mm thickness.
Ammonium persulfate (APS), 10% solution w/v. Prepare in a 10 mL volume.
Tetramethylethylenediamine (TEMED).
Tx elution buffer: 10 mM Tris pH 7.5, 200 mM NaCl, 0.5 mM EDTA. Prepare in a 25 mL volume.
Ethanol, 100% and 90% solution, chilled (−20 °C).
Vertical PAGE system (16.5 cm × 28 cm or 33 cm × 39 cm gel).
Plastic film.
Handheld UV lamp.
Scalpel or razor blade.
Shaker/mixer for 1.5-mL microcentrifuge tubes (temperature controlled or in refrigerated environment, 4 °C).
Temperature controlled benchtop centrifuge.
Centrifugal vacuum concentrator.
UV spectrophotometer.
2.3. RNase H Processing Components
Purified RNA transcripts containing a 5′ leader sequence.
DNA oligonucleotide complementary to 5′ RNA leader sequence.
RNase H enzyme.
RNase H buffer, final concentration (1×): 50 mM Tris pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT) (see Note 3). Prepare 1 mL as a 10× stock solution.
2.4. RNA Assembly and Nondenaturing PAGE Components
Purified RNA transcripts.
Tris–borate (TB) buffer, final concentrations (1×): 89 mM Tris, 89 mM boric acid, pH 8.3. Prepare 1 L as a 10× stock solution.
Assembly buffer, final concentrations (1×): 1× TB buffer, 2 mM Mg(OAc)2. Prepare 1 mL as a 5× concentrated solution.
Non-denaturing PAGE gel: 10% acrylamide–bis-acrylamide (19:1), 1× TB buffer, 2 mM Mg(OAc)2; 0.8 mm thickness.
Native gel running buffer: 1× TB buffer, 2 mM Mg(OAc)2. Prepare 1 L.
Native gel loading buffer: 1× assembly buffer, 0.005% (w/v) xylene cyanol, 0.005% (w/v) bromophenol blue, 50% (v/v) glycerol (see Note 4). Prepare in a 1 mL volume.
Cold room or refrigerated environment (4 °C).
Vertical PAGE system (16.5 cm × 28 cm or 33 cm × 39 cm gel).
Nucleic acid stain solution (such as: ethidium bromide solution, 0.5 μg/mL; SYBR Gold, 1×; SYBR Green II, 1×).
Fluorescence gel scanner (see Note 5).
Plastic film.
Handheld UV lamp.
Scalpel or razor blade.
Flat-bottom microcentrifuge tubes.
2.5. Cell Culture, Transfection, and Analysis Components
MDA-MB 231 cell line stably expressing eGFP (see Note 6).
Dicer substrate RNA designed against the endogenous RNA trigger molecule (for the CTGF/eGFP switch described here, an anti-CTFG Dicer substrate is used).
Complete growth medium: DMEM high glucose medium supplemented with 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin.
Opti-MEM medium (Gibco).
Lipofectamine 2000 (Invitrogen) (see Note 7).
Cell dissociation buffer. Available from various commercial sources.
Phosphate buffered saline (PBS), 1× concentration: 1.47 mM KH2PO4, 4.3 mM Na2HPO4, 2.7 mM KCl, 137 mM NaCl, pH 7.4, sterile. Can be purchased from commercial source, or prepare 1 L of 1× concentration.
Grid slide.
Optical microscope.
Flow cytometer.
3. Methods
3.1. Overview of RNA Sequence Design
Both the anti-trigger and the anti-target strand of the switch must adhere to strict sequence constraints across long stretches of each molecule (Fig. 1). Much of the anti-trigger strand sequence is defined by its necessity to be complementary to the RNA biomarker trigger. Within the anti-target strand, the 5′ and 3′ regions must encode the sense and anti-sense sequences of the siRNA against the gene targeted for knockdown, respectively. These two constraints establish the starting point for RNA sequence design.
The anti-trigger sequence is designed to contain the single-stranded toehold (approximately 25–30 nt) at its 5′ end before hybridizing with the anti-target strand to form helices H5 and H4, and then forms the intramolecular hairpin containing helix H3. All three of these helical sequences are defined by the RNA trigger sequence. The 3′ end of the anti-trigger strand is designed to be complementary to a portion of the siRNA region within the anti-target strand, forming helix H2.
The anti-target strand begins at its 5′ end by encoding the sense sequence for the target siRNA. The central portion of the molecule is designed to be complementary to the anti-trigger strand, forming helices H4 and H5, before forming intrastrand pairings with its own 5′ end (helix H1). Additional nucleotides are then incorporated to ensure that the 3′ end of the molecule, that encodes the siRNA antisense sequence, forms a stable hairpin denoted helix H6. Single-stranded poly-A regions are incorporated into the anti-trigger and anti-target strands at various places to provide structural flexibility.
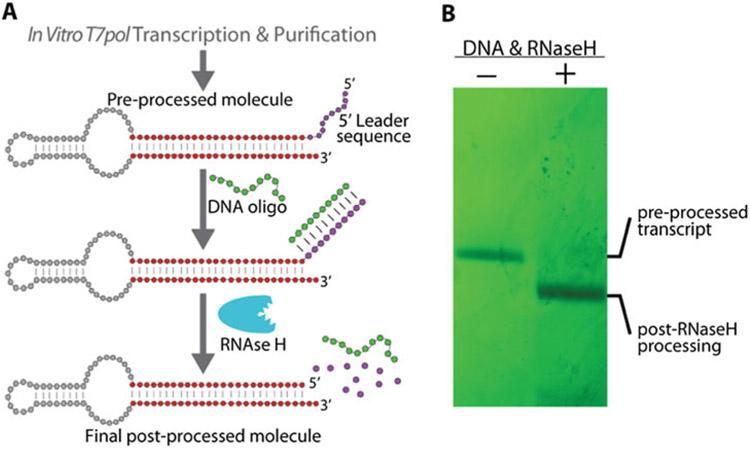
Importantly, both anti-trigger and anti-target RNAs are initially transcribed containing a purine rich 5′ leader sequence that helps increase synthesis yield by T7 RNA polymerase. The leader sequence is later removed by processing with RNase H (Fig. 2). The DNA templates for each switch strand should be designed to contain the T7 promoter sequence, followed by a 14 nucleotide RNA leader sequence at positions +1 to +14, relative to the start of transcribed regions. The sequence of the final desired RNA should begin at position +15 on the template. Typically, we use the promoter sequence 5′TTCTAATACGACTCACTATA at positions −20 to −1, followed by the leader sequence 5′GGGAAAGGAAGAGC at positions +1 to +14, after which the desired switch strand sequence is encoded. The process of removing the leader sequence is outlined in Subheading 3.4.
Fig. 2.
5′-End processing by RNase H. (a) RNA molecules are transcribed containing a purine rich 5′ leader sequence which increases transcription yield by T7 RNA polymerase. The 5′ leader sequence can be removed by processing the transcript with RNase H. A short DNA oligonucleotide is added, complementary to the 5′ leader sequence of the RNA, forming an RNA–DNA hybrid duplex. The RNA strand of the hybrid duplex is degraded by addition of RNase H. (b) Transcripts processed by RNase H can be purified by denaturing PAGE. Molecules not subjected to the RNase H processing can be used as a full-length negative control. Adapted and reprinted with permission from [18], copyright 2016 American Chemical Society
3.2. In Vitro Transcription Using T7 RNA Polymerase
Prepare in vitro transcription reactions in a 1.5-mL microcentrifuge tube using approximately 20 pmol DNA template.
Transcription reactions can be prepared to a final volume of up to 200 μL. Based on your desired reaction volume, add the appropriate volume of 10× transcription buffer to achieve a 1× concentration.
Add NTPs to a final concentration of 2.5 mM each.
Add IPP to a final concentration of 0.01 U/μL.
Add DTT to a final concentration of 2 mM.
Add MnCl2 to a final concentration of 0.5 mM.
Bring to the final reaction volume by addition of water.
Initiate transcription by adding T7 RNA polymerase to the transcription mix, to a final concentration of 2.5 U/μL. Pipet to mix; avoid vortexing.
Incubate the reaction at 37 °C for 4 h.
Terminate the transcription reaction by adding 5 U of DNase 1 per 100 μL of transcription volume.
After addition of DNase 1, incubate the transcription reaction for 30 min at 37 °C to ensure digestion of the DNA template.
It is recommended that transcribed RNA be purified immediately following transcription termination and DNA template digestion. However, if necessary, the transcription reaction can be stored at −20 °C overnight and purified the following day (see Note 8).
3.3. Purification of RNA by Denaturing PAGE
Prepare a 1.6 mm thick denaturing PAGE gel. For a 16.5 cm × 28 cm gel, prepare 100 mL of denaturing gel solution and initiate polymerization by adding 600 μL of 10% APS solution and 80 μL TEMED. For a 33 cm × 39 cm gel, increase the volume of gel solution to 300 mL and polymerize by adding 1800 μL of 10% APS solution and 240 μL TEMED (see Note 9). Insert a comb that produces wells with a volume of 300–400 μL.
One polymerized, set up the PAGE system at room temperature using 1× TBE as the running buffer.
Using a syringe or plastic transfer pipet, rinse the wells of the gel with the running buffer to remove any debris or air bubbles.
Prerun the gel for 20–30 min. For a 16.5 × 28 cm gel, run at a constant power of 30 W. For a 33 × 39 cm gel, run at a 60 W.
Add 100 μL urea blue loading dye to the RNA sample(s) to be purified.
Heat to 94 °C for 2 min to denature RNA molecules, then remove from heat.
Rerinse the wells of PAGE gel and load RNA in the cleaned wells. For small gels (16.5 cm × 28 cm), perform electrophoresis at a constant 30 W. For large gels (33 cm × 39 cm), electrophoresis should be performed using a constant power of 60–70 W. Gels should be run for approximately 2–3 h (see Note 10).
Following electrophoresis, carefully open the glass plates used to cast the gel. The gel should adhere to one of the two plates.
With the gel adhered to one of the plates, cover the exposed gel surface with plastic film. Flip the gel over so the plastic film is down on the benchtop.
Carefully peel the gel off the second plate and onto the plastic film. Cover the newly exposed side of the gel with plastic film.
Locate the RNA within the gel by examining the gel under UV light. For greater contrast a UV reflective surface can be placed under the gel, but a white surface should be sufficient. Circle the location of RNA in the gel using a fine-tipped marker.
Use a scalpel blade to cut the band of RNA from the rest of the gel and place the excised gel piece in a 1.5-mL microcentrifuge tube.
Add 600 μL of Tx elution buffer to the tube and place on a shaker at 900 rpm and 4 °C overnight (~12–14 h).
The following morning, transfer the elution buffer to a new 1.5-mL microcentrifuge tube.
Add 900 μL of cold 100% ethanol to the elution buffer. Invert the tube several times to mix.
Cool the RNA to −20 °C by placing in a −20 °C freezer for 1 h. Alternatively, the sample can be placed in dry ice for 3–5 min, but should not be allowed to freeze.
Centrifuge the RNA at 14,000 × g, at 4 °C for 30 min to precipitate RNA.
Remove the supernatant (leaving about 100 μL of liquid) and wash the pelleted RNA by adding 900 μL of cold 90% ethanol.
Centrifuge the RNA at 14,000 × g at 4 °C for 10 min.
Repeat the wash step described in steps 18 and 19.
Following the second wash with 90% ethanol, remove the supernatant, leaving ≤50 μL of liquid in the tube. Be careful not to disturb the pelleted RNA.
Dry the RNA by centrifuging under vacuum to remove any remaining solvent.
Reconstitute the purified RNA in 30 μL of ultrapure water. RNA concentration can be determined by OD260 using the molecule’s molar extinction coefficient.
RNA should be stored at −20 °C. For long-term storage, store RNA at −70 °C.
3.4. 5′-End Processing of RNA Transcripts Using RNase H
The reaction should be conducted at a RNA concentration of 10 μM. Based on the concentration of purified RNA obtained from in vitro transcription, adjust the final reaction volume to achieve a 10 μM solution (see Note 11).
Add the appropriate volume of 10× RNase H buffer to produce a final 1× solution.
Add 500 pmol of the DNA oligonucleotide that is complementary to the 5′ RNA leader sequence you wish to remove from the RNA transcript.
Bring to the final volume with water.
Heat the reaction to 94 °C for 2 min.
Remove from the heat block and allow 5 min for the tube to cool to room temperature.
Add 6 U of RNase H per 1 nmol RNA. Mix by pipetting.
Incubate the reaction at 37 °C for 1 h.
Follow Subheading 3.3 to purify the RNase H processed molecules by denaturing PAGE (see Note 12 and Fig. 2).
3.5. Two-Stranded Switch Assembly from Individual Component Strands
For a 10 μL final assembly volume, combine stoichiometric quantities of RNA monomer stands in a 1.5-mL microcentrifuge tube to achieve the desired nanoconstruct concentration (see Note 13). Add water to bring the total volume to 8 μL (see Note 14).
Heat the assembly mixture to 94 °C for 2 min to denature the monomer strands.
Quickly transfer the assembly mixture from 94 °C to a 55 °C heat block. Allow the mixture to incubate at 55 °C for 10 min (see Note 15).
Add 5× assembly buffer to the mixture to achieve a final concentration of 1× assembly buffer with respect to the final volume. Incubate the assembly mixture for an additional 10–15 min.
Remove the assembly from the heat block and allow to cool to room temperature.
Spin the assembly mixture in a tabletop centrifuge for 30 s at room temperature to allow any condensation on the walls of the tube to collect in the bottom of the tube.
Assembled RNA nanoconstructs can be stored short term (hours) at 4 °C. For longer term storage, store assembled constructs at −20 °C (see Note 16).
See Subheading 3.7 for verification of switch assembly and function by nondenaturing PAGE.
3.6. Native Purification of RNA Switch by Nondenaturing PAGE
Before purifying a large quantity of assembled RNA switch for the first time, it is recommended to first verify that strand assembly is occurring. For a detailed description and protocol for analytical PAGE analysis, see Subheading 3.7.
Prepare a nondenaturing PAGE gel with a thickness of 0.8 mm. For a 16.5 cm × 28 cm gel prepare 50 mL of nondenaturing gel solution. Initiate polymerization by adding 300 μL of 10% APS solution and 40 μL TEMED (see Note 9). Insert a comb that produces wells with a volume of 80–100 μL. This should result in 13–15 wells across a 16.5 cm wide gel.
Once polymerized, set up the PAGE system in a refrigerated environment (4–6 °C) using 1× native gel running buffer.
Using a syringe or transfer pipet, rinse the wells of the gel with 1× native gel running buffer to remove any gel debris or air bubbles.
Prerun the gel at 4 W for 10 min while preparing RNA samples for electrophoresis.
Prepare approximately 0.5 nmol of assembled RNA switch in a 50 μL volume according to the protocol presented in Subheading 3.5 (see Note 17).
Add 0.2 volumes of native gel loading buffer to each assembled sample to be purified (for 50 μL of assembled construct, add 10 μL of loading buffer).
Load the nondenaturing PAGE gel with the constructs to be purified. If running a 16.5 cm × 28 cm gel, perform electrophoresis at a constant power of 6–8 W. This can be increased to 14–16 W if using a larger 33 cm × 39 cm gel. Run the electrophoresis until sufficient separation has been achieved, approximately 4 h (see Notes 18 and 19).
Following electrophoresis remove the gel from the glass plates and visualize the location of the assembled construct using a UV lamp. Refer to Subheading 3.3, steps 8–11 for details.
Cut out the area of gel containing the assembled construct using a scalpel blade.
Carefully transfer the excised gel piece to a flat-bottomed 2-mL microcentrifuge tube. Place the piece of gel in tube so that the gel lies flat on the bottom of the tube (see Note 20 and Fig. 3).
Add 50–60 μL of 1× assembly buffer directly on top of the gel piece(s) in the flat-bottom tube. This volume should be sufficient to completely cover the gel piece if lying flat on the bottom. Store overnight at 4 °C to allow the construct to elute from the gel.
The following day, remove the assembly buffer used to elute the gel-purified RNA construct and transfer it to a new tube. The concentration of the assembled nanoconstruct can be determined by OD260 (see Note 21). The assembled construct can be used immediately or stored at −20 °C (see Note 16).
Verify the purity of the gel-purified construct by running on a nondenaturing PAGE gel along with an unpurified assembly and monomeric strand controls (Fig. 3). Refer to Subheading 3.7 for details regarding analytical nondenaturing PAGE analysis.
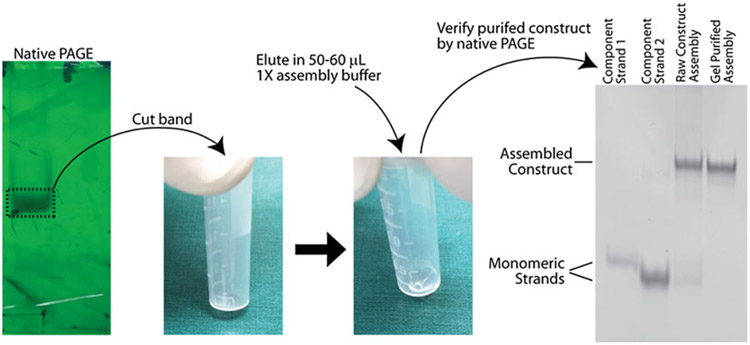
Fig. 3.
RNA nanoconstructs purified by nondenaturing PAGE. After performing electrophoresis in non-denaturing conditions, the RNA can be located by visualizing the gel under UV light. The RNA is cut form the gel and eluted in 1× assembly buffer overnight. It is important the gel lies flat on the bottom of a centrifuge tube to ensure maximal interface between the gel and buffer. The purity of the eluted nanoconstruct can be qualitatively assessed by performing analytical nondenaturing PAGE with monomeric and unpurified assembly controls. Assembly of the switch from component anti-trigger and anti-target strands is not 100% efficient and some monomeric strands remain. Native PAGE purification removes any unassembled monomer strands, yielding only the assembled switch
3.7. Verification of Switch Assembly and/or Function by Nondenaturing PAGE Analysis
Prepare a nondenaturing PAGE gel as described in Subheading 3.6, steps 1–4.
Prepare RNA samples in 10 μL volumes containing a 1× concentration of assembly buffer. The concentration of each RNA should be approximately 1 μM. If assessing switch assembly, prepare one tube containing the assembled switch and control tubes containing individual component strands (anti-trigger and anti-target). If assessing switch function, prepare one tube containing the gel-purified switch incubated with the trigger RNA, as well as appropriate molecular controls. These controls may include the gel-purified switch, individual RNA molecules (anti-trigger, anti-target, trigger RNA), and combinations of the RNA molecules (anti-trigger with trigger, anti-target with trigger) (see Note 22 and Fig. 4).
Add 0.2 volumes of native gel loading buffer to each sample tube (for a 10 μL sample add 2 μL of loading buffer).
Rinse the wells after prerunning the nondenaturing PAGE gel and load the samples.
Perform electrophoresis by applying a constant power of 8–10 W for a 16.5 cm × 28 cm gel. Increase this to 16–20 W for 33 cm × 39 cm gel. Run the gel at constant power for approximately 3 h (see Note 18).
Following electrophoresis, remove the gel from the stand and carefully remove one of the glass plates. Place the plate that retained the gel in a secondary container, gel side up.
Pour a nucleic acid stain over the gel; enough to cover the entire gel with a thin layer of stain solution (see Note 23). Allow the stain to sit and permeate the gel for 10–15 min. If the gel appears dry, add additional stain solution.
Remove the stain solution by tilting the gel and allowing the stain to flow off into the secondary container (see Note 24).
Rinse the gel with deionized water to remove any excess stain. Use paper towels to blot excess water around the gel and remove any gel or salt residue from the bottom of the plate, as these can decrease the quality of the image taken.
Use a fluorescence gel scanner to image the gel. Use appropriate excitation and emission filters based on the nucleic acid stain used.
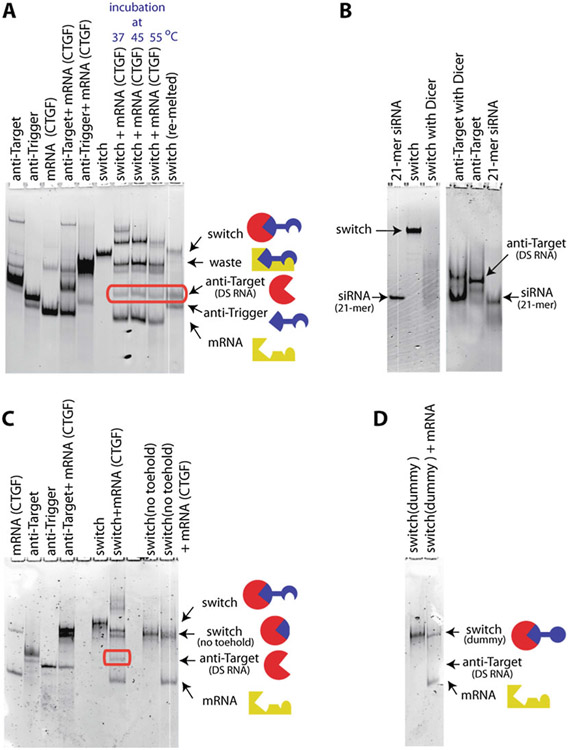
Fig. 4.
Total staining RNA native PAGE results demonstrating the proper assembly and function of the RNA switch. (a) Lanes 1–5 contain molecular controls, including various combinations of anti-target strand, anti-trigger strand and mRNA fragment. The lane labeled “switch” corresponds to the gel-purified switch, assembled from anti-target and anti-trigger strands. Lanes 7–9 demonstrate for several temperatures that the RNA switch releases the functional anti-target RNA in the presence of the trigger mRNA fragment. (b) Dicer processing of the RNA switch. Lanes 1 & 6: control of 21-mer siRNA. Lanes 2–3 show that Dicer can degrade the assembled 2-stranded switch. The degradation products do not correspond, however, to distinct bands (lane 3). Lanes 4–5 demonstrate that the Dicer enzyme processes the anti-target strand into a 21-mer siRNA strand. (c) A version of the RNA switch where the anti-trigger strand has no toehold does not lead to the release of the functional anti-target strand. (d) Similarly, a version of the RNA switch where the anti-trigger strand has a toehold sequence with scrambled (i.e., randomized) nucleotide content does not lead to the release of the functional anti-target strand. Adapted and reprinted with permission from [18], copyright 2016 American Chemical Society
3.8. Verification of Switch Function in Cultured Human Cells
It is important to use a cell line that that expresses both the trigger and target gene. In the case of the CTGF/eGFP switch, we use a human breast cancer cell line, MDA-MB 231, that stably expresses eGFP. If other trigger–target pairs are to be used, consult the literature to find an appropriate cell line. All cell culture experiments should be performed in an appropriate biosafety cabinet. All cell culture experiments should be prepared using RNase-, DNase-, and endotoxin-free water and solutions. Cells are cultured at 37 °C with 5% CO2 and 85–90% humidity. All cell culture media should be pre-equilibrated to 37 °C before use.
3.8.1. Preparing Cells in a 24-Well Plate for RNA Transfection
Culture cells in a flask with complete growth medium until 90% confluence is reached.
Add cell dissociation buffer to lift cells. Incubate the flask at 37 °C until cells have completely detached, but no longer than 20 min (see Note 25).
Add an equal volume of complete growth medium to the flask.
Transfer dissociation buffer/medium/cell solution to a 15-mL tube.
Spin down the cells in a centrifuge at 500 × g for 5 min.
Aspirate medium from the 15-mL tube, leaving pelleted cells in the bottom.
Add 1 mL of complete growth medium to cells. Mix well by pipetting.
Count cells using a grid slide and an optical microscope.
Add between 5000 and 30,000 cells in complete growth medium to each well of a 24-well plate, depending on the length of the experiment. For longer transfection experiment, fewer cells should initially be plated, so they do not become overconfluent. The final well volume should be 500 μL.
Allow cells to grow in incubator for 24 h.
3.8.2. Verification of Gene Silencing by Two-Stranded RNA Switch
Follow Subheading 3.7, step 1 to prepare a 24-well plate seeded with 30,000 cells per well.
In sterile microcentrifuge tubes, prepare RNA switch and individual strand controls at 50× concentration in a 10 μL volume. The final 1× RNA concentration should be between 1 and 50 nM. Each sample should be prepared in 1× assembly buffer. A 10 μL sample of only 1× assembly buffer should be prepared as a negative control.
Prepare a second set of sterile tubes containing 2 μL Lipofectamine 2000 (L2K) and 20 μL Opti-MEM medium.
Add 10 μL of the prepared 50X RNA solution to the tubes containing L2K–Opti-MEM. Mix by pipetting.
Incubate RNA–L2K–Opti-MEM mixture at room temperate for 20 min.
Add 470 μL Opti-MEM to the RNA–L2K–Opti-MEM mixture. Mix by pipetting.
Aspirate growth media from a well of the 24-well plate and quickly add 500 μL of the corresponding RNA–L2K–Opti-MEM mixture.
After addition of all samples to the plated cells, incubate the plate at 37 °C for 4 h.
After 4 h, aspirate the transfection mixture from each well of the 24-well plate and replace with 500 μL of complete growth medium.
Place the 24-well plate in the incubator for additional 68 h (3 days total).
After 3 days, aspirate the growth medium and wash wells with 1× PSB.
Add 325 μL of cell dissociation buffer to each well and place in incubator for 15–20 min.
Rinse the bottom of each well with the added dissociation buffer and transfer to a new vessel appropriate for use in flow cytometry analysis. Place the vessels in ice.
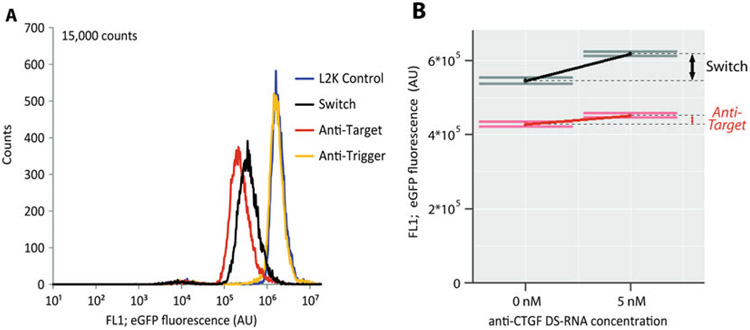
Perform flow cytometry to analyze the extent of switch function by measuring the level of eGFP expression in each sample (Fig. 5a).
Fig. 5.
Analysis of switch function using flow cytometry. As the RNA switch described throughout the protocol targets the knockdown of eGFP, flow cytometry can be used to measure switch activity. (a) Analysis of eGFP expression reveals that MDA-MB 231 cells transfected with the CTGF/eGFP RNA switch show a significant reduction in eGFP levels 3 days post transfection. (b) To assess how trigger RNA (CTGF mRNA) expression levels affect switch function, MDA-MB 231 cells were initially transfected with a Dicer substrate RNA (DS-RNA) against CTGF. After 2 days, cells were transfected with the RNA switch or anti-target control, targeting eGFP knockdown. Three days later eGFP expression level was analyzed. Cells that were initially transfected with the anti-CTGF DS-RNA saw a large increase in eGFP expression compared to cells were no anti-CTGF DS-RNA was transfected, indicating reduced switch activation. Cells transfected with the anti-target control displayed minimal changes in eGFP expression as a function of anti-CTGF DS-RNA. Adapted and reprinted with permission from [18], copyright 2016 American Chemical Society
3.8.3. Sequential Transfection to Knock Down RNA Trigger Expression
This protocol is use to knock down expression of the endogenous RNA trigger molecule prior to transfection with the RNA switch. It allows for examination of switch activity as a function of trigger expression level (Fig. 5b).
Follow Subheading 3.8.1 to prepare a 24-well plate seeded with 5000 cells per well.
In sterile microcentrifuge tubes prepare a Dicer-substrate RNA, targeting the knockdown of the RNA trigger, at 50× concentration in a 10 μL volume. The final 1× RNA concentration should be between 1 and 50 nM. Each sample should be prepared in 1× assembly buffer. A 10 μL sample of only 1× assembly buffer should be prepared as a negative control.
Follow Subheading 3.8.2, steps 3 through 9.
Place the 24-well plate in the incubator for additional 44 h (2 days total).
After 2 days, prepare individual samples of the RNA switch and the anti-target strand at 50× concentration in a 10 μL volume in sterile microcentrifuge tubes (see Note 26). The final 1× RNA concentration should be between 1 and 50 nM. Each sample should be prepared in 1× assembly buffer. A 10 μL sample of only 1× assembly buffer should be prepared as a negative control.
Follow Subheading 3.8.2, steps 3 through 14.
4. Notes
DNA templates for transcription can be purchased from a commercial source or generated by PCR.
The conditions of this buffer have been optimized for use with an in-house produced T7 RNA polymerase enzyme, but also work well with many commercially available T7 polymerases. However, buffer conditions may need to be optimized for different T7pol enzymes. If commercial T7 polymerase is used for transcription, we recommend initially using the buffer provided or suggested by the commercial source.
This buffer may need to be optimized based on the source of the enzyme. Generally, commercial vendors of RNase H will provide or specify a particular reaction buffer for the enzyme. If obtaining RNase H from a commercial source, we recommend initially using the buffer conditions given by the supplier.
Depending on the excitation and emission filters used to image the gel following electrophoresis, xylene cyanol and bromophenol blue may produce a fluorescence signal when imaging.
If this is problematic, either or both dyes can be omitted from the gel loading buffer.
If a fluorescence gel scanner is not available, imaging can be performed by staining the gel with ethidium bromide, visualizing the gel on a UV-transilluminator, and capturing the image with a digital camera. Due to the reduced sensitivity of this technique, the concentration of ethidium bromide solution used for staining may need to be increased above the 0.5 μg/ mL suggested in the main text.
Choose an appropriate cell line based on the trigger and target of the RNA switch. Based on the choice of cell line used, the complete growth medium may need to be adjusted.
We find that Lipofectamine 2000 works very well for transfection of cultured cells and the transfection protocol outlined in Subheading 3.8 is based on the use of Lipofectamine 2000. Other transfection agents could be used in conjunction with the RNA switch; however, transfection protocols would likely need to be tailored and optimized for different transfection agents.
The transcription buffer contains a relatively high concentration of Mg2+ ions, which may promote backbone hydrolysis of the RNA and reduce full-length transcript yield if left for an extended duration. It is advised to purify RNA transcripts immediately following transcription.
TEMED should always be added immediately prior to pouring the gel, as polymerization will begin as soon as TEMED is added.
For denaturing PAGE gels, the power supplied should be sufficient to heat the gel approximately 45–50 °C. If the gel feels extremely hot to the touch while running, reduce the applied power. Many power supplies support the use of an electronic temperature probe that can be attached to the outside of the glass plate during electrophoresis, if desired. Adhesive temperature indicator strips can also be purchased and placed on the glass plate. The duration of electrophoresis should be optimized based on the length of molecules and the conditions of the gel to ensure adequate separation of the full-length and incomplete transcripts. Marker dyes within the urea blue loading buffer (xylene cyanol and bromophenol blue) can be used to estimate the migration distance of transcripts.
We suggest a final reaction volume in the range of 50–200 μL. A 50 μL reaction should contain enough RNA to visualize within a PAGE gel by shadowing with a UV lamp. However, we do not suggest larger reaction volumes than 200 μL to ensure the entire reaction contents can be loaded into a single well of a polyacrylamide gel for the purpose of purification.
To ensure that cleavage of the 5′ RNA leader sequence by RNase H has occurred, prepare a negative control sample of the transcript that has not be subjected to the RNase H protocol. Run this control side by side with the RNase H processed transcript on the denaturing PAGE gel used for purification as a means to visually confirm the reduction in length of the RNase H processed molecules.
For the purpose of verifying switch assembly by nondenaturing PAGE, preparing a 10 μL sample at a final construct concentration of 1 μM is generally satisfactory. If the assembly is to be gel-purified, we suggest preparing a 50 μL sample at a final assembled concentration of approximately 10–12 μM.
Because a concentrated 5× assembly buffer will be added at a later step, the initial preparation of monomer strands in water should be prepared to 4/5 of the final desired volume.
The incubation step at 55 °C is designed to allow the thermodynamically most-favorable intermolecular interactions to form, while preventing formation of less stable alternative folds. We find that incubation at 55 °C works best for the CTGF/eGFP two-stranded switch. However, if variant switches are being assembled, we suggest trying assembly temperatures ranging between 45 and 65 °C to find the optimal condition to promote proper nanoconstruct assembly.
If assembled RNA constructs have been stored for an extended duration of time, or repeatedly thawed and frozen, we recommend checking the composition of the construct by both native and denaturing PAGE to verify that the assembly is intact and no strand degradation has occurred.
Due to several factors including the efficiency of nanoconstruct assembly, the need to identify the nanoconstruct’s location in the gel by UV shadowing, and the efficiency of RNA elution from polyacrylamide gels, the starting quantity of the RNA nanoconstruct must be relatively large to purify by nondenaturing PAGE. Generally, a 50 μL volume of assembled construct at a concentration of 10 μM is sufficient to perform purification.
The power supplied to the gel should be low enough that the gel does not warm above ~10 °C. Keeping the gel cool will ensure that the assembled nanoconstructs do not denature during electrophoresis.
Due to the large quantity of RNA loaded in an individual well during nondenaturing PAGE purification, running the gel slowly at fairly low power helps to reduce the extent of streaking tails as the band of RNA migrates through the gel. The duration of electrophoresis should be sufficient to clearly separate the assembled nanoconstruct from any partial assemblies or monomeric strands. This can be optimized by performing nondenaturing PAGE electrophoresis using relatively small amounts of assembled RNA and visualizing the migration using fluorescent nucleic acid stains (described in Subheading 3.7)
It may be necessary to cut the gel piece into multiple smaller sections so that the pieces fit flat on the bottom of the tube. If this is the case, place each gel section from a single band into a single tube to maximize the final concentration of RNA construct eluted. Try to position the gel pieces to minimize gel overlap and maximize exposure to the elution buffer. Use of a pipette tip may be helpful to position the sections of gel flat on the bottom of the tube.
Due to the extensive base pairing formed within an assembled RNA nanoconstruct, the RNA should be denatured to obtain an accurate measure of concentration by OD260. This can be accomplished by measuring absorbance at a temperature above the melting temperature of the RNA, or by adding a denaturant and briefly heating the RNA to 94 °C. We find that adding a sample of the RNA assembly to a 6 M urea, 20 mM EDTA solution (final concentrations), heating for 1 min to 94 °C, and allow the solution to cool to room temperature is sufficient to denature most RNA constructs.
The trigger RNA can be synthesized using in vitro transcription and PAGE purification as described in Subheadings 3.2 and 3.3. If the switch is designed to trigger upon interaction with an endogenous mRNA, the entire mRNA does not need to be synthesized for use in nondenaturing PAGE analysis, but only a portion of the mRNA sequence that contains the region complementary to the anti-trigger molecule.
Nucleic acid stains pose potential health hazards. These stains should be handled and disposed of in accordance with local environmental health and safety practices.
Nucleic acid stains such as ethidium bromide, SYBR Gold, and SYBR Green II can be collected post-staining and reused multiple times until the extent of staining is no longer satisfactory. Dispose of the stain in accordance with local environmental health and safety practices.
If cells have not completely detached after 20 min, try washing the bottom of the flask with the cell dissociation buffer that has already been added to the flask.
The anti-target molecule should be used as an internal control and be subjected to identical conditions as the switch samples. The ability of the anti-target molecule to silence eGFP should not be affected by the knockdown of RNA trigger levels. As such, transfection of the anti-target molecule provides a control measurement indicating the maximal extent of possible target knockdown, regardless of endogenous RNA trigger expression levels.
Acknowledgments
This work has been funded in whole or in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under Contract No. HHSN261200800001E. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Grabow WW, Jaeger L (2014) RNA self-assembly and RNA nanotechnology. Acc Chem Res 47(6):1871–1880 [DOI] [PubMed] [Google Scholar]
- 2.Guo P (2010) The emerging field of RNA nanotechnology. Nat Nanotechnol 5 (12):833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batey RT, Rambo RP, Doudna JA (1999) Tertiary motifs in RNA structure and folding. Angew Chem Int Ed Engl 38(16):2326–2343 [DOI] [PubMed] [Google Scholar]
- 4.Butcher SE, Pyle AM (2011) The molecular interactions that stabilize RNA tertiary structure: RNA motifs, patterns, and networks. Acc Chem Res 44(12):1302–1311 [DOI] [PubMed] [Google Scholar]
- 5.Parlea L et al. (2016) Ring Catalog: a resource for designing self-assembling RNA nanostructures. Methods 103:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bindewald E, Hayes R, Yingling YG, Kasprzak W, Shapiro BA (2008) RNAJunction: a database of RNA junctions and kissing loops for three-dimensional structural analysis and nano-design. Nucleic Acids Res 36(Database issue): D392–D397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parlea LG, Sweeney BA, Hosseini-Asanjan M, Zirbel CL, Leontis NB (2016) The RNA 3D motif atlas: computational methods for extraction, organization and evaluation of RNA motifs. Methods 103:99–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabow WW et al. (2011) Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett 11(2):878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Severcan I, Geary C, Verzemnieks E, Chworos A, Jaeger L (2009) Square-shaped RNA particles from different RNA folds. Nano Lett 9 (3):1270–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J et al. (2013) Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol Ther 21(1):192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonin KA et al. (2015) Triggering of RNA interference with RNA-RNA, RNA-DNA, and DNA-RNA nanoparticles. ACS Nano 9 (1):251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delebecque CJ, Lindner AB, Silver PA, Aldaye FA (2011) Organization of intracellular reactions with rationally designed RNA assemblies. Science 333(6041):470–474 [DOI] [PubMed] [Google Scholar]
- 13.Afonin KA et al. (2010) In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat Nanotechnol 5(9):676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Liu Z, Jiang W, Wang G, Mao C (2015) De novo design of an RNA tile that self-assembles into a homo-octameric nanoprism. Nat Commun 6:5724. [DOI] [PubMed] [Google Scholar]
- 15.Zalatan JG et al. (2015) Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160 (1–2):339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afonin KA et al. (2016) The use of minimal RNA toeholds to trigger the activation of multiple functionalities. Nano Lett 16 (3):1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochrein LM, Schwarzkopf M, Shahgholi M, Yin P, Pierce NA (2013) Conditional Dicer substrate formation via shape and sequence transduction with small conditional RNAs. J Am Chem Soc 135(46):17322–17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bindewald E et al. (2016) Multistrand structure prediction of nucleic acid assemblies and design of RNA switches. Nano Lett 16(3):1726–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakrevsky P, Bindewald E, Shapiro BA (2016) RNA toehold interactions initiate conditional gene silencing. DNA RNA Nanotechnol 3 (1):11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res 15 (21):8783–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Li T, Dang Y, Feng Y, Huang P (2005) A novel in vitro transcription method for producing siRNAs without specific sequence requirements. Mol Biotechnol 31(3):187–192 [DOI] [PubMed] [Google Scholar]