ABSTRACT
Background
Amikacin monotherapy is recommended for urinary tract infection (UTI) treatment with multi-resistant pathogens. Even though amikacin efficacy in the treatment of UTIs is dependent on its urinary concentration, there are no robust data proving that sufficiently high urinary concentration is reached in patients with reduced glomerular filtration rate (GFR).
Methods
A prospective study to monitor amikacin penetration into urine of 70 patients [40 males, median (interquartile range) age 70 (65–79) years] with different levels of glomerular filtration decline, including patients treated by dialysis, was conducted. The bactericidal efficacy of amikacin in urine samples has been evaluated.
Results
Patients with estimated GFR (eGFR) <30 mL/min had significantly lower median amikacin urinary concentration than patients with eGFR >30 mL/min (89.75 vs 186.0 mg/L, P < .0001; 200.5 vs 830.0 mg/L, P < .0001; and 126.0 vs 408.0 mg/L, P < .0001 for minimal, maximal and minimal together with maximal concentrations, respectively). The amount of amikacin eliminated in the first 10–13 h after dose administration was dependent on eGFR (r2 = 0.6144, P < .0001). The urinary concentration of amikacin in patients treated by dialysis was indirectly proportional to pH of urine. The plasma concentrations of amikacin did not correlate with urinary levels in patients in either of the GFR categories. Microbiological evaluation showed that the critical urinary concentration for efficacy of amikacin during UTI monotherapy in patients treated by dialysis is 100 mg/L. We found that 4 out of 11 patients treated by dialysis did not reach this level during the treatment.
Conclusion
Systemic administration of amikacin monotherapy in patients treated by dialysis is questionable as the concentrations of amikacin in their urine are often below the threshold of effectivity. Amikacin plasma concentrations are not a major determinant of amikacin concentration in urine, therefore pulse dosing is neither necessary nor safe in patients treated by dialysis, and may cause undesirable toxicity.
Keywords: aminoglycosides, dialysis, kidney impairment, pharmacokinetics, UTI
KEY LEARNING POINTS.
What was known:
Amikacin clearance depends on glomerular filtration rate.
Amikacin concentration in urine of patients with impaired kidney function should therefore be lower than in patients with preserved renal function.
Direct measurement of amikacin concentration in urine of patients with kidney impairment was not available.
This study adds:
Information about urinary amikacin concentration in patients with various severity of kidney dysfunction.
Direct microbiological evaluation of the bactericidal activity of amikacin in the urine of patients with kidney impairment.
Evaluation of covariates that influence the amikacin concentration in the urine of patients with kidney impairment.
Potential impact:
Systemic administration of amikacin as monotherapy for urinary tract infection in dialysis-dependent patients does not provide reliable amikacin concentrations in the urine; therefore, other administration routes (e. g. direct instillation into urinary bladder) should be considered.
INTRODUCTION
Amikacin (AM) is an antimicrobial agent recommended for the treatment of acute complicated urinary tract infections (UTIs) and acute pyelonephritis caused by antibiotic-resistant G-organisms [extended spectrum beta-lactamase (ESBL)-producing, multi-drug resistant Pseudomonas and Acinetobacter spp.] [1]. It is a highly hydrophilic drug virtually unbound to plasma proteins that is almost entirely eliminated by glomerular filtration. Kirby et al. recovered 94% of AM dose in the urine collected during 24 h after administration to healthy volunteers [2], confirming the theoretical expectation of high concentrations of AM in urine and making it an optimal antimicrobial agent for the treatment of UTIs. High drug penetration into the urine was also proved for a similar antibiotic, gentamicin, in patients with preserved kidney function treated for UTIs [3, 4]. However, there are no direct data on AM urinary exposure in patients with decreased glomerular filtration rate (GFR). There is only one study that included patients treated by aminoglycosides (AG) with reduced GFR, which reported mean urinary concentrations of gentamicin and tobramycin at 48 h after therapy, but no correlation of these urinary drug concentrations with patients’ GFR can be inferred from the sporadic data [5]. Since the clearance of AM is strongly dependent on the GFR [6], the theoretical assumption is that a significantly lesser amount of drug is filtered into the urine in patients with reduced GFR, while reliable data quantifying urinary AM concentrations in these patients are virtually nonexistent. Decreased urinary AM concentrations could be expected particularly in patients in whom reduced GFR is accompanied by preserved urine output.
Moreover, the pharmacodynamic activity of AM may be decreased in urine, possibly requiring higher target concentrations to be achieved compared with those seen in plasma. It has been reported that AG efficacy is highly pH dependent. The minimum inhibitory concentration (MIC) of gentamicin for Staphylococcus aureus rises up to 70-fold at pH 5, which is not an unusual urine pH value, when compared with the MIC at pH 7.4 [7]. Similar results were also described for gentamicin/Escherichia coli and tobramycin/Pseudomonas aeruginosa, while the post-antibiotic effect was also shortened in an acidic environment [8]. Another study further revealed that ion concentrations greatly influenced the efficacy of gentamicin [9].
As there are no robust experimental data available for correct dose selection in this patient population, several recommendations propose switching to lower AM doses with prolonged dosing interval (i.e. a “conventional” dosing regimen with extended dosing intervals) when estimated GFR (eGFR) decreases below 20–40 mL/min [10–12]. The appropriateness of AM for the treatment of UTIs in patients with significantly reduced GFR is controversial as it has never been demonstrated that the drug reaches an efficacious concentration in urine in this patient population and extrapolated evidence from subjects with normal GFR is likely not relevant.
The aim of this study was to determine pharmacokinetic (PK)/pharmacodynamic suitability of systemic administration of AM for the treatment of UTI by measuring the concentrations of AM in the urine of patients with decreased GFR, including patients treated by dialysis. Furthermore, the efficacy of antibiotics in the urine of patients with various GFRs was directly measured via microbiological methods.
MATERIALS AND METHODS
Patients and sampling
A prospective study was performed in The Nephrology Department of the General University Hospital, Prague, Czech Republic and 3rd Department of Surgery of the Motol University Hospital, Prague, Czech Republic between August 2019 and December 2022. All patients signed informed consent and the study was approved by the local ethic committee under the number 69/17. The study was carried out in compliance with the ethical principles of the Helsinki Declaration.
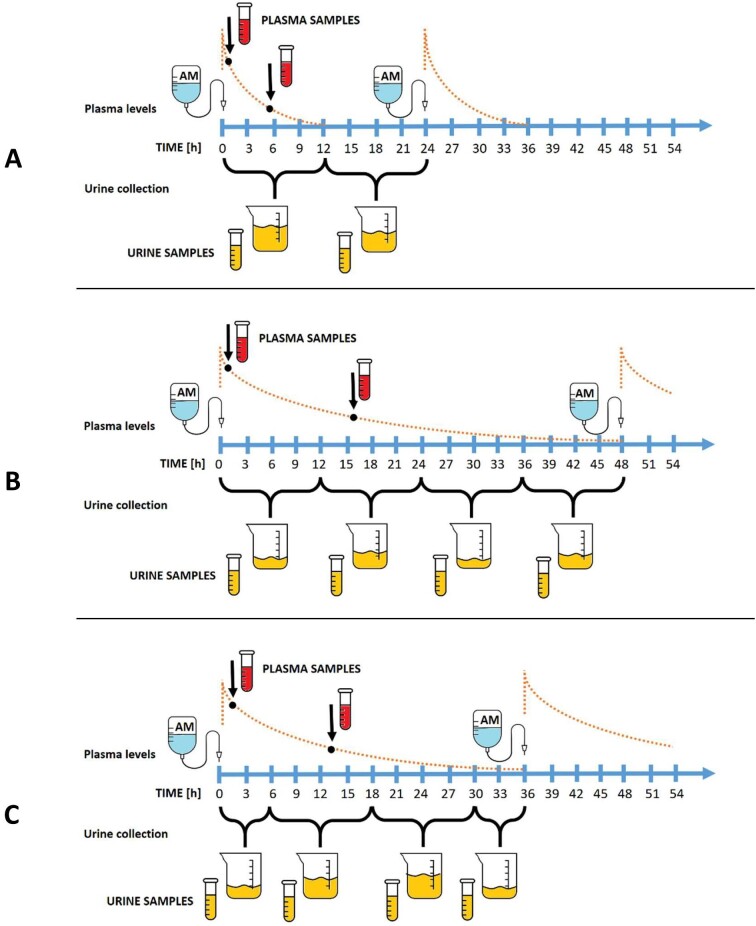
Patients were offered participation in the study if they were treated by AM according to recommendation of antibiotic center, regardless of the indication. The choice of the antibiotic was fully independent of the study. Urine from patients willing to participate in the study was collected for further analysis of AM concentration. The urine samples were collected according to clinical suitability most commonly in two daily intervals, 12 h each. The total length of urine collection was determined according to AM dosing interval with the intention to cover at least one complete dosing interval. During the one dosing interval, also two AM serum concentrations were drawn according to hospital therapeutic drug monitoring (TDM) guidelines. Figure 1 depicts the scheme of the study sampling. The beginning of sample collection was preceded by complete emptying of the bladder or replacement of urinary bag if the patient had an indwelling urinary catheter, and the urine collection started with the beginning of AM infusion administration. The volume and pH of each collected urine sample was recorded immediately after the collection. The aliquot samples (∼30 mL) were taken from each collected urine sample and stored in the deep freeze at −80°C until analyzed for AM levels. After the AM concentrations were determined, chosen samples were tested for microbiological activity. The samples were selected to cover the whole range of AM concentrations found in the urine and to test the efficacy of AM monotherapy and AM combinations with beta-lactams and other antibiotics. Information about dosing and serum AM concentrations were recorded from standard TDM protocols. Current creatinine level was recorded and individual eGFR to categorize kidney functions was calculated according to the Chronic Kidney Disease Epidemiology Collaboration 2009 formula normalized for body surface are (BSA). Weight and height was recorded, and BSA calculated according to DuBois formula. Other antibiotic co-medication was also recorded.
Figure 1:
Scheme of plasma and urine sampling. (A) Patient with normal GFR with 24-h dosing interval; (B) patient with severely decreased GFR with 48-h dosing interval; (C) patient with moderately reduced GFR with 36-h dosing interval. The urine sampling was irregular due to the clinical issues. AM: infusion of amikacin.
AM biochemical analysis
AM levels in plasma samples were determined using an EMIT (Enzyme Multiplied Immunoassay Technique) method (Siemens Healthcare Diagnostics Inc., USA), measured on a DxC 700 AU analyzer. The analytical measuring range for plasma samples is 2.5–50 mg/L. When higher levels were detected, the samples were diluted to obtain measurable values that were subsequently multiplied according to the dilution factor. The method was validated for urine samples by measuring a series of AM concentrations between 6.25 and 50 mg/L. The calibration curve derived from sample triplets is depicted in Fig. 2. A high level of reproducibility was obtained when samples were reevaluated after a year in the deep freeze.
Figure 2:
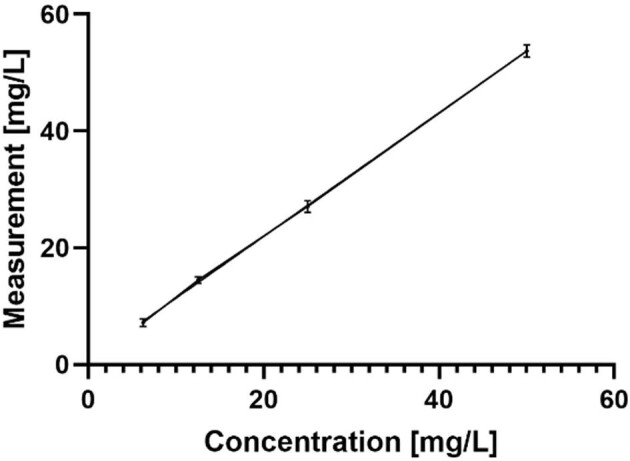
Calibration curve of AM in urine.
Microbiological analysis
The microbiological evaluation of the bactericidal activity of the urine samples with AM was adapted from the study by Wise et al. [13]. The urine samples were diluted with tryptic soy broth in dilution sequences 1:2 from 1- to 1024-fold. Strain of E. coli CNCTC 5276 was cultivated for 24 h in 37°C on Columbia blood agar. Bacterial suspensions of 0.5 McF (108 CFU/mL) were subsequently prepared. These suspensions were further diluted in tryptic soy broth in a ratio of 1:100. Furthermore, 100 µL of diluted samples were then transferred to every well in a 96-well plate and incubated for 24 h at 37°C. Ten microliters of broth in wells that were not cloudy were then transferred to the Columbia blood agar and incubated for 48 h at 37°C. The first dilution was regarded as bactericidal if 99.9% of bacteria growth was suppressed.
Pharmacokinetic analysis
As recommended approach for TDM in our facilities is to measure at least two levels in one dosing interval to characterize PK parameters, we were able to determine the elimination constant according to the formula:
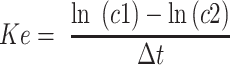 |
where Ke is the elimination constant; c1 and c2 are the first and second plasma concentrations; and ∆t is the time between c1 and c2, and half-life according to formula:
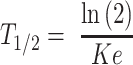 |
where Ke is the elimination constant; and T1/2 is the half-life.
From T1/2 and measured plasma AM concentrations, peak concentration 1 h after the beginning of the infusion was calculated for each patient to compare the levels between the patients and for further analysis.
Statistical analysis
Median and interquartile range (IQR) were calculated using MS Excel 2010 (Microsoft Corporation, Redmond, WA, USA). The effects of categorical and continuous variables on AM PK were assessed using the Mann–Whitney U test and linear regression model, respectively. GraphPad Prism 8.2.1 software (GraphPad Inc., La Jolla, CA, USA) was used for evaluation of all relationships and P < .05 was considered as statistically significant.
RESULTS
During the study period, 70 patients (40 men, 30 women) were enrolled into the study with 224 urine samples and 200 AM plasma levels available for PK analysis. Fifty-five urine samples were tested by microbiological methods. Basic demographic parameters of the studied population are listed in Table 1.
Table 1:
Basic demographic characteristics of patients in the study.
| Characteristic | Median (IQR) |
|---|---|
| Age (years) | 70 (65–79) |
| Weight (kg) | 81 (68.25–94.75) |
| Body surface area (BSA) according to DuBois (m2) | 1.97 (1.78–2.11) |
| eGFR (mL/min) | 46.5 (16.25–86.5) |
| Treated by dialysis | 11 |
| eGFR <15 mL/min not treated by dialysis | 6 |
| eGFR 15–30 mL/min | 11 |
| eGFR 30–60 mL/min | 16 |
| eGFR 60–90 mL/min | 12 |
| eGFR >90 mL/min | 14 |
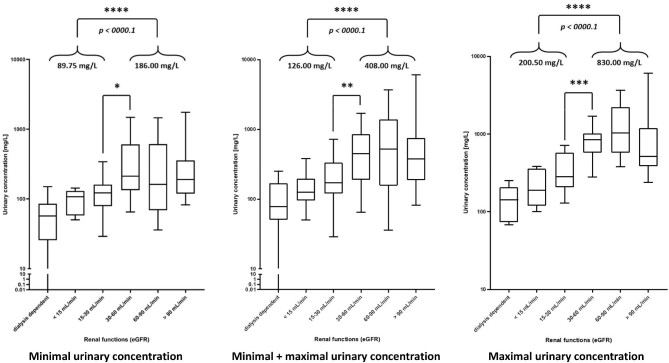
Figure 3 depicts the comparison of the median concentrations measured in urine of patients with various eGFR. The patients are divided according to eGFR into GFR categories. Even though some of the patients had unstable kidney functions, eGFR was calculated according to current creatinine level. As the number of samples differed between the patients, only minimal, maximal and minimal together with maximal concentrations from each patient were used for calculation of the medians in Fig. 3 to ensure equal representation of each patient. In this analysis, patients with eGFR 30–60 mL/min had significantly higher urinary AM concentrations than patients with eGFR 15–30 mL/min [122.0 vs 211.0 mg/L (P = .0151), 282.5 vs 846.0 mg/L (P = .0009) and 171 vs 450.5 mg/L (P = .0057) for minimal, maximal and minimal together with maximal concentrations, respectively] and when taken as a whole, patients with eGFR <30 mL/min had significantly lower AM urinary concentration than patients with eGFR >30 mL/min [89.75 vs 186.0 mg/L (P < .0001), 200.5 vs 830.0 mg/L (P < .0001) and 126.0 vs 408.0 mg/L (P < .0001) for minimal, maximal and minimal together with maximal concentrations, respectively]. This means that urinary concentration is significantly influenced by eGFR. Also, the difference between maximal and minimal levels of AM in the urine is much larger in patients with preserved GFR. The medians of maximal levels increase more rapidly from lower to higher eGFR groups than the medians of minimal levels. Altogether, this shows how patients with lower eGFR lose their ability to concentrate the drug into urine.
Figure 3:
AM urinary concentration (median, 1st and 3rd quartile and IQR) of in patients with various eGFRs.
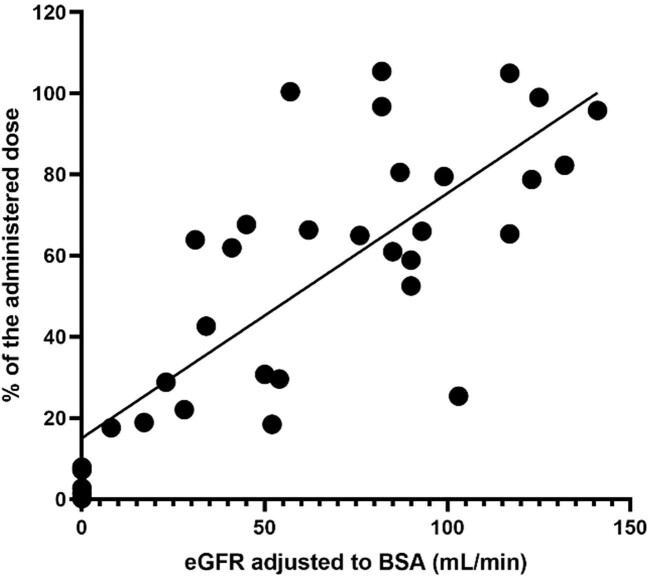
We found a strong correlation of the amount of AM eliminated into the urine and eGFR during the first 10–13 h after AM administration (r2 = 0.6144, P < .0001, Fig. 4).
Figure 4:
Elimination of AM (as % of administered dose) in the first urine collection sample (10–13 h after dosing).
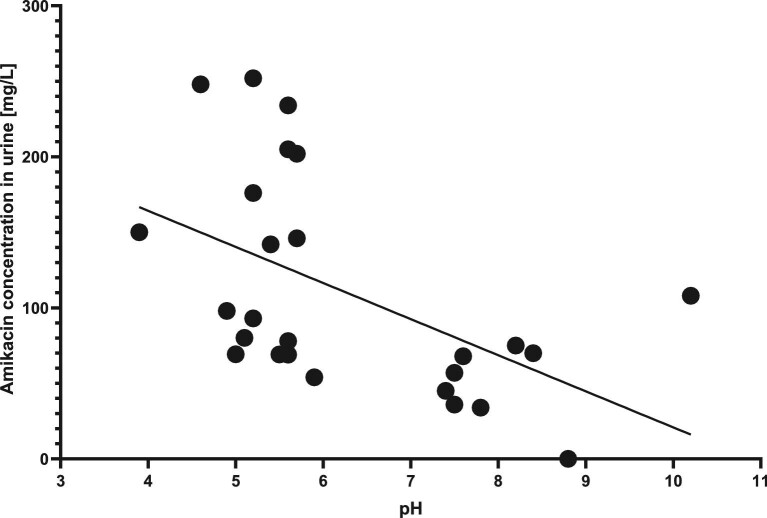
We also found a slight inverse correlation of urinary pH and AM concentration in patients treated by dialysis (r2 = 0.2685, P = .0067) with higher concentrations in lower pH, which supports the mechanism of ion trapping (Fig. 5). We did not find any significant differences between urinary pH in various eGFR groups.
Figure 5:
Linear regression of AM concentration in urine of patients treated by dialysis and urinary pH.
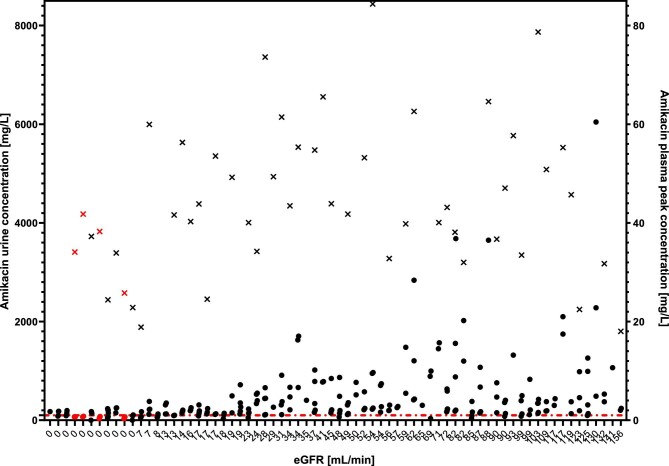
Figure 6 depicts all measured urine concentrations of AM sorted according to patients’ eGFR regardless of urine output. The crosses in Fig. 6 show calculated peak plasma levels of AM. No significant correlation of calculated plasma peak concentrations with the highest or mean urinary concentration in the whole group or in any of the eGFR subgroups was detected.
Figure 6:
All measured urine levels of AM and calculated peak plasma levels of AM. Circles: measured AM urinary concentration; crosses: calculated peak AM plasma concentrations; red line: minimum concentration for reliable efficacy against E. coli (100 mg/L). Patients with all measured urinary concentrations below target concentration are colored in red. 0: patients treated by dialysis.
Microbiological testing
We found a linear correlation between concentration of AM in the urine and bactericidia of the samples of patients where AM was used in monotherapy or with vancomycin (r2 = 0.6677, P < .0001). In the urine of patients treated by dialysis, AM was in relatively low concentrations and almost ineffective against E. coli when used as a single agent or combined with vancomycin, but samples with beta-lactams showed remarkable bactericidia. On the other hand, similar concentrations of AM monotherapy in patients with preserved GFR showed reliable efficacy and the difference between AM monotherapy and combination with beta-lactams was not pronounced (Table 2).
Table 2:
The bactericidal effect of urine of patients treated with AM.
| Pt No. | AM concentration (mg/L) | eGFR (mL/min/1.73 m2) | Antibiotic co-medication | Dilution with bacterial growth (E. coli) |
|---|---|---|---|---|
| Patients treated by dialysis | ||||
| 1 | 69 | N/A | Vancomycin | 1:2 |
| 80 | N/A | <1:2 | ||
| 2 | 0 | N/A | No | <1:2 |
| 176 | N/A | <1:2 | ||
| 142 | N/A | <1:2 | ||
| 3 | 36 | N/A | Vancomycin | 1:2 |
| 57 | N/A | 1:2 | ||
| 75 | N/A | 1:2 | ||
| 70 | N/A | <1:2 | ||
| 4 | 69 | N/A | Meropenem | 1:64 |
| 69 | N/A | 1:128 | ||
| 5 | 205 | N/A | Ampicillin/sulbactam | 1:32 |
| 202 | N/A | 1:128 | ||
| 78 | N/A | 1:128 | ||
| 146 | N/A | 1:256 | ||
| 234 | N/A | 1:256 | ||
| 54 | N/A | 1:128 | ||
| 6 | 150 | N/A | Piperacillin/tazobactam | 1:512 |
| 252 | N/A | 1:256 | ||
| 248 | N/A | >1:1024 | ||
| 7 | 68 | N/A | Imipenem/cilastatin | 1:4 |
| 45 | N/A | 1:8 | ||
| 34 | N/A | 1:4 | ||
| 8 | 108 | N/A | Piperacillin/tazobactam | 1:64 |
| 98 | N/A | 1:32 | ||
| 93 | N/A | 1:32 | ||
| Patients not treated by dialysis | ||||
| 9 | 671 | 75 | No | 1:16 |
| 173 | 1:8 | |||
| 1073 | 1:16 | |||
| 145 | 1:8 | |||
| 10 | 382 | 8 | No | 1:8 |
| 225 | 1:16 | |||
| 218 | 1:16 | |||
| 120 | 1:8 | |||
| 11 | 3681 | 94 | No | 1:32 |
| 1557 | 1:32 | |||
| 878 | 1:16 | |||
| 183 | 1:8 | |||
| 205 | 1:8 | |||
| 12 | 768 | 75 | Amoxicilin/clavulanate | 1:16 |
| 514 | 1:32 | |||
| 1206 | 1:16 | |||
| 116 | 1:8 | |||
| 13 | 846 | 46 | Amoxicilin/clavulanate | 1:16 |
| 193 | 1:16 | |||
| 150 | 1:16 | |||
| 211 | 1:8 | |||
| 14 | 719 | 21 | Metronidazole | 1:8 |
| 268 | 1:4 | |||
| 126 | 1:4 | |||
| 352 | 1:4 | |||
| 183 | 1:4 | |||
| 15 | 1704 | 32 | Ampicillin/sulbactam | 1:64 |
| 664 | 1:64 | |||
| 1628 | 1:32 | |||
The concentration in some of the samples with unmeasurable bactericidia in patients treated by dialysis was as high as 176 mg/L. Owing to the fact that during the microbiological testing the first tested concentration was obtained by diluting the urine by half with the broth, a target concentration in urine >100 mg/L is necessary to achieve a reliable bactericidal effect. This concentration is marked by red line in Fig. 6 and patients that did not reach this concentration in any of their sample are depicted in red. Four out of 11 patients treated by dialysis (36%) did not reach the AM urine concentration >100 mg/L which makes its use questionable in this patient group.
DISCUSSION
We measured AM concentrations in the urine of 70 patients with various eGFRs, including patients treated by dialysis. Urinary concentration was influenced by eGFR (Figs 3 and 4) and urinary pH (Fig. 5). Figure 6 clearly shows that patients with lower eGFR were not able to concentrate the AM in the urine to the same extent as patients with well-preserved GFR. Nevertheless, in patients with well-preserved GFR the concentrations of AM in urine varied largely as they were very high in the initial portions after the drug administration and fell rapidly to often very low levels in the subsequent portions, which is in line with the once daily dosing that allows AM to be fully eliminated in the first half of the dosing interval with subsequent washout period. This difference between the initial and washout period was completely missing in patients with eGFR below 18 mL/min. We found out that the maximal achievable AM concentrations in urine of patients with diminished GFR are approximately 1-fold lower than in patients with well-preserved GFR, and plasma levels do not correlate with urinary concentration in any of eGFR groups. This is of high importance, as during UTI treatment the urinary levels of antibiotics are crucial [14], and our finding therefore makes the use of once daily dosing strategy for UTI treatment in patients with reduced kidney function questionable. Administration of lower doses over extended intervals would lead to similar urinary concentration and potentially lower risk of systemic toxicity. This is in line with various current guidelines that do not recommend once-daily dosing in patients with markedly reduced GFR [12].
By means of direct microbiological testing of urine samples we found that, in a rather high number of patients treated by dialysis, AM monotherapy is not effective against susceptible bacteria even though the reported MIC is exceeded. When AM is combined with beta-lactam in the urine of patients treated by dialysis, the bactericidal effect is much more reliable, probably driven by the beta-lactam efficacy or synergy. We did not observe this difference between AM monotherapy and combined therapy in patients that were not treated by dialysis (Table 2). This may point to the importance of urine composition which is altered in patients treated by dialysis and may hamper the efficacy of AM. It is well described that AGs are more effective in alkaline pH [7]. Nevertheless, pH is not the only factor influencing AGs efficacy. Minuth et al. proved that the urine of uremic patients decreased the effect of gentamicin on P. aeruginosa and E. coli even when adjusted to alkaline pH. The inhibitory effect of uremic urine may therefore be the result of an increased concentration of urinary solutes that directly inhibit AG efficacy rather than the effect of osmolality or pH. When the authors diluted gentamicin in pooled human urine and then further diluted these samples, they found out that the more concentrated samples were less bactericidal against P. aeruginosa than the diluted samples, even though the pH was adjusted to 5.5 in all samples [9]. This shows that sometimes a larger amount of preserved diuresis may be of an advantage even though it dilutes the relatively smaller amount of eliminated AM to lower concentrations in patients treated by dialysis.
Four out of 11 patients treated by dialysis in our study did not reach reliable levels for AM monotherapy in their urine (red symbols in Fig. 6). This questions the administration of AM as monotherapy in patients treated by dialysis simply because it does not reach effective concentrations to overcome the antagonistic effect of uremic urine itself. This is a similar situation to that formerly described for nitrofurantoin which does not reach effective concentrations in urine of patients with eGFR <60 mL/min [15], and our finding is also in line with that of Bailey et al. who determined the concentration of another AG, sisomicin, in urine produced by either kidney in patients with unilateral kidney damage. They found that even though the relative concentration of antibiotic normalized for urine creatinine concentration was higher in damaged kidneys than in healthy ones, the absolute concentrations in kidneys with eGFR <15 mL/min was ineffective and they recommended treatment with cephalosporins [16].
As we showed that systemic treatment with AM is unreliable for reaching sufficiently high concentrations of AM in urine of patients treated by dialysis, we propose that at least for patients that already have indwelling urinary catheter, direct local instillation of AM into the urinary bladder may be helpful. This was formerly proposed for neomycin, which is too toxic for systemic administration [17], as well as for colistin [18]. AG administration via clean intermittent catheterization was proven to be effective and safe alternative of systemic antibiotic administration for UTI prophylaxis [19]. AM was also proven to be a highly effective prophylaxis for catheter-associated UTIs in neurosurgical patients when administered twice daily via indwelling urinary catheter [20].
Our study has a few limitations. Even though we proved that urine pH influenced the amount of eliminated AM into urine (Fig. 5), we were not able to measure directly the influence of pH on the AM efficacy by microbiological methods, due to the characteristics of the method used (i.e. multiple dilution of the sample by broth). Furthermore, we did not prove that the amount of urine produced significantly influenced the AM concentrations as other covariates probably made it insignificant. We also included patients treated with AM regardless of the indication. Therefore, patients not suffering from UTI were also included. In theory, inflammation of the kidneys may influence the elimination of the antibiotics into the urine. Nevertheless, we believe that this would be done by measurable GFR changes during the UTI and as we calculated eGFR according to current creatinine level we would include these patients into particular eGFR group. We did not evaluate the clinical efficacy as many of the patients were not treated for UTI and most of the patients were on combination therapy. Therefore, further study on the clinical efficacy of AM in monotherapy of UTI in patients with reduced kidney function may provide more insight into its applicability in this indication. On the other hand, our results suggest that this represents a theoretical risk of treatment failure and clinical reevaluation of these results may represent an ethical problem.
CONCLUSION
We determined that AM concentrations in urine of patients treated by dialysis are often insufficient to treat E. coli UTIs when AM is used as monotherapy. The plasma concentrations did not correlate with the urinary concentration which was determined more by urinary pH, eGFR and probably volume of diuresis. This makes intravenous administration of large doses in this patient group questionable, as it would probably not improve the efficacy but may result in toxicity. AM monotherapy probably does not allow sufficient concentrations to be reached to sterilize the urine of a large number of uremic patients when AM is administered systematically. This might be improved by instillation of AM directly to the bladder via the route of indwelling urinary catheter or intermittent catheterization.
Contributor Information
Jan Miroslav Hartinger, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Eliška Dvořáčková, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Vojtěch Krátký, Department of Nephrology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Zdenka Hrušková, Department of Nephrology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Marek Mysliveček, Department of Nephrology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Daniel Bobek, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Hana Benáková, Institute of Medical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Jan Závora, Institute of Medical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Gabriela Kroneislová, Institute of Medical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Barbora Agatha Halouzková, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Martina Brejníková, 3rd Department of Surgery, First Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, Czech Republic.
Vendula Martínková, 3rd Department of Surgery, First Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, Czech Republic.
Vladimír Tesař, Department of Nephrology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Ondřej Slanař, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
FUNDING
This study was supported by research initiatives of the Ministry of Health of Czech Republic RVO-VFN 64165 and Ministry of Education, Czech Republic grant COOPERATIO 207034 Internal Disciplines and Pharmaceutical sciences.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors declare they do not have any conflict of interest regarding this article.
REFERENCES
- 1. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med 2017;129:242–58. 10.1080/00325481.2017.1246055 [DOI] [PubMed] [Google Scholar]
- 2. Kirby WM, Clarke JT, Libke RDet al. . Clinical pharmacology of amikacin and kanamycin. J Infect Dis 1976;134 Suppl:S312–5. 10.1093/infdis/135.Supplement_2.S312 [DOI] [PubMed] [Google Scholar]
- 3. Labovitz E, Levison ME, Kaye D. Single-dose daily gentamicin therapy in urinary tract infection. Antimicrob Agents Chemother 1974;6:465–70. 10.1128/AAC.6.4.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riff LJ, Jackson GG. Pharmacology of gentamicin in man. J Infect Dis 1971;124 Suppl:S98–105. 10.1093/infdis/124.Supplement_1.S98 [DOI] [PubMed] [Google Scholar]
- 5. Fang GD, Brennen C, Wagener Met al. . Use of ciprofloxacin versus use of aminoglycosides for therapy of complicated urinary tract infection: prospective, randomized clinical and pharmacokinetic study. Antimicrob Agents Chemother 1991;35:1849–55. 10.1128/AAC.35.9.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sima M, Hartinger J, Cikankova Tet al. . Estimation of once-daily amikacin dose in critically ill adults. J Chemother 2018;30:37–43. [DOI] [PubMed] [Google Scholar]
- 7. Baudoux P, Bles N, Lemaire Set al. . Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J Antimicrob Chemother 2007;59:246–53. 10.1093/jac/dkl489 [DOI] [PubMed] [Google Scholar]
- 8. Gudmundsson A, Erlendsdottir H, Gottfredsson Met al. . Impact of pH and cationic supplementation on in vitro postantibiotic effect. Antimicrob Agents Chemother 1991;35:2617–24. 10.1128/AAC.35.12.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minuth JN, Musher DM, Thorsteinsson SB. Inhibition of the antibacterial activity of gentamicin by urine. J Infect Dis 1976;133:14–21. 10.1093/infdis/133.1.14 [DOI] [PubMed] [Google Scholar]
- 10. Melinda Monteforte BS. Aminoglycoside dosing and monitoring guidelines for adult patients at Stony Brook University Hospital. 2015, 14p. https://www.stonybrookmedicine.edu/sites/default/files/amino.pdf (15 January 2024, date last accessed).
- 11. Stankowicz MS, Ibrahim J, Brown DL. Once-daily aminoglycoside dosing: an update on current literature. Am J Health Syst Pharm 2015;72:1357–64. 10.2146/ajhp140564 [DOI] [PubMed] [Google Scholar]
- 12. Halouzková BA, Hartinger JM, Krátký Vet al. . Dosing of aminoglycosides in chronic kidney disease and end-stage renal disease patients treated with intermittent hemodialysis. Kidney Blood Press Res 2022;47:448–58. 10.1159/000523892 [DOI] [PubMed] [Google Scholar]
- 13. Wise R, Andrews JM. The bactericidal activity of gatifloxacin in plasma and urine. Clin Microbiol Infect 1998;4:392–6. 10.1111/j.1469-0691.1998.tb00083.x [DOI] [PubMed] [Google Scholar]
- 14. Stamey TA, Fair WR, Timothy MMet al. . Serum versus urinary antimicrobial concentrations in cure of urinary-tract infections. N Engl J Med 1974;291:1159–63. 10.1056/NEJM197411282912204 [DOI] [PubMed] [Google Scholar]
- 15. Sachs J, Geer T, Noell Pet al. . Effect of renal function on urinary recovery of orally administered nitrofurantoin. N Engl J Med 1968;278:1032–5. 10.1056/NEJM196805092781902 [DOI] [PubMed] [Google Scholar]
- 16. Bailey RR, Peddie B, McRae CU. Antibiotic concentrations in the urine from kidneys of unequal function. Clin Nephrol 1982;17:258–61. [PubMed] [Google Scholar]
- 17. Stamey TA, Govan DE, Palmer JM. The localization and treatment of urinary tract infections: the role of bactericidal urine levels as opposed to serum levels. Medicine (Baltimore) 1965;44:1–36. 10.1097/00005792-196501000-00001 [DOI] [PubMed] [Google Scholar]
- 18. Giua R, Pedone C, Cortese Let al. . Colistin bladder instillation, an alternative way of treating multi-resistant Acinetobacter urinary tract infection: a case series and review of literature. Infection 2014;42:199–202. 10.1007/s15010-013-0507-y [DOI] [PubMed] [Google Scholar]
- 19. Bilsen MP, van Uhm JIM, Stalenhoef JEet al. . Intravesical aminoglycoside instillations as prophylaxis for recurrent urinary tract infection: patient satisfaction, long-term safety and efficacy. JAC Antimicrob Resist 2023;5:dlad040. 10.1093/jacamr/dlad040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zacharias S, Dwarakanath S, Agarwal Met al. . A comparative study to assess the effect of amikacin sulfate bladder wash on catheter-associated urinary tract infection in neurosurgical patients. Indian J Crit Care Med 2009;13:17–20. 10.4103/0972-5229.53110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.