Abstract
Introduction
Tissue injury (TI) and hemorrhagic shock (HS) are the major contributors to trauma-induced coagulopathy (TIC). However, the individual contributions of these insults are difficult to discern clinically because they typically coexist. TI has been reported to release procoagulants, while HS has been associated with bleeding. We developed a large animal model to isolate TI and HS and characterize their individual mechanistic pathways. We hypothesized that while TI and HS are both drivers of TIC, they provoke different pathways; specifically, TI reduces time to clotting, whereas, HS decreases clot strength stimulates hyperfibrinolysis.
Methods
After induction of general anesthesia, 50 kg male, Yorkshire swine underwent isolated TI (bilateral muscle cutdown of quadriceps, bilateral femur fractures) or isolated HS (controlled bleeding to a base excess target of − 5 mmol/l) and observed for 240 min. Thrombelastography (TEG), calcium levels, thrombin activatable fibrinolysis inhibitor (TAFI), protein C, plasminogen activator inhibitor 1 (PAI-1), and plasminogen activator inhibitor 1/tissue-type plasminogen activator complex (PAI-1-tPA) were analyzed at pre-selected timepoints. Linear mixed models for repeated measures were used to compare results throughout the model.
Results
TI resulted in elevated histone release which peaked at 120 min (p = 0.02), and this was associated with reduced time to clot formation (R time) by 240 min (p = 0.006). HS decreased clot strength at time 30 min (p = 0.003), with a significant decline in calcium (p = 0.001). At study completion, HS animals had elevated PAI-1 (p = 0.01) and PAI-1-tPA (p = 0.04), showing a trend toward hyperfibrinolysis, while TI animals had suppressed fibrinolysis. Protein C, TAFI and skeletal myosin were not different among the groups.
Conclusion
Isolated injury in animal models can help elucidate the mechanistic pathways leading to TIC. Our results suggest that isolated TI leads to early histone release and a hypercoagulable state, with suppressed fibrinolysis. In contrast, HS promotes poor clot strength and hyperfibrinolysis resulting in hypocoagulability.
Keywords: Trauma-induced coagulopathy, Fibrinolysis, Tissue injury, Hemorrhagic shock animal models
Introduction
Trauma remains a leading cause of death worldwide [2], with death within 6 h being most commonly due to uncontrolled hemorrhage [3, 4] associated with trauma-induced coagulopathy (TIC) [5, 6]. TIC is defined as any change in the coagulation system resulting from trauma, and manifests in various phenotypes ranging from hypocoagulable/hyperfibrinolyic resulting in bleeding to hypercoagulable/hypofibrinolytic promoting pathologic clotting [7]. Early TIC is dominated by the former, i.e., typically ineffective hemostasis due to impaired clot formation, compounded by hyperfibrinolysis [7]. Several mechanisms have been proposed as drivers of this early hypocoagulative state, but no specific driver of TIC has been identified. Notably, severe trauma is usually combined hemorrhagic shock (HS) and tissue injury (TI), and it is assumed that both insults are important for severe TIC to occur. However, their distinct effects are poorly defined [8]. HS leads to hypoperfusion and tissue hypoxia producing metabolic acidosis which contributes to poor clot formation [9, 10]. In contrast, TI causes endothelial disruption [11] with tissue factor exposure [7] and provokes inflammatory responses such as release of histones and damage associated molecular patterns (DAMPs) [12] that promote clotting. In trauma, HS and TI synergistically lead to endothelial and immune system activation resulting in clotting factor consumption, platelet dysfunctions, impaired fibrinogen, and an ineffective endothelial barrier C. These mediators of coagulation alter thrombin generation, fibrin crosslinking, platelet fibrin interaction with platelets and incorporation of red blood cells collectively known as clot strength, as well as clot durability.
While impaired coagulation dominates the acute resuscitation phase following trauma, hypercoagulopathy occurs later in the hospital course and is associated with delayed preventable deaths [13]. Patients with the most severe early hypocoagulopathy and severe tissue injury appear to be most at risk for later developing hypercoagulopathy [7]. The switch from a hypocoagulative state to a hypercoagulative usually occurs within 12 h following injury [14] and is a significant risk for later complications such as acute respiratory distress syndrome and multiple organ failure [15]. Identification of mechanistic pathways driving early hypocoagulability and subsequent hypercoagulability could improve our ability to treat at risk patients for delayed thrombotic complications.
Varying levels of hypoperfusion and with different degrees of soft tissue injury limit our ability to identify the dominant clinical pathways driving TIC. To investigate this complex environment, animal models can provide isolated insults to identify specific pathways leading to TIC. Rodent models with isolated TI, isolated HS, and combined trauma (TI + HS) groups have been published by several coagulation laboratories. Early work by the San Francisco group showed that in a murine model of HS + TI, increasing levels of activated protein C were associated with TIC, and increased fibrinolytic activity [16]. Previous work in rats by our group has suggested that HS drives tPA release with subsequent tPA-mediated fibrinolysis, while TI impairs fibrinolysis [17]. However, the limitation of murine models is their poor simulation of the human coagulation system, especially hypocoagulable states [18], and the limited blood volume in rats precludes sequential sampling. More recently, swine models due to their increased similarity to human anatomy and physiology have been developed to study TIC and novel resuscitation strategies following multisystem trauma. These models can assist in establishing the individual mechanistic pathways specifically associated with HS and TI. Thus, we evaluated drivers of TIC in our established model of isolated HS and isolated TI in swine, using novel assays to detect changes in coagulation proteins, histones, and myosin. We hypothesized that while TI and HS both promote TIC, they stimulate different pathways: TI reduces time to clotting, whereas HS decreases clot strength and promotes hyperfibrinolysis.
Methods
Animal model
This animal study was conducted in compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. Results are reported in accordance with the ARRIVE guidelines [1]. The University of Colorado Institutional Animal Care and Use Committee approved this animal study under protocol #0323, and the research was conducted in a fully accredited Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) facility.
Healthy adolescent, male Yorkshire swine (average weight 50.0 ± 2.7 kg) were used for this study. Prior to study, swine were acclimated in the animal facilities for a minimum of 3 days and examined by a veterinarian on day of experimentation. This model has been described in detail previously [19]. In brief, on the day of surgery, swine anesthesia was induced via intramuscular injection of ketamine (20.0 mg/kg), xylazine (2.0 mg/kg), and acepromazine (0.2 mg/kg) followed by intubation under direct laryngoscopy. Continuous infusion anesthesia was maintained through a superficial ear vein using propofol (3 mg/kg/h) and fentanyl (3 mcg/kg/h). Lactated Ringer’s solution was administered at a rate of 10 mL/kg/h to overcome insensible losses. 12 French Mahurkar catheters were placed in the femoral arteries for vascular access.
Randomization/Sample size: Swine were randomized 2:1 to control (SHAM, n = 5), tissue injury (TI, n = 10), or hemorrhagic shock (HS, n = 10). Sample size/power analysis was conducted using our previously identified physiologic TEG MA values for swine [20]. Using the reported MA mean of 72.5 mm and a standard deviation of 2 mm, a sample size of 5 controls to 10 injury groups allowed us to detect minimum differences in 3.3 mm with 80% power and 95% confidence.
Procedures
The SHAM group underwent no further manipulation after the anesthesia and monitoring instrumentation. For the TI group, injury consisted of open, bilateral, comminuted femur fractures: surgical cutdown through the quadricep muscle provided direct access to the femurs, where we fired a captive bolt stun gun (Blitz-Kerner, Turbocut JOBB GmbH, Germany). Visual inspection post-fracture was completed and packing of 4 × 4 gauze at fracture site protected against unwanted post-fracture blood loss. For the HS group, fixed-pressure HS was produced through controlled bleeding from the 12 Fr Mahurkar catheters into 450 mL, prefilled CPDA-1 blood transfusion bags (Jorgensen Laboratories, Inc. Loveland, CO). To induce hypoperfusion, blood was collected directly into the transfusion bags using the femoral catheters. Removal of blood volume was initiated rapidly with the target of reaching a mean arterial pressure (MAP) of 30 mmHg in 5 min. Blood removal was stopped once the MAP reached 30 mmHg, marking the beginning of the HS phase. If the MAP pressure increased above 30 mmHg, additional blood was removed until MAP once again dropped below 30 mmHg. Base excess (BE) was monitored throughout the 45–60 min HS period, and once − 5 mmol/L was reached resuscitation with shed blood was initiated. Animals were monitored for 240 min following end of TI or HS or end of instrumentation for the SHAM group. Serial blood draws were taken at baseline (prior to any injuries) and then again at 30, 60, 120, 180, and 240 min post-injury. After 240 min of observation, swine were euthanized using pentobarbital (86.6 mg/kg).
Data collection
Vital signs including systolic blood pressures (SBP), diastolic blood pressures (DBP), MAP, heart rate (HR), and respiratory rate (RR), as well as EtCO2 were monitored continuously. At all timepoints (baseline, 30, 60, 120, 180, and 240 min) citrated whole blood was used for citrate native (CN) thrombelastography (TEG) to obtain viscoelastic properties of clot formation. CN TEG results include the speed of clot initiation (R-time), rate of clot propagation (angle), maximum clot strength (maximum amplitude, MA), and percent lysis at 30 min (LY30). Platelet poor plasma from the citrated blood samples was separated for protein analysis. Plasma levels of active plasminogen activator inhibitor 1 (PAI-1), tissue plasminogen activator (tPA) -PAI-1 complex, and histones (TI and SHAM groups only) were measured at each timepoint using enzyme-linked immunosorbent assay kits (Innovative Research, Novi, MI, product #s POPAIT-PAKT-COM, POPAIKT, and MyBioSource, San Diego, CA) per the manufacturer’s instructions.
Proteomic analysis of plasma proteins was conducted according to a previously described protocol [21, 22]. Briefly, proteins underwent reduction and alkylation of cysteines, followed by digestion with trypsin. Reverse phase cleanup of the digests was used, and then samples underwent liquid chromatography separation and electrospray ionization-tandem mass spectrometry characterization. The raw outputted data were queried for peptide identification using the Uniprot database and corresponding gene names [23]. The razor, total, and unique spectral counts were averaged across each timepoint to generate protein level measurements for protein-C and thrombin activatable fibrinolysis inhibitor (TAFI). Measurements were recorded as mean spectral count (MSC).
Immunoblot assay was used to measure skeletal muscle myosin (SkM) [24]. Briefly, anti-SkM heavy chain antibodies were screened using immunoblotting for their ability to identify skeletal muscle myosin heavy chain isoforms in pig plasma. The proteins were transferred to FL-PVDF membranes using a semi-dry system (Trans-Blot Turbo Transfer System, BioRad). The membrane was blocked using LiCor blocking buffer in phosphate buffered saline, then incubated with anti-SkM chain monoclonal antibodies (22D4, 10 μg/mL), washed, and then incubated with IRDye-conjugated donkey anti-mouse antibodies. After a final wash, the signal of bound IRDye-conjugated secondary antibodies to the primary anti-myosin antibodies on the membrane was detected by the LiCor imaging system. The SkM band migrating at 250 kDa represents intact SkM heavy chain (HC), while the 160 and 140 kDa bands represent heavy meromyosin (HMM) of SkM HC, and the 100 and 95 kDa bands represent S1 fragment of SkM HC. Levels of plasma SkM bands are reported as mean relative abundance (MRA) where 100% was defined as mean baseline value for each cohort [24].
Data analysis
Data were entered in Microsoft Excel 2010 (Microsoft Corporation, Redmond, USA) and analyzed using SAS Studio 2021 and SAS vs 9.4 (SAS Institute, Cary, NC). For continuous variables, distribution was determined by the Kolmogorov–Smirnov statistic along with visually inspecting histograms. Linear mixed models for repeated measures were used to compare the temporal trends of the groups, with contrasts between groups adjusted by false discovery rate. The incidence of hyperfibrinolysis (LY30 > 3.0%) [25] was compared using the Fisher’s Exact Test. A p value < 0.05 was considered statistically significant. Numerical data are presented as mean ± standard deviation for normally distributed data and represented by median (interquartile range) if nonparametric distribution.
Results
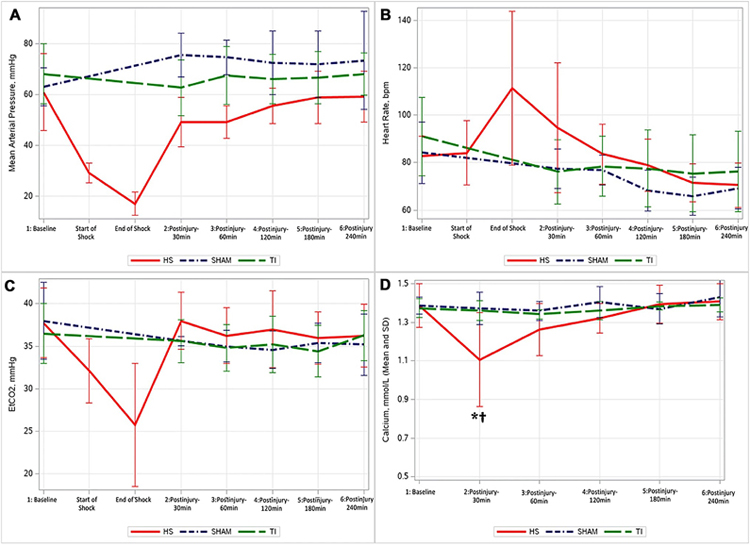
Twenty-five swine were randomized for the experiment (SHAM, n = 5; TI, n = 10; HS, n = 10). One swine in the HS group decompensated 20 min into shock resulting in early expiration, leaving the HS group with n = 9 for analysis. Prior to injury, all groups had similar baseline means for MAP, EtCO2, and heart rate (p > 0.4, Fig. 1). For the SHAM group, there were no significant changes in MAP, EtCO2, or heart rate throughout the model; however, MAP trended upward and HR appear to trend downward as the experiment progressed. The TI group showed a significant decline in HR at the 180 and 240 min timepoints. For the HS group, the mean time to reach the BE goal was 56.1 min (± 10.5 min). The mean MAP at the start of the HS phase was 28.7 ± 3.9 mmHg and at the end of the HS period was 17.9 ± 5.2 mmHg.
Fig. 1.
Changes in mean arterial pressure (MAP, Panel A), heart rate (Panel B), end tidal carbon dioxide (EtCO2, Panel C), and ionized calcium (Panel D) through the experiment. Statistical significance is only shown in Panel D, where *significant difference in HS compared to other groups (TI and SHAM); †significant change from baseline in HS group; (HS Hemorrhagic Shock, TI tissue injury);
Ionized calcium (Ca) levels remained unchanged throughout the experiment for the TI and SHAM group. For the HS group, Ca levels decreased significantly following the shock phase at the 30 min post-shock timepoint (baseline 1.38 ± 0.11–1.10 ± 0.24 mmol/L at 30 min, p = 0.001).
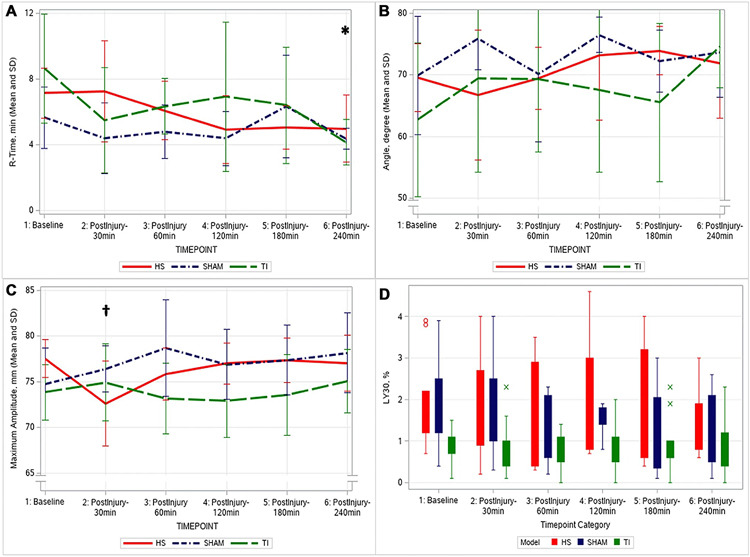
Thrombelastography
The TEG outcomes were similar among the three groups at baseline and at the final 240 min (p > 0.40), as shown in Fig. 2. The R-time trended to shortening in all groups; however, this decrease was significant only in the TI group (baseline 8.64 ± 3.33–4.16 ± 1.39 min at 240 min, p = 0.005).
Fig. 2.
Native thromboelastography as a function of time. R-Time, time to clot formation (Panel A). Angle, rate of clot propagation (Panel B). Maximum amplitude, clot strength (Panel C). LY30, percentage of clot of lysis at 30 min (Panel D); *significant change from baseline in TI group; †significant change from baseline in HS group; (HS Hemorrhagic Shock, TI tissue injury)
The HS group was the only group that showed a downward trend in angle following injury (not significant), while the TI and SHAM group showed a trend toward increased angle after injury/anesthesia (not significant). There were no significant changes in any group throughout the model for TEG angle. In the HS group, MA, a measure of clot strength, decreased significantly following HS at the 30 min post-shock (baseline 77.56 ± 2.12–72.65 ± 4.63 mm at 30 min, p = 0.003), which normalized with continued resuscitation.
The incidence of TEG measured hyperfibrinolysis (defined as using the clinical cutoff of LY30 > 3.0%) during the experiment trended higher in the HS group, with four out of the nine pigs (44%) achieving a post-shock LY30 value > 3.0%, compared to zero pigs in the TI group and one in the SHAM group (p = 0.06).
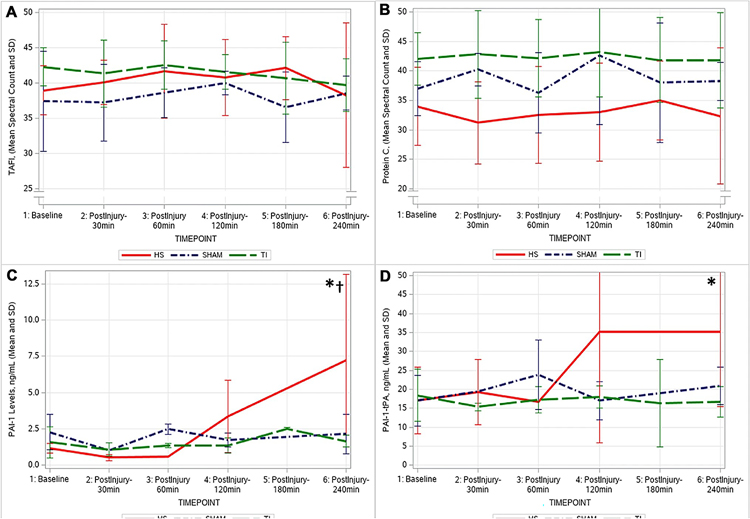
Coagulation factors and calcium
Protein-C and TAFI protein plasma levels and PAI-1 and PAI-1-tPA complex plasma levels measured by ELISAs are shown in Fig. 3. Protein-C and TAFI plasma levels showed no significant changes throughout the experiment in any group. For PAI-1 and the PAI-1-tPA complex plasma levels, the TI and SHAM showed no changes in these protein levels. In the HS group, active PAI-1 began to increase 60 min following shock, reaching a statistically significant higher level above baseline at the end of the experiment (baseline 1.17 ± 0.32–7.21 ± 5.95 ng/mL at 240 min, p = 0.002). The PAI-1-tPA complex followed a similar pattern: increasing in the HS group after the 60 min post-shock timepoint and continuing to increase to the end of the experiment (baseline 17.05 ± 8.77–32.21 ± 19.86 ng/mL at 240 min, p = 0.04).
Fig. 3.
Thrombin activatable fibrinolysis inhibitor (TAFI, Panel A), Protein C (Panel B), Plasminogen activator inhibitor 1 (PAI-1, Panel C), and plasminogen activator inhibitor 1/tissue-type plasminogen activator complex (PAI-1-tPA, Panel D). *significant change from baseline in HS group; †significant difference between HS group compared to SHAM and TI groups. (HS Hemorrhagic Shock, TI tissue injury; SHAM: control)
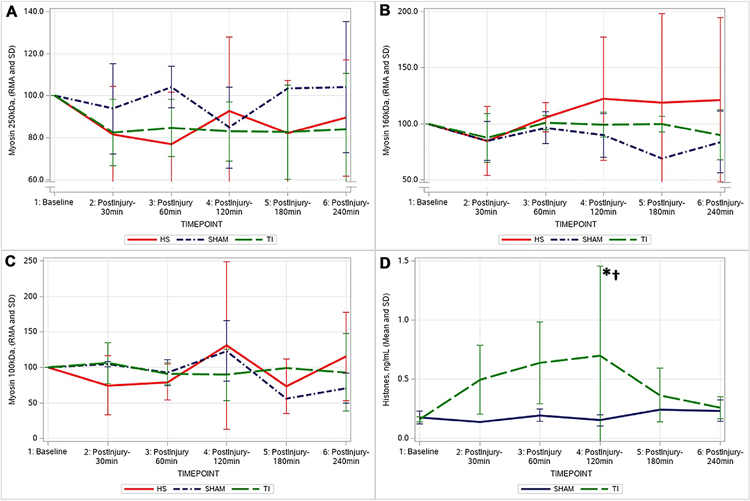
Skeletal muscle myosin and histone release
Patterns in the skeletal muscle myosin (SkM) immunoblot assays in the 250 kDa chain and the 160 kDa chain, and relative abundance of myosin are shown in Fig. 4. There was a sustained, yet nonsignificant decrease in the relative plasma abundance of the 250 kDa chain in the HS (89.48 ± 27.78%) and TI (84.13 ± 26.45%) groups, while the SHAM group levels did not change. For the 160 kDa chain levels, all groups showed a slight (nonsignificant) decrease in the relative abundance of this chain 30 min following the injury phase (SHAM 85.05 ± 17.37%; TI 87.60 ± 21.51%; HS 84.88 ± 30.54%).
Fig. 4.
Relative abundance of myosin levels trended downward in the 250 kDa Heavy chains (Panel A) in the HS and TI groups. For the 160 kDa chain levels (Panel B), all groups showed a slight (nonsignificant) decrease in the relative abundance of this chain 30 min following the injury phase. No significant changes were detected in the 100 kDa chains in any group (Panel C). The TI group had increased histone levels (Panel D) following injury. *significant change from baseline in TI group; †significant difference compared to SHAM. (HS Hemorrhagic Shock, TI tissue injury)
The TI group showed increased histone levels following injury, which peaked at 120 min and were significantly elevated compared to baseline (baseline 0.16 ± 0.02–0.7 ± 0.75 ng/mL at 120 min, p = 0.02). At this 120 min timepoint, the TI group’s histones were significantly elevated compared to the corresponding SHAM levels (0.15 ± 0.05 ng/mL at 120 min, p = 0.05).
Discussion
Clinically, trauma patients present with heterogenous injuries with varying levels of shock and tissue injury severity. Consequently, it is difficult to determine which insult drives a specific coagulopathic pathway. In this large animal study, we have shown that HS and TI provoke distinct pathways that contribute to the development of TIC. HS led to increased levels of PAI-1 activity and PAI-1-tPA complex, suggesting increasing tPA release. HS was associated with poor clot formation and increased fibrinolysis, while TI trended toward a suppressed fibrinolytic state.
Previous animal models have suggested a link between hypoperfusion and protein C activation (aPC) as a possible driver in early TIC. It is proposed that aPC exerts an anticoagulant effect by inactivating factors Va and VIIIa [26] and has a role in upregulating fibrinolysis by degrading PAI-1 [27]. In this study, we reported no changes in protein C levels (which can serve as a surrogate for aPC) through proteomic analysis; however, others have reported associated changes of protein C and aPC levels with TIC. The San Francisco lab suggested that aPC was a potential driver of TIC because hypocoagulation was associated with significant increases in aPC levels [28]. Furthermore, in their analysis of 300 trauma patients, aPC was elevated in patients with severe shock (BE < − 6 mmol/L) [16]. In their murine model, they observed that aPC levels did not increase in isolated injury groups, but only in animals subjected to both TI and HS [29]. This requirement for HS and TI to act synergistically on the aPC pathway may explain the lack of significant changes in plasma aPC levels in our isolated insult swine models.
Alternatively, the decreased clot strength in the HS group could be related to reduced calcium levels post-shock while the hyperfibrinolysis could represent overwhelming endogenous tPA release. Previous clinical data suggested tPA release was overcome by increased PAI-1 production in severely injured patients [24]. Previous work by our group has also showed a significant correlation between hypocalcemia and weaker clot strength [30]. Calcium is an essential component in platelet activation [31] and is also involved in Factor XIII activation, which is responsible for cross-linking fibrin in clots to prevent premature fibrinolysis [32]. The HS group had a statistically significant decline in ionized Ca levels; however, the clinical relevance of a decrease from 1.38 to 1.10 mmol/L in swine is unknown.
While we did not measure plasma tPA levels, our HS group showed significant increases in PAI-1 and the PAI-1-tPA complex. Few porcine trauma models have evaluated PAI-1, tPA, and PAI-1-tPA complex plasma levels; Brannstrom et al. found that in their polytrauma porcine model tPA remained unchanged while PAI-1 had nonsignificant increases at their end of their observation (120 min) [33]. Our study, as well as septic shock models, suggest that elevated PAI-1 are a delayed response occurring 3–5 h after injury [34, 35]. Regarding tPA, prior studies suggest that the concentration of active tPA exits in a relatively stable range, balanced by endothelial secretion, PAI-1 inhibition, and rapid hepatic clearance [36, 37]. Clinical studies have shown that elevated tPA is associated with increasing fibrinolysis [38]. The measured increases in the plasma PAI-1-tPA complex levels suggests that tPA can overwhelm nearby PAI-1 reserves, prompting temporary, unchecked plasmin activation and hyperfibrinolysis [39]. Because free tPA is rapidly cleared compared to tPA complexed with PAI-1, the complex may be a better measure for tPA release [40]. We speculate that in the isolated HS animals, post-shock tPA release fueled the increase in measured levels of the PAI-1tPA complex, prompting hyperfibrinolysis in 44% of the HS animals at the 120 and 180 min timepoints.
While the aPC pathway and PAI-1-tPA pathway are known to change in TIC, much less is known about the role of histones and myosin in contributing to coagulopathy. Skeletal myosin exhibits procoagulant activity and has been shown to promote thrombosis [41, 42]. Unlike our findings, previous animal and human studies observed elevated markers of plasma skeletal myosin following injury. However, these studies assessed sports related muscle injuries (not isolated trauma) and employed different detection techniques [43–45]. Additionally, we recently showed that trauma patients who had depressed full chain SkM plasma levels exhibited slower clot propagation, weaker clot strength, and an increased rate of clot degradation [24]. Thus, while isolated injures and shock did not appear sufficient to provoke SkM release into circulation, a pre-existing deficiency of circulating SkM may predispose patients to poor hemostasis following severe trauma. Alternatively, depressed full-chain SkM levels post-injury, such as the non-significant decline seen in this study, could be due to consumption at the injury site leading to an impaired hemostasis [24].
Histones have been shown to be pro-thrombotic [46, 47]. Previous animal models demonstrated that histones are released into circulation from apoptotic cells and neutrophil extracellular traps (NETs) following injury [48, 49]. Furthermore, the intravascular presence of histones results in thrombosis [50]. Kutcher et al. established further links between histones, coagulopathy, and organ damage. In their clinical study of 123 trauma patients, they found that elevated plasma histone levels were associated with higher injury severity and markers of coagulopathy including d-dimers, tPA, TAFI and aPC [46]. In our investigation, bilateral femur fractures with quadricep muscle injury provided the stimulus to promote histone release. The tendency of the TI animals to exhibit decreased levels of fibrinolysis along with elevated histones, combined with their known cytotoxic effects on organ damage [51–53], supports the hypothesis that the association of multiple organ failure with fibrinolysis shutdown may be partially mediated by histone-activated pathways.
Our study has limitations; thus, the conclusions should be considered within the context of the severity of the injuries and the limited battery of analyses performed. Due to the costs associated with large animal models such as swine, loss of animals during the experiment can result in inadequate samples size for analysis at later timepoints. The powered analysis was based on detectable changes in MA, and this sample size may be underpowered to support statistically significant detection of fibrinolysis. To maximize survival through the entire monitoring period, depth of shock was set to a moderate base excess goal of − 5 mmol/L. The base excess goal was achieved utilizing pressure-targeted hemorrhagic shock. Exact bleeding volumes were not measured, and this limits the ability to compare the level of hemorrhage utilized in this study with other similar studies. Additionally, the bilateral femur fractures in the TI group are also a moderate injury. However, the contribution of tissue injury to the development of TIC has been poorly defined [54, 55], and femur fractures remain a frequent addition to polytrauma models because they are common in civilian blunt trauma [56, 57]. Expanded analyses to include additional proteases (especially tPA, plasminogen, and plasmin), coagulation factors activity, thrombin generation, and functional fibrinogen would provide a more comprehensive understanding when evaluating HS-induced or TI-induced mechanisms responsible for TIC phenotypes. Improved knowledge of animal-specific normal ranges would advance our understanding of the physiologic significance of detected changes. For example, the human clinical cutoff of LY30 > 3.0% to define hyperfibrinolysis [13] in this swine model may not be an optimal threshold. Swine are innately hypercoagulable, and thus generating a hypocoagulable state in this species is difficult, even in the most severe models. However, swine share anatomical and physiologic similarities, making them an attractive animal choice for studying TIC. Many researchers have overwhelmed their hypercoagulable state by employing dilutional fluid resuscitation to induce a dilutional coagulopathy [58, 59]. As trauma resuscitation protocols have shifted away from crystalloid-based resuscitation and toward component or blood-based resuscitation [60], we elected to avoid a dilutional coagulopathy in this model. While the use of shed blood adequately modulates product/blood first resuscitation, anticoagulants within the blood transfusion bags may have influenced some of our results. However, as these products are also standard in clinical resuscitation their use represents a “real-world” scenario that we sought to replicate in the model. Lastly, the different techniques utilized in this study to measure the various proteins limited our ability to draw conclusions regarding the relative abundance of the different proteins, and unanticipated COVID challenges resulted in incomplete evaluation of the histone levels in the HS models, leading to comparisons only in the TI and SHAM groups.
Conclusion
Isolated injury animal models are important to elucidate the mechanistic pathways leading to TIC. Our results suggest that isolated TI leads to early histone release and a hypercoagulable state with a shortened R-time. Our HS group indicated decreased clot strength and elevated PAI-1 and PAI-1-tPA complex levels, as well as a tendency to increased fibrinolysis. The elevated PAI-1 and PAI-1-tPA complex levels support the mechanistic pathway identified in HS patients with systemic fibrinolysis.
Funding
This research is funded in part by the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC) contract number UM1-HL120877. Research support is also provided by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315). The current major funding source is an RM1 grant (1RM1GM131968–01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest E.E.M. has patents pending related to coagulation and fibrinolysis diagnostics and therapeutic fibrinolytics and is a cofounder with stock options in ThromboTherepeutics. E.E.M. has received grant support from Haemonetics, Inc., Stago, Hemosonics, Instrumentation Laboratories, Inc, and Diapharma outside the submitted work.
Ethical approval This animal study was conducted in compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. Results are reported in accordance with the ARRIVE guidelines [1]. The University of Colorado Institutional Animal Care and Use Committee approved this animal study under protocol #0323, and the research was conducted in a fully accredited Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) facility.
This work was presented as an oral presentation at the 21st annual European Congress on Trauma and Emergency Surgery in Oslo, Norway on April 26 2022. We confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
References
- 1.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577–9. 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact Sheets: Injuries and violence. 2021. https://www.who.int/news-room/fact-sheets/detail/injuries-and-violence#:~:text=Injuries%20%E2%80%93%20both%20unintentional%20and%20violence,nearly%208%25%20of%20all%20deaths. Accessed 11 Jun 2022.
- 3.Kalkwarf KJ, Drake SA, Yang Y, et al. Bleeding to death in a big city: An analysis of all trauma deaths from hemorrhage in a metropolitan area during 1 year. J Trauma Acute Care Surg. 2020;89(4):716–22. 10.1097/TA.0000000000002833. [DOI] [PubMed] [Google Scholar]
- 4.Moore HB, Moore EE, Chapman MP, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–91. 10.1016/S0140-6736(18)31553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42(5):857–61. 10.1097/00005373-199705000-00016 (Discussion 861-2). [DOI] [PubMed] [Google Scholar]
- 7.Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30. 10.1038/s41572-021-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frith D, Goslings JC, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–25. 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 9.Kutcher ME, Howard BM, Sperry JL, et al. Evolving beyond the vicious triad: differential mediation of traumatic coagulopathy by injury, shock, and resuscitation. J Trauma Acute Care Surg. 2015;78(3):516–23. 10.1097/TA.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 10.Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58(5):1002–9. 10.1097/01.ta.0000156246.53383.9f (Discussion 1009-10). [DOI] [PubMed] [Google Scholar]
- 11.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 12.Johansson PI, Windelov NA, Rasmussen LS, Sorensen AM, Ostrowski SR. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock. 2013;6(3):171–5. 10.4103/0974-2700.115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7. 10.1097/TA.0000000000000341 (Discussion 817). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE. Temporal Changes in Fibrinolysis following Injury. Semin Thromb Hemost. 2020;46(2):189–98. 10.1055/s-0039-1701016. [DOI] [PubMed] [Google Scholar]
- 15.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83(6):1014–22. 10.1097/TA.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davenport RA, Guerreiro M, Frith D, et al. Activated protein C drives the hyperfibrinolysis of acute traumatic coagulopathy. Anesthesiology. 2017;126(1):115–27. 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–92. 10.1016/j.surg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–12. 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cralley AL, Moore EE, Kissau D, Coleman JR, Vigneshwa N, Debot M, Schaid TR Jr, Moore HB, Cohen MJ, Hansen K, Silliman CC. A combat casualty relevant dismounted complex blast injury model in swine. J Trauma Acute Care Surg. 2022;93(2S Suppl 1):S110–S118. 10.1097/TA.0000000000003674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stettler GR, Moore EE, Moore HB, et al. Thrombelastography indicates limitations of animal models of trauma-induced coagulopathy. J Surg Res. 2017;217:207–12. 10.1016/j.jss.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Dzieciatkowska M, Hill RC, Hansen KC. Supernatant protein biomarkers of red blood cell storage hemolysis as determined through an absolute quantification proteomics technology. Transfusion. 2016;56(6):1329–39. 10.1111/trf.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzieciatkowska M, D’Alessandro A, Hill RC, Hansen KC. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics. 2015;120:1–6. 10.1016/j.jprot.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46(5):2699. 10.1093/nar/gky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman JR, Deguchi H, Deguchi TK, Cohen MJ, Moore EE, Griffin JH. Full-length plasma skeletal muscle myosin isoform deficiency is associated with coagulopathy in acutely injured patients. J Thromb Haemost. 2022. 10.1111/jth.15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman MP, Moore EE, Ramos CR, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–7. 10.1097/TA.0b013e3182aa9c9f (Discussion 967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 27.de Fouw NJ, de Jong YF, Haverkate F, Bertina RM. Activated protein C increases fibrin clot lysis by neutralization of plasminogen activator inhibitor–no evidence for a cofactor role of protein S. Thromb Haemost. 1988;60(2):328–33. [PubMed] [Google Scholar]
- 28.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoper-fusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–65. 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore HB, Tessmer MT, Moore EE, et al. Forgot calcium? Admission ionized-calcium in two civilian randomized controlled trials of prehospital plasma for traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2020;88(5):588–96. 10.1097/TA.0000000000002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay ZA, Fields AT, Nunez-Garcia B, et al. Dynamic effects of calcium on in vivo and ex vivo platelet behavior after trauma. J Trauma Acute Care Surg. 2020;89(5):871–9. 10.1097/TA.0000000000002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Dodt J, Volkers P, et al. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci Rep. 2019;9(1):11324. 10.1038/s41598-019-47815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brannstrom A, von Oelreich E, Degerstedt LE, et al. The swine as a vehicle for research in trauma-induced coagulopathy: Introducing principal component analysis for viscoelastic coagulation tests. J Trauma Acute Care Surg. 2021;90(2):360–8. 10.1097/TA.0000000000002997. [DOI] [PubMed] [Google Scholar]
- 34.Schochl H, Solomon C, Schulz A, et al. Thromboelastometry (TEM) findings in disseminated intravascular coagulation in a pig model of endotoxinemia. Mol Med. 2011;17(3–4):266–72. 10.2119/molmed.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saetre T, Lindgaard AK, Lyberg T. Systemic activation of coagulation and fibrynolysis in a porcine model of serogroup A streptococcal shock. Blood Coagul Fibrinolysis. 2000;11(5):433–8. 10.1097/00001721-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Chandler WL, Trimble SL, Loo SC, Mornin D. Effect of PAI-1 levels on the molar concentrations of active tissue plasminogen activator (t-PA) and t-PA/PAI-1 complex in plasma. Blood. 1990;76(5):930–7. [PubMed] [Google Scholar]
- 37.Bjorquist P, Brohlin M, Ehnebom J, et al. Plasminogen activator inhibitor type-1 interacts exclusively with the proteinase domain of tissue plasminogen activator. Biochim Biophys Acta. 1994;1209(2):191–202. 10.1016/0167-4838(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 38.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 39.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23. 10.1097/TA.0000000000000885 (Discussion 23-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96(3):761–8. 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 41.Coleman JR, Moore EE, Zilberman-Rudenko J, et al. Cardiac and skeletal muscle myosin exert procoagulant effects. Shock. 2019;52(5):554–5. 10.1097/SHK.0000000000001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deguchi H, Sinha RK, Marchese P, et al. Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood. 2016;128(14):1870–8. 10.1182/blood-2016-03-707679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlasakova K, Lane P, Michna L, Muniappa N, Sistare FD, Glaab WE. Response of novel skeletal muscle biomarkers in dogs to drug-induced skeletal muscle injury or sustained endurance exercise. Toxicol Sci. 2017;156(2):422–7. 10.1093/toxsci/kfw262. [DOI] [PubMed] [Google Scholar]
- 44.Burch PM, Greg Hall D, Walker EG, et al. Evaluation of the relative performance of drug-induced skeletal muscle injury biomarkers in rats. Toxicol Sci. 2016;150(1):247–56. 10.1093/toxsci/kfv328. [DOI] [PubMed] [Google Scholar]
- 45.Guerrero M, Guiu-Comadevall M, Cadefau JA, et al. Fast and slow myosins as markers of muscle injury. Br J Sports Med. 2008;42(7):581–4. 10.1136/bjsm.2007.037945 (Discussion 584). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg. 2012;73(6):1389–94. 10.1097/TA.0b013e318270d595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795–803. 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 48.Zeerleder S, Zwart B, Wuillemin WA, et al. Elevated nucleosome levels in systemic inflammation and sepsis. Crit Care Med. 2003;31(7):1947–51. 10.1097/01.CCM.0000074719.40109.95. [DOI] [PubMed] [Google Scholar]
- 49.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–21. 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleine TJ, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the role of protein and membrane structure. Am J Physiol. 1997;273(6):C1925–36. 10.1152/ajpcell.1997.273.6.C1925. [DOI] [PubMed] [Google Scholar]
- 52.Kleine TJ, Gladfelter A, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the conductive effect. Am J Physiol. 1995;268(5 Pt 1):C1114–25. 10.1152/ajpcell.1995.268.5.C1114. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187(5):2626–31. 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parr MJ, Bouillon B, Brohi K, et al. Traumatic coagulopathy: where are the good experimental models? J Trauma. 2008;65(4):766–71. 10.1097/TA.0b013e31818606d2. [DOI] [PubMed] [Google Scholar]
- 55.Ask A, Eltringham-Smith L, Bhakta V, Donkor DA, Pryzdial ELG, Sheffield WP. Spotlight on animal models of acute traumatic coagulopathy: an update. Transfus Apher Sci. 2022;61(2):103412. 10.1016/j.transci.2022.103412. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds PS, Fisher BJ, McCarter J, et al. Interventional vitamin C: a strategy for attenuation of coagulopathy and inflammation in a swine multiple injuries model. J Trauma Acute Care Surg. 2018;85(1S Suppl 2):S57–67. 10.1097/TA.0000000000001844. [DOI] [PubMed] [Google Scholar]
- 57.Martini WZ, Rodriguez CM, Cap AP, Dubick MA. Efficacy of resuscitation with fibrinogen concentrate and platelets in traumatic hemorrhage swine model. J Trauma Acute Care Surg. 2020;89(2S Suppl 2):S137–45. 10.1097/TA.0000000000002736. [DOI] [PubMed] [Google Scholar]
- 58.Spronk HM, Braunschweig T, Rossaint R, et al. Recombinant factor VIIa reduces bleeding after blunt liver injury in a pig model of dilutional coagulopathy under severe hypothermia. PLoS One. 2015;10(6): e0113979. 10.1371/journal.pone.0113979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson KM, Johansson KJ, Wingren C, Fries D, Nelander K, Lovgren A. Recombinant human prothrombin reduced blood loss in a porcine model of dilutional coagulopathy with uncontrolled bleeding. Blood Coagul Fibrinolysis. 2017;28(3):244–53. 10.1097/MBC.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 60.Kutcher ME, Kornblith LZ, Narayan R, et al. A paradigm shift in trauma resuscitation: evaluation of evolving massive transfusion practices. JAMA Surg. 2013;148(9):834–40. 10.1001/jamasurg.2013.2911. [DOI] [PubMed] [Google Scholar]