Abstract
Background:
Global longitudinal strain (GLS) is an echocardiographic method to identify left ventricular (LV) dysfunction after cardiac arrest that is less sensitive to loading conditions. We aimed to identify the frequency of impaired GLS following pediatric cardiac arrest, and its association with hospital mortality.
Methods:
This is a retrospective single-center cohort study of children <18 years of age treated in the pediatric intensive care unit (PICU) after in- or out-of-hospital cardiac arrest (IHCA and OHCA), with echocardiogram performed within 24 hours of initiation of post-arrest PICU care between 2013 and 2020. Patients with congenital heart disease, post-arrest extracorporeal support, or inability to measure GLS were excluded. Echocardiographic LV ejection fraction (EF) and shortening fraction (SF) were abstracted from the chart. GLS was measured post hoc; impaired strain was defined as LV GLS ≥ 2 SD worse than age-dependent normative values. Demographics and pre-arrest, arrest, and post-arrest characteristics were compared between subjects with normal versus impaired GLS. Correlation between GLS, SF and EF were calculated with Pearson comparison. Logistic regression tested the association of GLS with mortality. Area under the receiver operator curve (AUROC) was calculated for discriminative utility of GLS, EF, and SF with mortality.
Results:
GLS was measured in 124 subjects; impaired GLS was present in 46 (37.1%). Subjects with impaired GLS were older (median 7.9 vs. 1.9 years, p < 0.001), more likely to have ventricular tachycardia/fibrillation as initial rhythm (19.6% versus 3.8%, p = 0.017) and had higher peak troponin levels in the first 24 hours post-arrest (median 2.5 vs. 0.5, p = 0.002). There were no differences between arrest location or CPR duration by GLS groups. Subjects with impaired GLS compared to normal GLS had lower median EF (42.6% versus 62.3%) and median SF (23.3% versus 36.6%), all p < 0.001, with strong inverse correlation between GLS and EF (rho 0.76, p < 0.001) and SF (rho −0.71, p < 0.001). Patients with impaired GLS had higher rates of mortality (60% vs. 32%, p = 0.009). GLS was associated with mortality when controlling for age and initial rhythm [aOR 1.17 per 1% increase in GLS (95% CI 1.09–1.26), p < 0.001]. GLS, EF and SF had similar discrimination for mortality: GLS AUROC 0.69 (95% CI 0.60–0.79); EF AUROC 0.71 (95% CI 0.58–0.88); SF AUROC 0.71 (95% CI 0.61–0.82), p = 0.101.
Conclusions:
Impaired LV function as measured by GLS after pediatric cardiac arrest is associated with hospital mortality. GLS is a novel complementary metric to traditional post-arrest echocardiography that correlates strongly with EF and SF and is associated with mortality. Future large prospective studies of post-cardiac arrest care should investigate the prognostic utilities of GLS, alongside SF and EF.
Keywords: Cardiac arrest, Pediatrics, Myocardial dysfunction, Post-arrest outcomes, Mortality
Introduction
Over 20,000 children suffer an in- (IHCA) and out-of-hospital cardiac arrest (OHCA) in the United States every year.1,2 For those with sustained return of circulation, post-cardiac arrest care aims to mitigate the post-cardiac arrest shock syndrome, which frequently manifests as post-arrest myocardial dysfunction and/or hypotension.3 Both the presence4–6 and burden of post-arrest hypotension7 have been repeatedly associated with worse outcomes. Hypotension is likely a manifestation of myocardial dysfunction and systemic inflammation after cardiac arrest, and while they are both critical components of the post-cardiac arrest shock state,8 they have not been well characterized. Therefore, the ideal approach to post-cardiac arrest management balancing impaired ventricular function, vasoplegia, and systemic inflammation, is unknown.
To date, there have only been two pediatric studies assessing post-cardiac arrest myocardial dysfunction,9,10 with function measurements limited to ejection and shortening fractions with neither evaluating blood pressure. The more novel myocardial performance measure of global longitudinal strain (GLS) may be more sensitive for myocardial dysfunction, has been observed in other pediatric diseases,11–14 and is more independent of loading conditions (preload, afterload).15,16 To our knowledge, strain has never been examined in pediatric post-cardiac arrest patients.
For this study, we aimed to identify the prevalence of impaired GLS in a retrospective cohort of children who were cared for after cardiac arrest in the pediatric intensive care unit, and to examine the association of impaired GLS with hospital mortality. We hypothesized that worse left ventricular GLS on echocardiogram in the first 24 hours after cardiac arrest would be associated with higher hospital mortality.
Methods
Overview
This is a retrospective cohort study, examining clinically indicated post-cardiac arrest echocardiograms performed within 24 hours of initiation of post-arrest care in children <18 years of age cared for in the Children’s Hospital of Philadelphia (CHOP) pediatric intensive care unit (PICU) from 2013 to 2020. IHCA or OHCA were included; the time of initiation of ICU post-arrest care arrest was used as time zero. We excluded patients with unrepaired or single ventricle congenital heart disease, those who were treated with extracorporeal membrane oxygenation (ECMO) within 24 hours of cardiac arrest, and those without sufficient images to measure GLS. This work was deemed by exempt the CHOP Institutional Review Board (IRB 21–018881).
Exposures and outcomes
The primary exposure was left ventricular GLS. Clinical echocardiograms were obtained at the discretion of the providers, but guided through a clinical pathway for usual post-arrest care at our institution that was introduced in 201317: for patients on the “severe” pathway, defined as failure to return to neurologic baseline after cardiac arrest, echocardiogram was recommended.
The primary outcome was mortality prior to hospital discharge. The secondary outcome was unfavorable neurologic outcome at discharge, defined as a Pediatric Cerebral Performance Category (PCPC)18 score ≥ 3 or a worsening from baseline PCPC. For secondary outcomes, pre- and post-arrest PCPCs were documented within the hospital’s cardiac arrest database.
Echocardiographic review
Echocardiograms were reviewed for adequacy of post-hoc calculation of GLS by two echocardiographers blinded to patient history and outcome (YW, LMR). If multiple echocardiograms were performed, only the first echocardiogram obtained post-arrest was analyzed. Echocardiograms were obtained and stored within the Syngo Dynamics system (Siemens Healthcare, Erlangen, Germany). Left ventricular systolic function was reported qualitatively (normal/hyperdynamic or mildly, moderately, or severely diminished), and measured quantitatively with ejection fraction (EF) derived by Simpson’s method, where impaired function is classified as EF < 55% and shortening fraction (SF) measured by M-mode, where impaired function is SF < 28%.19 Left ventricular GLS was measured in three unique views with TomTec software (Cardiac Performance Analysis, Munich, Germany). Per convention, a more positive GLS (reported in % but all negative numbers) is associated with worse function; for example, −10% GLS is worse than −20% GLS. Impaired GLS was defined as two or more standard deviations above than age-dependent normative values,20 where a more positive value demonstrates worse GLS.
Data collection
All consecutive subjects with an IHCA and OHCA with care provided at CHOP are prospectively identified and compiled into a clinical database which collects Utsein-style variables21 including subject demographics, arrest details, and survival to hospital discharge (IRB 11–008115). For this study, additional data were abstracted from the medical record including complete echocardiographic reports, vasoactive and inotropic administration and dosing, administration of steroid medications (methylprednisolone, hydrocortisone, or dexamethasone), and laboratory values within the first 24 hours of post-cardiac arrest care including the biomarkers troponin and lactate. Blood pressure was abstracted for the first 24 hours of postcardiac arrest care and percentile adjusted for age, sex, and height.22 Invasive blood pressure measurements were preferentially analyzed when available, with noninvasive blood pressure measurements analyzed when invasive measures were not available. Lowest percentile adjusted systolic blood pressure was measured within the first 6 and 24 hours after cardiac arrest, as well as at time of echocardiogram. Lowest age, sex, and height percentile-adjusted systolic blood pressure <5th percentile was dichotomized into hypotension (yes/no) within these time intervals. In addition, percentile-adjusted systolic blood pressure was time-weighted,23 summing the duration of time between documented percentile-adjusted blood pressure (percentile systolic blood pressure × minutes until next documented BP + percentile systolic blood pressure × min until next documented BP + …) divided by the summated time of all documented blood pressures; this was performed within 6 and 24 hours after initiation of post-arrest care.
Vasoactive and inotrope titration were at the discretion of clinicians. Vasoactive-inotropic score (VIS) was calculated based on the medications and doses at the time of echocardiogram, and highest value within 6 and 24 hours after cardiac arrest based on the following equation: dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 10 × milri none dose (μg/kg/min) + 10 000 × vasopressin dose (unit/kg/min) + 100 × norepinephrine dose (μg/kg/min).24,25
Statistical analysis
We measured the correlation between GLS with other echocardiographic measurements (SF and EF) using Pearson correlation coefficients. We also compared the discrimination of SF, EF and GLS for mortality with calculation of areas under the receiver operator characteristic (AUROC) curves. The association of impaired GLS and mortality prior to hospital discharge was quantified with logistic regression, controlling for age26 and initial rhythm,27 chosen a priori as these are both important demographic and arrest characteristic affecting outcomes. Logistic regression was performed with GLS as a categorical variable, as well as a continuous variable. Final results were presented with the continuous Z-scored GLS values. While hypotension4–7 and higher VIS28 are associated with mortality, we did not control for these as we postulate that these are causal mediators along the pathway between myocardial dysfunction and mortality.
Variables are presented as n (%) or median (interquartile range, IQR) and compared based on presence or absence of impaired GLS utilizing Fisher’s exact test or Wilcoxon rank sum, as appropriate. All statistical analyses were performed with Stata SE Release 16 (College Station, TX; StataCorp LP). P-values of <0.05 were selected as statistically significant.
Results
Of the 170 subjects who had echocardiograms performed within 24 hours, 124 (73%) subjects had measurable GLS (Fig. 1). The cohort’s clinical and arrest characteristics, echocardiographic measures and outcomes are shown in Table 1. The median age at time of arrest was 4 years old (IQR 0.7, 11.1), with more subjects experiencing OHCA (58%) (Table 1). Of those with a first documented rhythm, pulseless electrical activity (PEA) was the most common, but 27% of the cohort had an unknown/undocumented first rhythm. Eighteen subjects (14%) were defibrillated at least once. A small percentage of subjects had post-arrest hypotension at time of echocardiogram (14%), but the majority of all subjects (62%) received vasoactive support in the first 24 hours after cardiac arrest, with a median peak VIS of 13 (IQR 0.5, 40). Fifty-three subjects died prior to hospital discharge (43%), and 74 (60%) had an unfavorable neurologic outcome.
Fig. 1 –
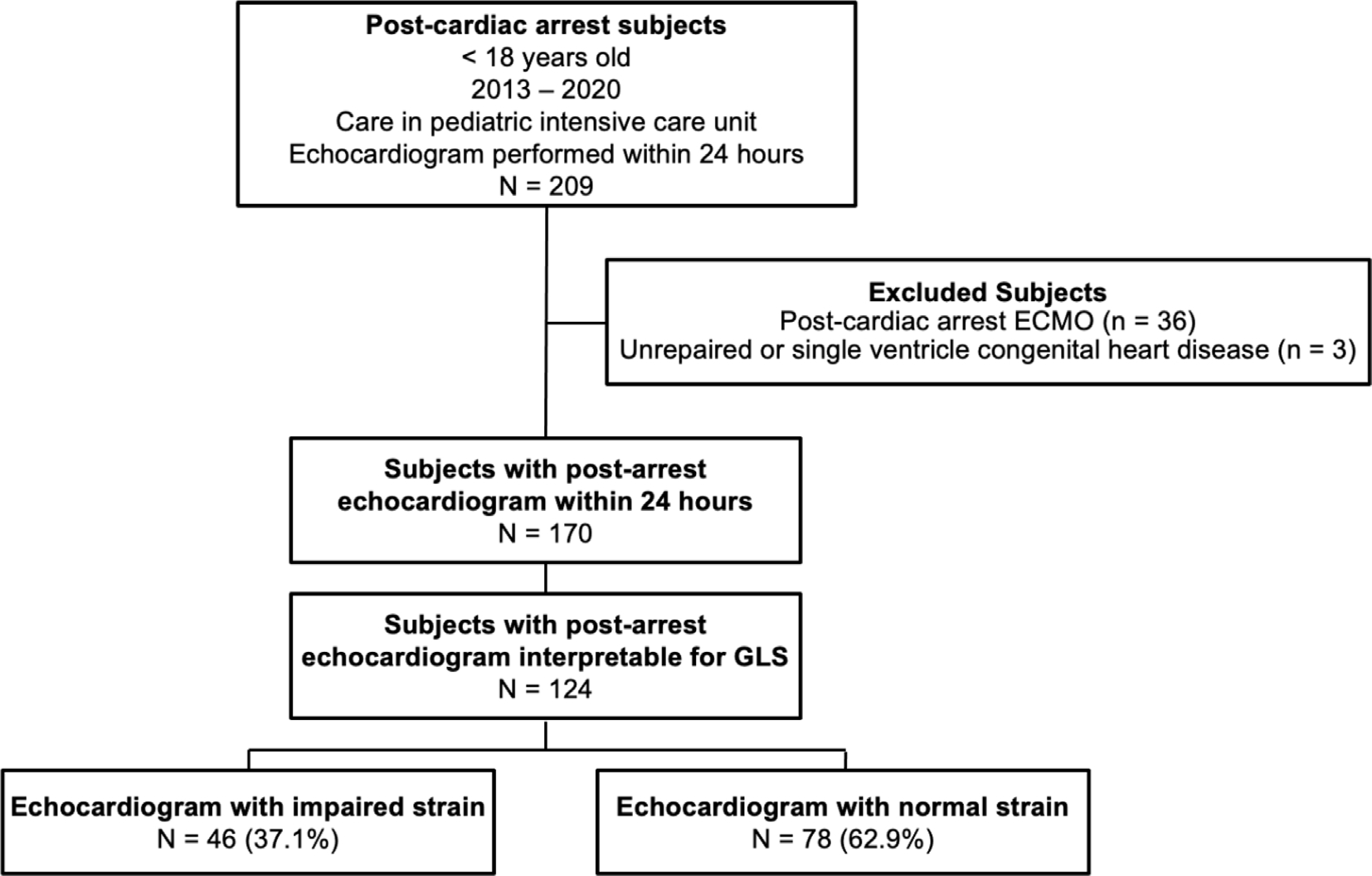
Consort diagram for retrospective post-arrest echocardiogram study.
Table 1.
Clinical characteristics and outcomes based on global longitudinal strain.
| All n = 124 | Impaired GLS1 n = 46 | Normal GLS n = 78 | P- value | |
|---|---|---|---|---|
|
| ||||
| Patient Characteristics | ||||
| Age (years) | 4.0 (0.7, 11.1) | 7.9 (2.5, 15.4) | 1.9 (0.6, 8.0) | <0.001 |
| Male gender | 66 (53.2%) | 28 (60.9%) | 38 (48.7%) | 0.190 |
| Pre-existing conditions | 37 (30.0%) | 14 (30.4%) | 23 (29.5%) | 0.911 |
| Pre-existing pressor use | 14 (11.3%) | 3 (6.5%) | 11 (14.1%) | 0.198 |
| Pre-arrest PCPC (n = 121) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | 0.644 |
| Arrest Characteristics | ||||
| Arrest location | 0.106 | |||
| OHCA | 72 (58.1%) | 31 (67.4%) | 41 (52.6%) | |
| ICHA | 52 (41.9%) | 15 (32.6%) | 37 (47.4%) | |
| Witnessed arrest (n = 121) | 91 (72.6%) | 30 (65.2%) | 61 (78.2%) | 0.170 |
| CPR duration (n = 104) | 6 (2.5, 15.0) | 8 (3, 15) | 6 (2, 15) | 0.564 |
| First documented rhythm | 0.017 | |||
| Pulseless electrical activity/asystole | 52 (24.2%) | 17 (40.0%) | 35 (44.9%) | |
| Bradycardia | 26 (21.0%) | 6 (13.0%) | 20 (25.6%) | |
| VT/VF | 12 (9.7%) | 9 (19.6%) | 3 (3.8%) | |
| Unknown/undocumented | 34 (27.4%) | 14 (30.4%) | 20 (25.6%) | |
| Defibrillation | 18 (14.5%) | 11 (23.9%) | 7 (9.0%) | 0.023 |
| Post-Arrest Laboratory & Hemodynamic Data | ||||
| Percentile adjusted SBP at echocardiogram2 | 46.2 (12.4, 90.1) | 45.1 (9.6, 94.9) | 46.3 (15.4, 84.7) | 0.885 |
| Hypotension (<5th percentile) at echocardiogram3 | 17 (13.7%) | 8 (17.4%) | 9 (11.5%) | 0.360 |
| VIS at time of echocardiogram | 2.25 (0, 15) | 4.5 (0, 25) | 1 (0, 13) | 0.197 |
| Lowest percentile adjusted SBP in first 6 hours2 (n = 104) | 7.5 (1, 54.4) | 1 (1, 57) | 14.5 (1, 52) | 0.107 |
| Hypotension BP <5th in the first 6 hours3 (n = 104) | 50 (41.8%) | 22 (47.8%) | 28 (35.9%) | 0.018 |
| Time-weighted percentile adjusted SBP in first 6 hours | 71.8 (42.0, 88.9) | 48.3 (32.3, 86.5) | 79.1 (51.1, 92.0) | 0.024 |
| Highest VIS within first 6 hours | 5 (0, 30) | 10 (0, 50) | 5 (0, 20) | 0.325 |
| Lowest percentile adjusted SBP in first 24 hours2 | 3 (1, 27) | 1 (1, 7) | 5 (1, 34) | 0.011 |
| Hypotension BP <5th in the first 24 hours3 | 65 (52.4%) | 29 (63.0%) | 36 (46.2%) | 0.028 |
| Time-weighted percentile adjusted SBP in first 24 hours | 75.5 (50.2, 89.1) | 75.5 (37.3, 89.6) | 75.2 (59.5, 88.2) | 0.456 |
| Highest VIS within first 24 hours | 13 (0.5, 40) | 23.2 (2, 85) | 11 (0, 30) | 0.030 |
| Any vasoactive support in first 24 hours (yes) | 77 (62.1%) | 31 (67.4%) | 46 (59.0%) | 0.351 |
| Highest lactate within first 24 hours (n = 67) | 6.2 (2.8, 8.9) | 3.1 (1.6, 8.0) | 7.2 (4.5, 10.4) | 0.005 |
| Highest troponin within first 24 hours (n = 58) | 0.9 (0.16, 4.23) | 2.5 (0.8, 8.6) | 0.5 (0.0, 1.2) | 0.002 |
| Post-Arrest Echocardiography | ||||
| Time to Echocardiogram (hours) | 9.7 (3.1, 17.6) | 7.6 (2.8, 20.7) | 11.6 (3.8, 17.8) | 0.896 |
| Qualitative LV Function (n = 63) | <0.001 | |||
| Normal + Hyperdynamic | 29 (46.0%) | 8 (21.6%) | 21 (80.8%) | |
| Mildly diminished | 9 (14.3%) | 5 (13.5%) | 4 (15.4%) | |
| Moderately diminished | 10 (8.1%) | 9 (24.3%) | 1 (16.7%) | |
| Severely diminished | 15 (12.1%) | 15 (40.5%) | 0 | |
| LV ejection fraction (n = 80) | 60.0 (48.8, 65.8) | 42.6 (29.8, 58.7) | 62.3 (59.3, 66.7) | <0.001 |
| LV shortening fraction (n = 106) | 35.0 (28.1, 39.3) | 23.3 (15.2, 30.1) | 36.6 (34.2, 40.8) | <0.001 |
| LV global longitudinal strain | −20.5 (−25.9, −15.3) | −12.8 (−15.74, −9.87) | −23.3 (−26.4, −20.7) | <0.001 |
| LV global longitudinal strain Z-score | 0.9 (−0.2, 2.7) | 3.5 (2.4, 4.9) | 0.1 (−1.2, 0.63) | <0.001 |
| Outcomes | ||||
| Hospital Discharge Mortality | 53 (42.7%) | 28 (60.8%) | 25 (32.1%) | 0.002 |
| Unfavorable Neurologic Survival4 | 74 (59.7%) | 31 (67.4%) | 43 (55.1%) | 0.179 |
Data presented as n (%) or median (interquartile range).
Impaired global longitudinal strain (GLS) defined as GLS ≥ 2 SD worse than age-defined normative values.
Systolic blood pressure at time of echocardiogram percentile-adjusted for age, sex, and height.
Hypotension defined as percentile-adjusted systolic blood pressure <5th percentile.
Survival with unfavorable neurologic outcome defined as Pediatric Cerebral Performance Category (PCPC) score as ≥3 or change from baseline.
The median time to echocardiogram was 9 hours (IQR 3, 17), with many subjects having normal or hyperdynamic function when qualitative assessment was reported (29/63, 46%). Median GLS for the whole cohort was −20.5% (IQR −25.9, −15.3). Impaired GLS was present in 46 subjects (37.1%).
Subjects with impaired GLS were older (median 7.9 vs. 1.9 years, p < 0.001), more likely to have ventricular tachycardia/fibrillation as initial rhythm (19.6% versus 43.8%, p = 0.017) and had higher peak troponin levels within 24 hours post-arrest (median 2.5 vs. 0.5, p = 0.002). There was no difference between frequency of IHCA and OHCA, or CPR duration based on GLS categories. On univariate analysis, subjects with impaired GLS had higher mortality (60.8% vs. 32%, p = 0.002), with no difference in unfavorable neurologic outcome.
Subjects with impaired GLS were more likely to have lower percentile-adjusted blood pressures and higher prevalence of hypotension (<5th percentile-adjusted blood pressure) within the first 6 hours of arrest and within the first 24 hours of arrest compared to those with normal GLS (Table 1). Subjects with impaired GLS had higher VIS scores within 24 hours of initiation of ICU care. Timeweighted average percentile adjusted blood pressure or VIS were not different in the 6 hours after initiation of post-arrest care.
Mortality prior to hospital discharge was more common in subjects who received pre-arrest vasopressors, had longer CPR duration, or had an unwitnessed arrest (Table 2). Hypotension, defined as percentile age-, sex- and height-adjusted blood pressure < 5th percentile, within 6 and 24 hours of cardiac arrest was more common in non-survivors. Non-survivors had higher vasoactive support at time of echocardiogram, within 6 hours of arrest and 24 hours of arrest. Time-weighted average blood pressure percentiles were not significantly different at any time point between survivors and non-survivors. All markers of ventricular function were worse in non-survivors, including qualitative measure of function, EF, SF and GLS (Table 2).
Table 2.
Clinical characteristics and outcomes based on mortality prior to hospital discharge.
| Mortality prior to hospital discharge n =53 | Survival to hospital discharge n =71 | P-value | |
|---|---|---|---|
|
| |||
| Patient Characteristics | |||
| Age (years) | 3.2 (0.7, 8.0) | 5.7 (0.8, 14.4) | 0.298 |
| Male gender | 29 (54.7%) | 37 (52.1%) | 0.774 |
| Pre-existing conditions | 17 (32.1%) | 20 (28.2%) | 0.638 |
| Pre-existing pressor use | 12 (22.6%) | 2 (2.8%) | 0.001 |
| Pre-arrest PCPC (n = 121) | 1 (1, 3) | 1 (1, 3) | 0.712 |
| Arrest location | 0.235 | ||
| OHCA | 34 (64.2%) | 38 (53.5%) | |
| ICHA | 19 (35.8%) | 33 (46.5%) | |
| Witnessed arrest (n = 121) | 31 (58.5%) | 60 (84.5%) | 0.009 |
| CPR duration (n = 104) | 10 (3, 17) | 5 (2, 14) | 0.038 |
| First documented rhythm | 0.031 | ||
| Pulseless electrical activity/asystole | 28 (52.8%) | 24 (33.8%) | |
| Bradycardia | 7 (13.2%) | 19 (26.8%) | |
| VT/VF | 2 (3.8%) | 10 (14.1%) | |
| Unknown/undocumented | 16 (30.2%) | 18 (25.4%) | |
| Defibrillation | 4 (7.5%) | 14 (19.7%) | 0.028 |
| Arrest Characteristics | |||
| Percentile adjusted SBP at echocardiogram1 | 47.5 (9.3, 96.4) | 45.6 (15.4, 79.7) | 0.772 |
| Hypotension (<5th percentile) at echocardiogram2 | 11 (20.8%) | 6 (8.5%) | 0.049 |
| VIS at time of echocardiogram | 10 (0.7, 30.0) | 0 (0, 9.0) | <0.001 |
| Lowest percentile adjusted SBP in first 6 hours1 (n = 104) | 1.5 (1.0, 20.5) | 20 (1.0, 62.5) | 0.040 |
| Hypotension BP <5th in the first 6 hours2 (n = 104) | 26 (49.1%) | 24 (33.8%) | 0.006 |
| Time-weighted percentile adjusted SBP in first 6 hours | 70.9 (40.8, 88.8) | 72.8 (43.3, 89.6) | 0.985 |
| Highest VIS within first 6 hours | 28 (5, 60) | 0 (0, 10) | <0.001 |
| Lowest percentile adjusted SBP in first 24 hours1 | 1 (1, 12) | 6(1, 34) | 0.023 |
| Time-weighted percentile adjusted SBP in first 24 hours | 75.5 (51.5, 89.1) | 71.9 (50.2, 88.2) | 0.841 |
| Hypotension BP <5th in the first 24 hours2 | 33 (62.3%) | 32 (45.1%) | 0.029 |
| Highest VIS within first 24 hours | 40 (15.3, 100.2) | 0 (0, 10) | <0.001 |
| Any vasoactive support in first 24 hours (yes) | 42 (79.2%) | 35 (49.3%) | 0.001 |
| Peak lactate within first 24 hours (n = 67) | 7.4 (2.8, 10.6) | 0 (0, 10) | 0.328 |
| Peak troponin within first 24 hours (n = 58) | 2.2 (0.5, 13.7) | 0.6 (0.04, 1.4) | 0.002 |
| Post-Arrest Echocardiography | |||
| Time to Echocardiogram (hours) | 7.2 (3.2, 18.0) | 12.0 (2.8, 17.9) | 0.713 |
| Qualitative LV Function (n = 63) | <0.001 | ||
| Normal + Hyperdynamic | 8 (36.4%) | 21 (51.2%) | |
| Mildly diminished | 5 (23.7%) | 4 (9.8%) | |
| Moderately diminished | 9 (10.2%) | 1 (2.4%) | |
| Severely diminished | 0 | 15 (36.6%) | |
| LV ejection fraction (n = 80) | 51.4 (29.3, 60.9) | 61.0 (57.0, 66.7) | 0.001 |
| LV shortening fraction (n = 106) | 31.1 (18.5, 36.2) | 36.5 (32.1,41.3) | <0.001 |
| LV global longitudinal strain | −15.8 (−22.5, −10.2) | −21.8 (−26.4, −17.5) | <0.001 |
| LV global longitudinal strain Z-score | 2.15 (0.18, 4.65) | 0.4 (−0.93, 2.02) | <0.001 |
| Impaired global longitudinal strain4 | 28 (52.8%) | 21 (30.0%) | 0.009 |
Data presented as n (%) or median (interquartile range).
Survival with unfavorable neurologic outcome defined as Pediatric Cerebral Performance Category (PCPC) score as ≥3 or change from baseline.
Systolic blood pressure at time of echocardiogram percentile-adjusted for age, sex and height.
Hypotension defined as percentile-adjusted systolic blood pressure <5th percentile.
Impaired global longitudinal strain defined as GLS ≥ 2 SD worse than age-defined normative values.
Subjects with impaired GLS compared to non-impaired GLS had lower median EF (42.6% versus 62.3%) and median SF (23.3% versus 36.6%), all p < 0.001, with strong inverse correlation between GLS and EF (rho −0.76, p < 0.001), and SF (rho −0.71, p < 0.001). GLS, EF and SF had similar discrimination for mortality, with better discrimination when all combined (Supplemental Table S2).
When controlling for age and initial rhythm, GLS was associated with mortality, with 1.17 times higher odds of mortality for every 1% increase in GLS (aOR 1.17, IQR 1.09–1.26, p < 0.001; Table 3).
Table 3.
Association of post-arrest impaired global longitudinal strain and mortality prior to hospital discharge.
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
|
| |||
| Logistic regression | |||
| Unadjusted dichotomous strain with mortality | 3.29 | 1.54–7.05 | 0.002 |
| Unadjusted continuous strain with mortality | OR 1.12 | 1.06–1.19 | <0.001 |
| When controlling for age and initial rhythm | aOR 1.17 | 1.09–1.26 | <0.001 |
Eight subjects (16.3%) with impaired GLS had qualitatively normal or hyperdynamic function; of those eight, two died prior to hospital discharge and one survivor had unfavorable neurologic outcome (Supplemental Table S1). These eight subjects were more likely to have received defibrillation during arrest and had higher VIS at time of echocardiography than those with impaired GLS and qualitatively abnormal function. In contrast, five subjects had abnormal qualitative function but normal GLS. This occurred in subjects with median age 1.4 years (IQR 0.5–6.8), all with an OHCA. Of these, 2 died prior to discharge, and 3 survived to hospital discharge, all with favorable neurologic function.
Discussion
Impaired left ventricular GLS was common in the pediatric post-arrest population with a prevalence of nearly 40% in this retrospective cohort study. These data establish that impaired GLS following pediatric cardiac arrest is associated with mortality when controlling for age and initial rhythm, with 17% increased odds of mortality for every 1% increase in GLS. Intriguingly, we found eight subjects (15%) had normal qualitative function but impaired GLS, suggesting that GLS could be a more sensitive measure of LV dysfunction in children post-cardiac arrest. To our knowledge, this study is the largest to assess pediatric post-arrest myocardial impairment9,10 and highlights that impaired left ventricular function deserves focused study as a possible modifiable risk factor impacting post-arrest outcomes.
Measuring post-arrest GLS may be advantageous over standard echocardiographic measures because it is potentially a more sensitive measure of ventricular function. While left ventricular EF and SF assume all portions of the ventricle move in consort and are based on simplified geometric features, strain assesses deformation across the ventricle, measuring movement of different portions of the ventricle. In other pediatric diseases, such as sepsis29 and multisystem inflammatory syndrome in children (MIS-C),30 GLS was more sensitive to assess deformation of the ventricular wall, even when SF appears normal, and could serve as markers of illness severity. Eight subjects had normal qualitative function but impaired GLS. While a small group in our study, subjects with discrepant measures of ventricular function could be a sub-population to focus on in future studies.
While we did not find GLS to be a superior discriminator of hospital mortality compared to standard echocardiographic EF and SF, we cannot draw conclusions from this study regarding the GLS compared to SF and EF. This may reflect our small cohort, and the retrospective nature of the study which required subjects to have the necessary three echocardiographic views to be able to measure strain. Assessing GLS prospectively along with SF and EF would potentially improve our ability to phenotype post-arrest myocardial dysfunction and understand the relationship between therapies utilized in the post-arrest period and hemodynamics, cardiac function, end-organ perfusion, and survival. This risk stratification and prediction has been demonstrated more so in the adult population with cardiomyopathy31 and myocardial infarction.32,33
We found similar rates of myocardial dysfunction to a prior publication of a non-overlapping earlier cohort from our center where SF was abnormal in 40%, with similar rates of vasoactive and inotropic support use (~60%).10 Compared to that study, our study had a lower mortality, which may reflect temporal improvements in survival rates of pediatric cardiac arrest.34,35 Like previous studies (Conlon et al. and Checchia et al.), we found that impaired ventricular function was associated with mortality.9,10 This adds to the limited body of work of assessing myocardial function in pediatrics, including more novel techniques to assess myocardial strain.
Peak lactate levels at 24 hours were higher in the normal GLS group. Notably higher lactates were associated with mortality. CPR duration was not different based on GLS, but longer durations of CPR were seen in those subjects who died. While we were unable to explore the association between arrest duration, lactate, echocardiographic findings and mortality in this small study, these data suggest that lactate does not completely explain the complex relationship of post-cardiac arrest myocardial dysfunction, intra-arrest ischemia, post-arrest ischemia and reperfusion, and mortality. Future work to understand this complex interplay.
There is an interesting pattern of impaired GLS with higher rates of VT/VF and defibrillation in the impaired GLS group. We also found a higher rate of defibrillation in the population with impaired GLS and qualitatively normal function. Our study does not permit us to disentangle whether myocardial injury prior to arrest caused arrhythmias, or if the arrhythmia and/or treatment caused subsequent myocardial injury and impaired strain: clinical data in adults and translational laboratory studies have established that cardiac arrest and/or CPR can result in post-cardiac arrest myocardial dysfunction.36,37 In addition, impaired GLS is associated with arrhythmias and discharge of internal defibrillation in the hypertrophic cardiomyopathy population.38,39
Our study was retrospective in nature, and thus there were some limitations. As the pre-hospital data available in our dataset was minimal, we used initiation of critical care on arrival to the PICU as time zero for both OHCA and IHCA patients in our cohort.7 However, there may be important differences in care between the groups and timing from cardiac arrest to echocardiogram; larger cohorts will allow for a more detailed comparison of IHCA and OHCA independently. Echocardiograms were obtained on a clinical basis, and the resultant convenience sample of patients likely differs from patients who experienced cardiac arrest but did not have an echocardiogram. Additionally, as our clinical pathway recommends echocardiography in the event of severe sequalae after arrest, the frequency and timing of post-arrest echocardiograms may be particular to our institution.8 Image quality was limited in several echocardiograms, requiring exclusion, which risks introducing a systemic bias of the association between GLS and mortality. These limitations bring to light the importance of prospective measurement of GLS and protocolization of echocardiograms in post-cardiac arrest subjects.
Conclusions
Post-cardiac arrest impaired myocardial function measured by left ventricular GLS is common after pediatric cardiac arrest, can be present in patients with normal qualitative function, and is independently associated with hospital mortality when controlling for age and initial rhythm. The importance of this finding on prognostication, treatment and future therapies necessitates post-arrest echocardiography prospectively alongside serial clinical, biochemical, and pharmacological data.
Supplementary Material
Funding
Dr. Adam Himebach receives funding from NIH NHLBI K23HL153759.
Dr. Robert Berg receives funding from NIH 5R01-HL147616-03, and NIH 1RL1HD107777-01.
Dr. Ryan Morgan receives funding from NIH K23-HL148541.
Dr. Nadir Yehya receives funding from NIH NHLBI K23-HL136688, and NIH NHLBI R01148054.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Monique M. Gardner: Conceptualization, methodology, formal analysis, writing – original draft, writing – review & editing, visualization. Yan Wang: Methodology, investigation, writing – review & editing. Adam Himebauch: Methodology, writing – review & editing. Thomas Conlon: Writing – review & editing. Kathryn Graham: Data curation, writing – review & editing. Ryan Morgan: Methodology, data curation, writing – review & editing. Rui Feng: Methodology, writing – review & editing. Robert Berg: Writing – review & editing. Nadir Yehya: Conceptualization, methodology, formal analysis, writing – original draft, writing – review & editing, supervision. Laura Mercer-Rosa: Conceptualization, methodology, writing – review & editing, supervision. Alexis Topjian: Conceptualization, methodology, formal analysis, writing – original draft, writing – review & editing, supervision.
Appendix A. Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resuscitation.2023.109936.
REFERENCES
- 1.Fink EL, Prince DK, Kaltman JR, et al. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 2016;107:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States.Circ Cardiovasc Qual Outcomes 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 3.Jentzer JC, Chonde MD, Dezfulian C. Myocardial dysfunction and shock after cardiac arrest. Biomed Res Int 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topjian AA, French B, Sutton RM, et al. Early post-resuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med 2014;42:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topjian AA, Telford R, Holubkov R, et al. Association of early postresuscitation hypotension with survival to discharge after targeted temperature management for pediatric out-of-hospital cardiac arrest: secondary analysis of a randomized clinical trial. JAMA Pediatr 2018;172:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topjian AA, Telford R, Holubkov R, et al. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation 2019;141:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laverriere EK, Polansky M, French B, Nadkarni VM, Berg RA, Topjian AA. Association of duration of hypotension with survival after pediatric cardiac arrest. Pediatr Crit Care Med 2020;21:143–9. [DOI] [PubMed] [Google Scholar]
- 8.Topjian AA, De Caen A, Wainwright MS, et al. Pediatric post–cardiac arrest care: a scientific statement from the American Heart Association. Circulation 2019;140:e194–233. [DOI] [PubMed] [Google Scholar]
- 9.Checchia PA, Sehra R, Moynihan J, Daher N, Tang W, Weil MH. Myocardial injury in children following resuscitation after cardiac arrest. Resuscitation 2003;57:131–7. [DOI] [PubMed] [Google Scholar]
- 10.Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out of hospital cardiac arrest. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intens Crit Care Soc 2015;16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haileselassie B, Su E, Pozios I, Fiskum T, Thompson R, Abraham T. Strain echocardiography parameters correlate with disease severity in children and infants with sepsis. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intens Crit Care Soc 2016;17:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himebauch AS, Yehya N, Wang Y, et al. Early right ventricular systolic dysfunction and pulmonary hypertension are associated with worse outcomes in pediatric acute respiratory distress syndrome. Crit Care Med 2018;46:e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi R, Dionne A, Ferraro A, et al. Detailed assessment of left ventricular function in multisystem inflammatory syndrome in children, using strain analysis. CJC open 2021;3:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel MD, Mariano K, Dunbar T, Cornell TT, Punn R, Haileselassie B. Cardiac dysfunction identified by strain echocardiography is associated with illness severity in pediatric sepsis. Pediatr Crit Care Med 2020;21:e192–9. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bergh A, Flameng W, Herijgers P. Parameters of ventricular contractility in mice: influence of load and sensitivity to changes in inotropic state. Pflügers Archiv-Eur J Physiol 2008;455:987–94. [DOI] [PubMed] [Google Scholar]
- 16.Park CS, Kim Y-K, Song HC, et al. Effect of preload on left atrial function: evaluated by tissue Doppler and strain imaging. Eur Heart J – Cardiovasc Imaging 2012;13:938–47. [DOI] [PubMed] [Google Scholar]
- 17.Topjian A, Hutchins L, Nadkarni V, et al. PICU and CICU clinicalpathway for the care of children post-CPR. 2022. [Google Scholar]
- 18.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J-Cardiovasc Imaging 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 20.Romanowicz J, Ferraro AM, Harrington JK, et al. Pediatric normal values and Z score equations for left and right ventricular strain by two-dimensional speckle-tracking echocardiography derived from a large cohort of healthy children. J Am Soc Echocardiogr 2023;36:310–23. [DOI] [PubMed] [Google Scholar]
- 21.Nolan JP, Berg RA, Andersen LW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry template for in-hospital cardiac arrest: a consensus report from a task force of the international Liaison committee on resuscitation (American heart association, European resuscitation Council, Australian and New Zealand Council on resuscitation, heart and stroke foundation of Canada, InterAmerican heart foundation, resuscitation Council of southern Africa, resuscitation Council of Asia). Circulation 2019;140:e746–57. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JS, Yanay O, Barry D. Age-based percentiles of measured mean arterial pressure in pediatric patients in a hospital setting. Pediatr Crit Care Med 2020;21:e759–68. [DOI] [PubMed] [Google Scholar]
- 23.Kilgannon JH, Roberts BW, Jones AE, et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest*. Crit Care Med 2014;42:2083–91. [DOI] [PubMed] [Google Scholar]
- 24.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intens Crit Care Soc 2010;11:234–8. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intens Crit Care Soc 2017;18:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation 2009;119:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Jama 2006;295:50–7. [DOI] [PubMed] [Google Scholar]
- 28.Taeck OY, Oh J, Min PS, et al. Vasoactive-inotropic score as a predictor of in-hospital mortality in out-of-hospital cardiac arrest. Signa Vitae: J Intesive Care Emerg Med 2019;15:40–4. [Google Scholar]
- 29.Lautz AJ, Wong HR, Ryan TD, Statile CJ. Myocardial dysfunction is independently associated with mortality in pediatric septic shock. Crit Care Explor 2020;2:e0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu S, Kim EJ, Sharron MP, et al. Strain Echocardiography and myocardial dysfunction in critically III children with multisystem inflammatory syndrome unrecognized by conventional echocardiography: a retrospective cohort analysis. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intens Crit Care Soc 2022;23:e145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barros MVL, Leren IS, Edvardsen T, et al. Mechanical dispersion assessed by strain echocardiography is associated with malignant arrhythmias in Chagas cardiomyopathy. J Am Soc Echocardiogr 2016;29:368–74. [DOI] [PubMed] [Google Scholar]
- 32.Haugaa Kristina H, Grenne Bjørnar L, Eek Christian H, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. J Am Coll Cardiol Img 2013;6:841–50. [DOI] [PubMed] [Google Scholar]
- 33.Ersbøll M, Valeur N, Andersen Mads J, et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. J Am Coll Cardiol Img 2013;6:851–60. [DOI] [PubMed] [Google Scholar]
- 34.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes 2013;6:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmberg MJ, Wiberg S, Ross CE, et al. Trends in survival after pediatric in-hospital cardiac arrest in the United States. Circulation 2019;140:1398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee DA. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med 1996;24:992–1000. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez MM, Berg RA, Nadkarni VM, et al. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation 2008;117:1864–72. [DOI] [PubMed] [Google Scholar]
- 38.Reant P, Mirabel M, Lloyd G, et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016;102:741–7. [DOI] [PubMed] [Google Scholar]
- 39.Tower-Rader A, Mohananey D, To A, Lever HM, Popovic ZB, Desai MY. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: a systematic review of existing literature. J Am Coll Cardiol Img 2019;12:1930–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


