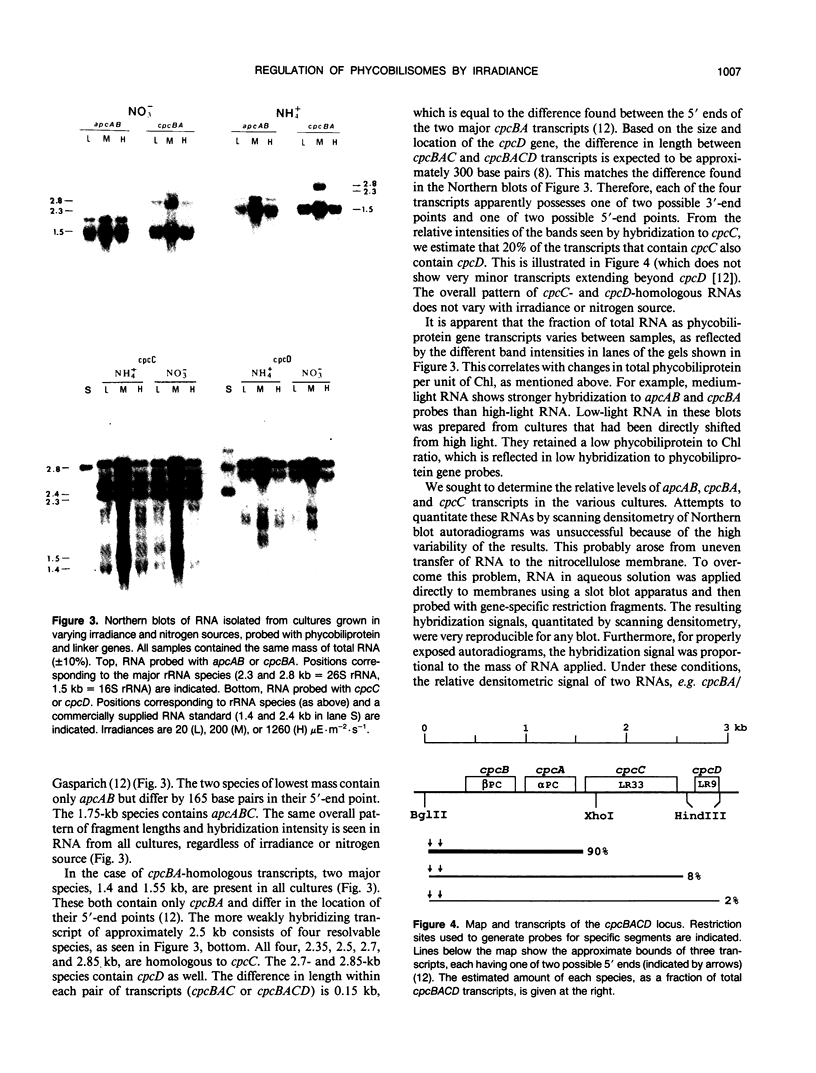
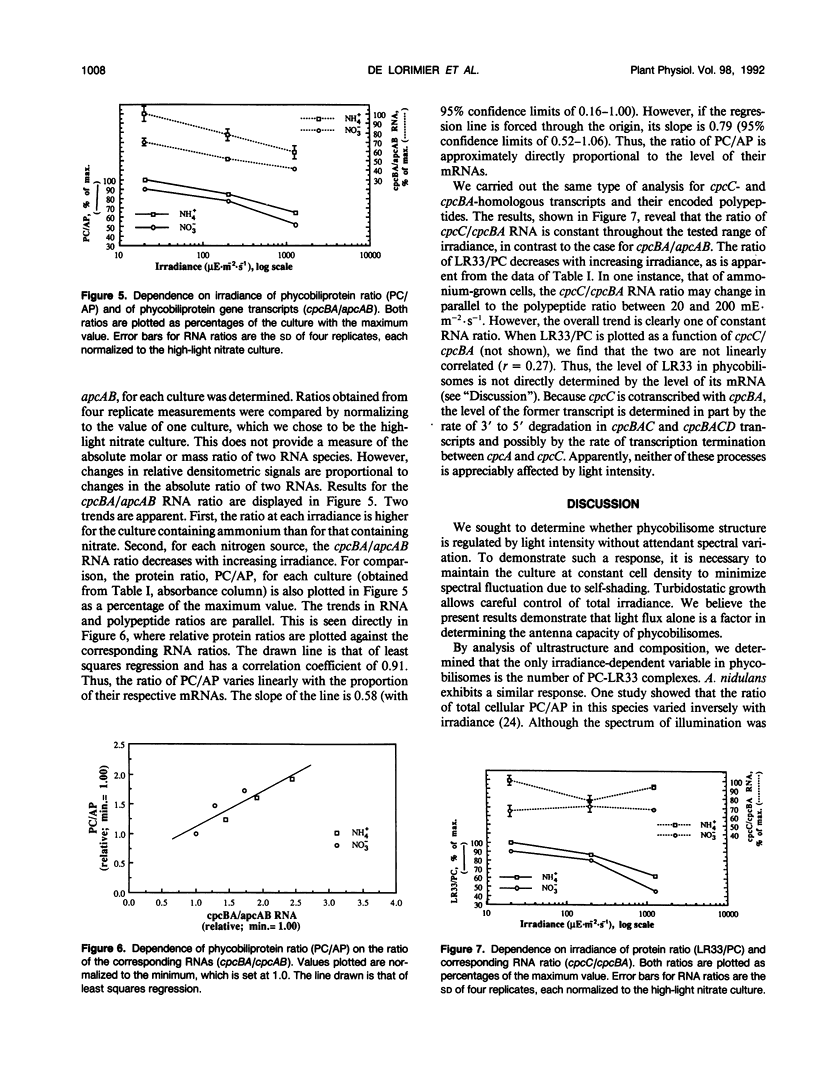
Abstract
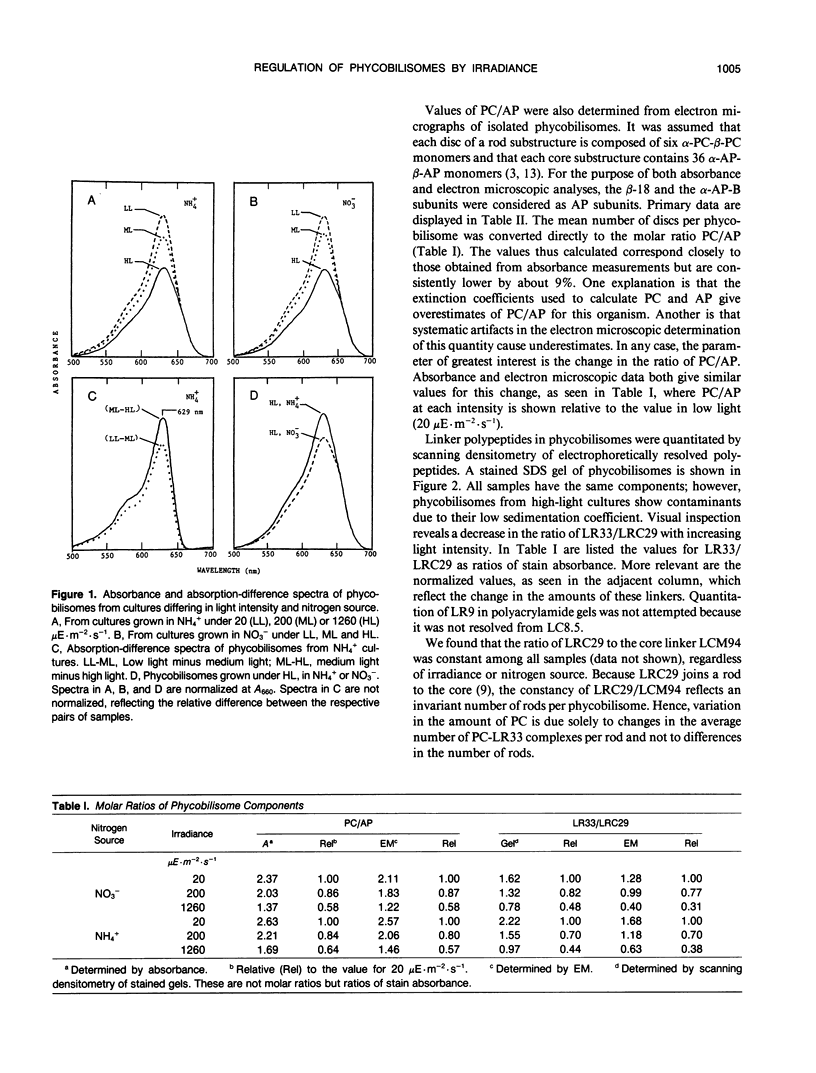
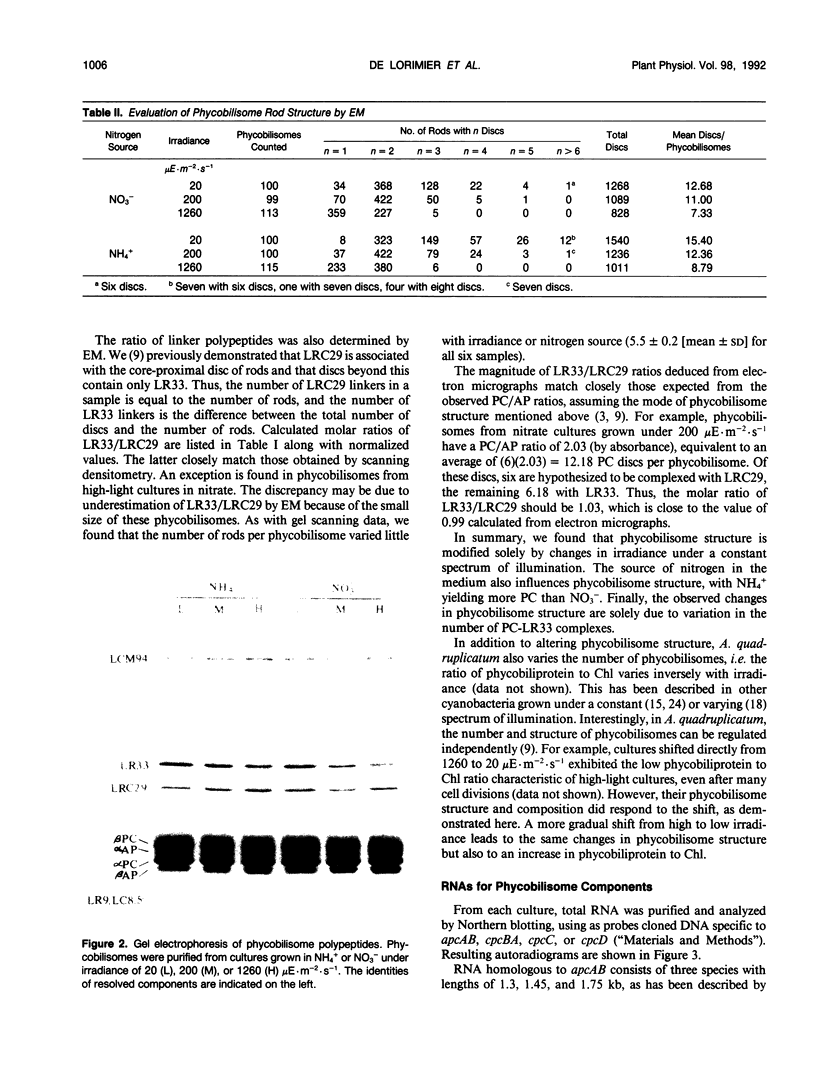
The cyanobacterium Agmenellum quadruplicatum PR-6 (Synechococcus sp PCC 7002) was grown turbidostatically in white light at three levels of irradiance: 20, 200, and 1260 microeinsteins per square meter per second. Phycobilisomes were isolated from each culture and analyzed by absorbance, gel electrophoresis, and electron microscopy. The ratio of phycocyanin to allophycocyanin decreased 1.8-fold from the lowest to highest irradiance. This change was due entirely to an approximately 2.5-fold decrease in one structural unit of rod domains, the complex of phycocyanin, and a 33-kilodalton linker polypeptide (LR33). For a given irradiance, phycobilisomes from cells grown on ammonium as the nitrogen source had 10 to 20% more phycocyanin than those from nitrate cultures. Total RNA was isolated from all cultures and probed with gene fragments specific to phycocyanin and allophycocyanin subunits and LR33. The relative level of RNAs encoding phycocyanin and allophycocyanin was found to vary with light intensity in parallel with the phycobiliprotein ratio. Hence, the light-harvesting capacity of phycobilisomes is directly regulated by relative levels of phycobiliprotein mRNA. The LR33 transcript occurs as a 3′ extension on about 10% of phycocyanin transcripts. The ratio of RNA encoding LR33 to that encoding phycocyanin did not vary with irradiance, although the protein ratio changed 1.7- to twofold between extremes. Based on these and other observations, we propose that the LR33 protein is constitutively synthesized at a rate higher than that required to complex with available phycocyanin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Guglielmi G., Stevens S. E., Jr Structural and compositional analyses of the phycobilisomes of Synechococcus sp. PCC 7002. Analyses of the wild-type strain and a phycocyanin-less mutant constructed by interposon mutagenesis. Arch Microbiol. 1990;153(6):550–560. doi: 10.1007/BF00245264. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. Ribosomal ribonucleic acid synthesis and maturation in the blue-green alga Anacystis nidulans. J Bacteriol. 1972 Aug;111(2):316–324. doi: 10.1128/jb.111.2.316-324.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J. C., Lundell D. J., Glazer A. N. Core substructure in cyanobacterial phycobilisomes. J Cell Biochem. 1983;22(1):1–14. doi: 10.1002/jcb.240220102. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Khanna R., Graham J. R., Myers J., Gantt E. Phycobilisome composition and possible relationship to reaction centers. Arch Biochem Biophys. 1983 Jul 15;224(2):534–542. doi: 10.1016/0003-9861(83)90241-2. [DOI] [PubMed] [Google Scholar]
- Kumano M., Tomioka N., Sugiura M. The complete nucleotide sequence of a 23S rRNA gene from a blue-green alga, Anacystis nidulans. Gene. 1983 Oct;24(2-3):219–225. doi: 10.1016/0378-1119(83)90082-3. [DOI] [PubMed] [Google Scholar]
- Lönneborg A., Lind L. K., Kalla S. R., Gustafsson P., Oquist G. Acclimation Processes in the Light-Harvesting System of the Cyanobacterium Anacystis nidulans following a Light Shift from White to Red Light. Plant Physiol. 1985 May;78(1):110–114. doi: 10.1104/pp.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raps S., Kycia J. H., Ledbetter M. C., Siegelman H. W. Light Intensity Adaptation and Phycobilisome Composition of Microcystis aeruginosa. Plant Physiol. 1985 Dec;79(4):983–987. doi: 10.1104/pp.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Stevens S. E., Jr Genetic analysis of a 9 kDa phycocyanin-associated linker polypeptide. Biochim Biophys Acta. 1990 Aug 9;1019(1):29–41. doi: 10.1016/0005-2728(90)90121-j. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Guglielmi G., Bryant D. A., Stevens S. E., Jr Structure and mutation of a gene encoding a Mr 33,000 phycocyanin-associated linker polypeptide. Arch Microbiol. 1990;153(6):541–549. doi: 10.1007/BF00245263. [DOI] [PubMed] [Google Scholar]