Abstract
Liquid biopsies—tests that detect circulating tumor cellular components in the bloodstream—have the potential to transform cancer by reducing health inequities in screening, diagnostics, and monitoring. Today, liquid biopsies are being used to guide treatment choices for patients and monitor for cancer recurrence, and promising work in multi-cancer early detection is ongoing. However, without awareness of the barriers to adoption of this new technology and a willingness to build mitigation efforts into the implementation of widespread liquid biopsy testing, the communities that could most benefit may be the last to access and use them. In this work, we review the challenges likely to affect the accessibility of liquid biopsies in both the general population and underserved populations, and recommend specific actions to facilitate equitable access for all patients.
Increasing access to liquid biopsy for cancer: a review of barriers and proposed solutions by @blood_pac.
INTRODUCTION
Cancer remains the second-leading cause of death in the United States and imposes tremendous financial and psychological strain on patients, families, and communities.1 Furthermore, the burden of a cancer diagnosis is compounded by social determinants of health—factors such as socioeconomic status, education and literacy skills, and race—which negatively affect access to and quality of care.2 The emergence of novel technologies provides an imperative to rethink existing health care delivery paradigms, to identify barriers to access, and to reduce health disparities through effective engagement, education, and thoughtful implementation.
Cancer care is being transformed by the emergence of precision or personalized medicine to improve risk assessment, screening, diagnosis, treatment, and monitoring. Examples of precision medicine tools include germline testing for genes such as BRCA1 and BRCA2, recommended for most women with breast cancer and men with prostate cancer, DNA-based tests, which have added to the number of options to provide minimally invasive screening tests for colorectal cancer, and comprehensive genomic profiling (CGP) that can detect driver mutations in tumors in hundreds of genes with data suggesting improved patient outcomes with adoption.3 Indeed, there is a steady decrease in cancer mortality due, at least in part, to the adoption of emerging technologies to detect cancers earlier and treat cancers based upon their specific genetics.1
In this work, we focus on the accessibility promises and challenges of liquid biopsy, an emerging tool already used to enable detection, interception, and more efficient treatment of cancer that will become increasingly available over the next decade for patients. As liquid biopsy tests are approved for additional use cases and accepted into standard of care, proactively addressing anticipated barriers to access is critical to avoid perpetuating existing disparities (Fig 1). In this paper, we will evaluate the following:
1. The role of liquid biopsy technology in improving care for all
2. The potential for liquid biopsy to reduce disparities in outcomes for underserved populations
3. Barriers to accessing liquid biopsy across all populations
4. Barriers to accessing liquid biopsy among underserved populations
5. Opportunities for stakeholders to collaborate and address barriers to access
FIG 1.
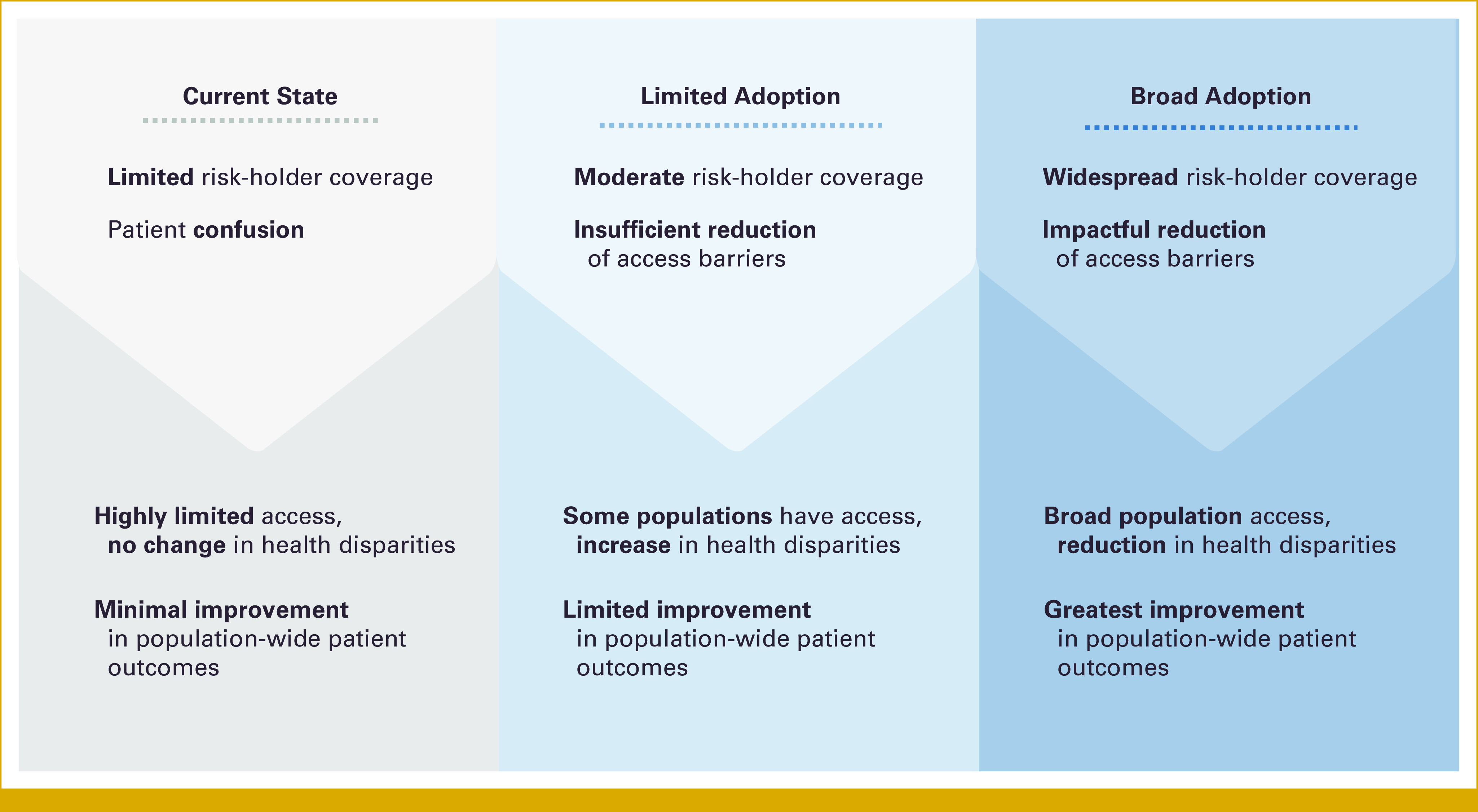
Pathways to liquid biopsy implementation and predicted impacts on health outcomes and disparities.
LIQUID BIOPSY: POPULATION-WIDE PROMISE ACROSS THE CANCER CARE CONTINUUM
Timely and accurate cancer diagnosis and treatment is critical to improving patient outcomes. The COVID-19 pandemic drastically reduced access to health care and an estimated 9.5 million Americans missed regular cancer screenings.4 Modeling suggests that over the next decade, this will lead to 10,000 excess cancer deaths from breast and colon cancer alone because of significant increases in late diagnosis and treatment.5 As cancer screening rates were already significantly lower in underserved communities prepandemic, these groups experienced a disproportional impact on access to cancer screening and care.6 As a result, the equitable adoption of technologies that can overcome the known obstacles to screening, diagnosis, and treatment of cancer is even more pressing.
Liquid biopsy can be easily performed as part of routine medical care and thus has the potential to mitigate many of the access barriers patients face across the cancer care continuum.7 The applications of liquid biopsy to cancer care can be divided into three general use cases: cancer early detection, treatment decision making, and post-treatment monitoring/molecular residual disease (MRD) detection in both curative and palliative settings (Fig 2).
FIG 2.
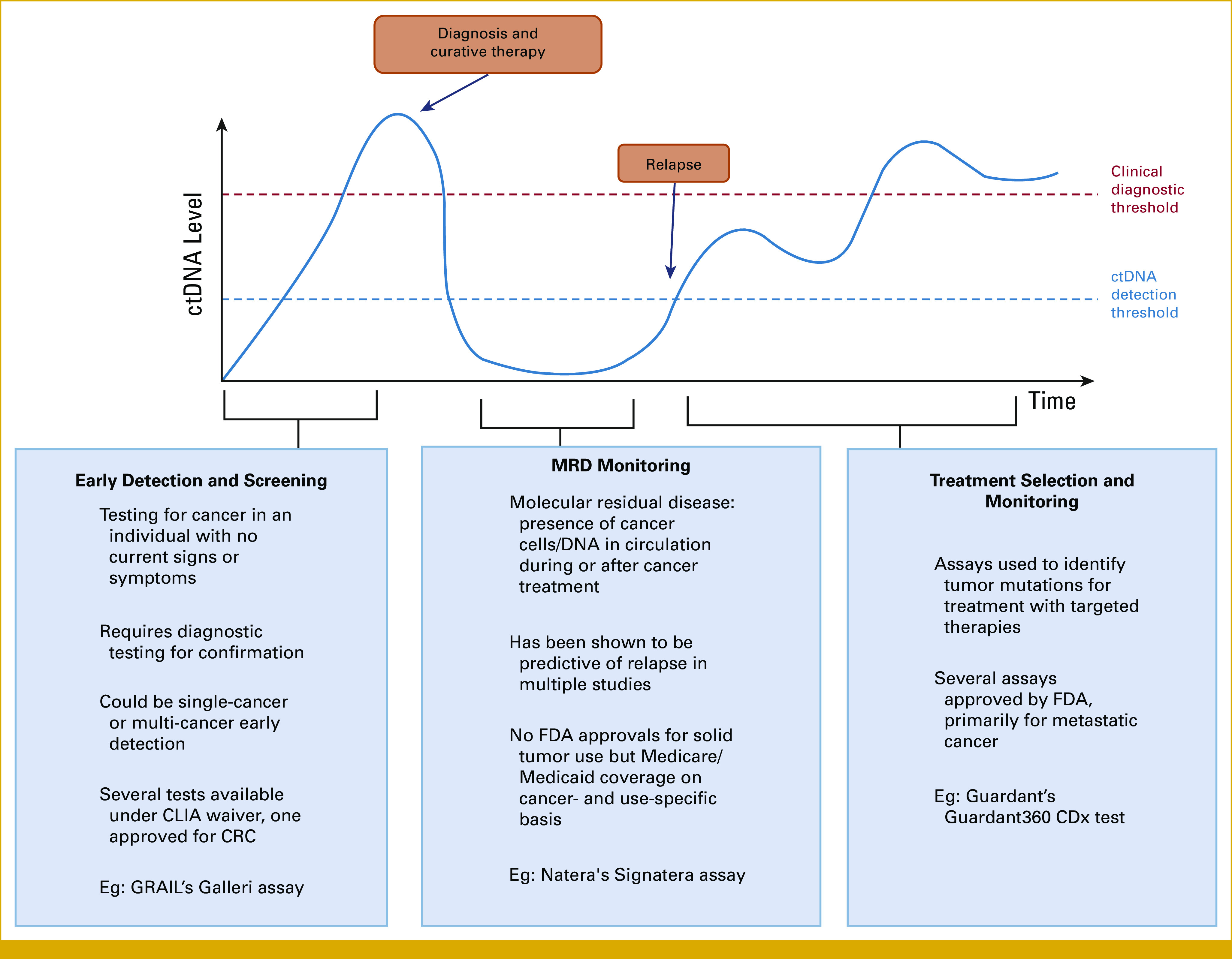
Use cases for liquid biopsy assays across the cancer patient journey. CLIA, Clinical Laboratory Improvement Amendments; CRC, colorectal cancer; ctDNA, circulating tumor DNA; FDA, US Food and Drug Administration.
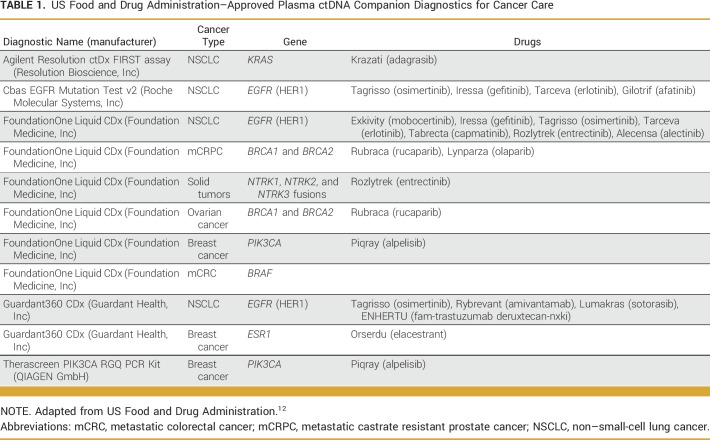
Currently, liquid biopsies are mostly used to identify targetable variants within a patient's tumor to guide management, helping patients access targeted therapies that can dramatically improve outcome.8-10 There are currently five liquid biopsy companion diagnostic assays approved by the US Food and Drug Administration (FDA) for clinical use to inform eligibility for over 17 different therapies in breast cancer, non–small-cell lung cancer, prostate cancer, colorectal cancer, ovarian cancers11 as well as one companion diagnostic for all solid tumors (Table 1).12 Although most often used for patients without sufficient tissue for molecular analysis, liquid biopsies are rapidly becoming embedded into treatment decision making for patients with cancer, especially given the faster turnaround time compared with tissue testing.13,14
TABLE 1.
US Food and Drug Administration–Approved Plasma ctDNA Companion Diagnostics for Cancer Care
In addition to treatment selection, there is growing literature on the validity of leveraging liquid biopsies for MRD detection.15,16 Although there are currently no FDA-approved MRD liquid biopsy tests, Clinical Laboratory Improvement Amendments–developed, single-site clinical tests are available and reimbursed by Medicare for colon, bladder, and breast cancers as well as to monitor response to immunotherapy cancer, and many are under development across multiple types of tumors and clinical contexts.17,18
Liquid biopsy technologies have also shown promise in identifying cancer at an early stage (screening and detection), which could be particularly impactful for cancers that are often diagnosed late.7 Although there are existing screening tests for colon, breast, cervical, lung, and prostate cancers, most require access to medical facilities for imaging and/or procedures. Adherence to screening recommendations remains low, particularly across underserved populations.2 To be introduced as a complement or alternative to existing screening methods, it is imperative that blood-based tests perform at least as well as the current standard of care.
Finally, liquid biopsy also has the potential to improve care for patients during and after treatment. Given the low risk of taking a blood draw, liquid biopsies can enable repeated sampling over time, providing insight into how a patient's cancer is changing and helping doctors provide the best possible care. During treatment, assays are being developed to monitor treatment efficacy and inform whether additional treatment options should be considered or if less aggressive treatment approaches are appropriate. After treatment, liquid biopsy assays have the potential to enable less burdensome and more timely monitoring for recurrence.
LIQUID BIOPSY—UTILITY IN MITIGATING HEALTH DISPARITIES
As for any new precision medical technology, liquid biopsy holds both the promise of improved care and the risk that only certain populations will benefit.19,20 Theoretically, the potential for liquid biopsies to improve health care access is immense. In practice, we are at a fork in the road: these assays can either deepen or decrease disparities, depending on how successful the community is in addressing accessibility barriers.
Treatment Selection and Monitoring
As noted earlier, treatment selection in solid tumors is the most common application of liquid biopsy. However, research shows significant racial and income disparities in access to genomic profiling for lung cancer treatment selection, with one recent study demonstrating a more than 10 percentage point difference in receipt of this testing between Black and White patients in the United States.21 While these studies are largely documenting disparities in tissue-based testing, the underlying factors that drive these disparities are likely to affect access and adoption of liquid biopsy testing as well. Although the causes for these disparities are understudied, studies indicate that they are driven by the socioeconomic determinants of health discussed later in this work.22,23
If these barriers are addressed, the widespread implementation of liquid biopsy for cancer treatment selection and disease monitoring could have a significant impact on the practical challenges of a cancer diagnosis. Reducing the likelihood that patients will be prescribed unnecessary treatment regimens may minimize the burden of repeated hospital/clinic visits and attendant travel, time, and childcare expenses. Moreover, liquid biopsy for recurrence monitoring may increase ease of accessing care—regular blood draws at a nearby site are more likely to increase compliance compared with repeated rounds of imaging or other more complex screening tests.24 Finally, liquid biopsy can be a route for informed treatment decision making in patients who are not candidates for tissue biopsy because of tumor location or health status.
Early Detection and Screening
Liquid biopsy could ameliorate several access barriers to routine cancer screening, potentially increasing adherence to screening guidelines within underserved communities. Currently, the majority of cancer screening options, such as colonoscopies and mammograms, require access to medical centers that are equipped with these screening capabilities. These procedures also require individuals to have consistent access to health care professionals and facilities, as well as time off from work and access to transportation and childcare. Individuals belonging to underserved groups such as racial and ethnic minorities and those living in rural areas are more likely to lack the support that makes regular and timely cancer screenings realistic.25-27 Consequently, these communities have lower screening rates and higher mortality rates across many cancer types.28,29
Several cancer early detection assays are currently in late-stage development or clinical trials, and one single-cancer detection test for colorectal cancer is FDA-approved for patients who have declined all other screening methods. In contrast to traditional cancer screening methodologies, noninvasive liquid biopsies can be completed at a laboratory facility, as part of a doctor's visit, or in a variety of other settings via mobile phlebotomy. In particular, direct-to-patient sampling for liquid biopsy assays has been explored in at least one pilot program (Foundation Medicine's FMI-Liquid@Home initiative during the COVID-19 pandemic) and shown to result in significant savings of patient and caregiver time and cost.30 Implemented on a wider scale, these alternative care delivery models could increase access to care among patients who face transportation barriers and help address the geographic challenges of health care in rural communities. Furthermore, it is well documented that the earlier a cancer is diagnosed, the lower the overall cancer-associated health care costs.31,32 Liquid biopsy–enabled early detection therefore has the potential to significantly decrease the financial burden on the individual as well as the health care systems in which they are receiving care, which would be most impactful to those with the lowest incomes.
It is important to note that although liquid biopsy can bring cancer screening to more individuals, a positive test and treatment if cancer is found will still necessitate scans, surgeries, hospital visits, and all the attendant socioeconomic challenges that accompany them. In addition, for those who have a positive test but negative diagnostic evaluation, there is the potential for increased out-of-pocket expenses from unnecessary or nondiagnostic procedures and the emotional challenges of being told, sometimes incorrectly, that one has cancer. These issues remain to be addressed by the broader cancer care community to ensure that patients can continue to access care after screening. However, by starting with a blood-based screening test, resources can be focused on those individuals who are screening test-positive and at high risk for having cancer.
POPULATION-WIDE BARRIERS TO CLINICAL ADOPTION AND PATIENT ACCESS
Uncertainty Around Technology Performance
The evidence to support use of liquid biopsies is quickly evolving, yet there is heterogeneity in the strength and availability of data on different intended uses and patient populations.33,34 The pace at which evidence is developing challenges patients, providers, health care systems, regulators, and payers to remain up to date on key developments. To achieve test coverage and adoption, it is important that each group become confident in the performance of liquid biopsies.
Ensuring the development of an evidence package that clearly outlines the analytic validity (a test's ability to measure a given analyte/biomarker), clinical validity (a test's ability to identify true diagnosis and/or differentiate groups with significantly different clinical outcomes), and clinical utility (a test's ability to inform clinical decisions and improve outcomes) is critical for clinical adoption and payer coverage. Evidence to support each specific use case for liquid biopsy, including screening/detection, treatment selection, and monitoring of recurrence for patients, is needed.35
Lack of Familiarity
Education barriers for the application of liquid biopsy are substantial in both provider and patient populations. Additionally, tests in development use a variety of different quantification, analysis, and interpretation methods. For providers educating on the emerging data, use cases and interpretation of data that complement or provide alternatives to standard of care will become increasingly necessary. For patients, communicating the essential elements of the technology along with risks, benefits, and misconceptions is of paramount importance.
Each use case of liquid biopsy—screening, treatment selection, and monitoring—presents unique educational challenges. In screening, there will be a high need for educational support for both providers and patients around decision making for positive results and the issue of false positives. Additionally, educating providers on when to order liquid biopsy screening tests, given that many cancers do not currently have early screening regimes, will be an important task. In the context of patient monitoring and treatment choice, one use challenge is the need for guidance and education on integrating liquid biopsy results with traditional imaging and tissue biopsy data.
It is likely that the increased use of liquid biopsies will create new patient categories for providers. Already, some have proposed modifying traditional TNM staging to include a blood-based detection.36 Understanding and educating health care providers on how to manage patients with positive screening tests without confirmation via traditional diagnostic workup or positive monitoring tests without radiologic correlation will be critical to the clinical implementation of liquid biopsies.
Test developers are well placed to educate payers and providers on the clinical utility of liquid biopsy assays and appropriate interpretation of results. Health systems will clarify the best management for patients after a positive screening or monitoring test. Because of the numerous use cases and abundance of different technologies that will be commercialized in the coming years, a coordinated effort to build a strong foundational understanding of circulating tumor DNA technologies should be undertaken. This will help empower providers to understand and use liquid biopsy tests to their best effect.
Inconsistent Payer Coverage
Cost is a major variable in a patient's medical decision making and is expected to play a significant role in utilization of liquid biopsy.37 Securing payer coverage, including Medicare and Medicaid coverage, will be critical to widespread adoption of this new technology as most commercially available tests face an inconsistent coverage landscape in the United States.38 Although a handful of states have recently passed legislation requiring health plans to cover biomarker tests that meet certain evidentiary requirements, including some liquid biopsy assays, the majority of states do not have such minimum coverage laws.37,39,40
Many US payers elect to not cover liquid biopsy assays, considering them experimental and investigational, and payers also express a lack of concordance between liquid biopsy and tissue-based tests.40 Liquid biopsy and tissue-based CGP share many of the same coverage challenges: payers often require testing to be deferred to smaller panels or single-gene testing as opposed to broad panels or CGP.40 Providers experience variable coverage among Medicare and commercial payers, and they also face inconsistent use case scenarios and previous authorization criteria. Although liquid biopsy CGP can have a faster turnaround time because of the noninvasive nature of testing,13,14 if not mitigated, the above issues can still delay appropriate treatment selection for late-stage, complex patients. Because of the rapidly evolving science, continued evidence generation to demonstrate clinical utility is important in supporting greater consistency in policy coverage.40
Unfortunately, these barriers to adoption are not unique to North America. On behalf of European Society for Medical Oncology, Bayle et al41 published a recent survey of European nations' adoption of biomarker technologies used in oncology. Responses were issued from 201 hospital-affiliated clinicians, primarily oncologists (72%), and biologists/pathologists (22%). Of note, very low availability was reported for liquid biopsies, which were available in clinical trials or research only in high-income countries, and never in low- or middle-income countries. Exceptions were liquid biopsies in lung cancer.41
SPECIFIC BARRIERS FOR UNDERSERVED POPULATIONS
Access to novel technologies in medicine is not shared equally across all stakeholders. Furthermore, technology adoption is not a single event and there can be significant delays between initial use and eventual adoption across diverse communities. In the context of liquid biopsy, understanding how to proactively address concerns within and across communities is critical to minimize differential adoption and resulting health inequities. Specific barriers to adoption for underserved populations include discrimination in the health care setting, mistrust of the medical establishment, and difficulty with the terminology used in educational materials.
Discrimination in Health Care
The health disparities revealed during the COVID-19 pandemic have led to increased awareness of how social determinants can affect a patient's care and health outcomes.42 Discrimination in the health care setting is a major obstacle to adequate health care. Discrimination can be defined as any negative actions or lack of consideration given to an individual or group because of bias or unjustified opinion.43 In one recent study, over 20% of respondents indicated that they experienced instances of discrimination while accessing health care.44 This proportion is similar to what has been reported in other studies, and some work has found even higher rates of discrimination when surveying specific underserved communities.45,46
Without awareness and concerted effort, discrimination may directly affect the development and utilization of liquid biopsies. First, to understand the performance of liquid biopsies across all patient populations, there must be inclusion and representation in developmental and validation studies. Enrollment of racial and ethnic minorities in clinical studies remains a challenge across most institutions but success can be realized. In the ECLIPSE study testing a blood-based screening test for colon cancer, 13% of participants self-identified as Black or African American and 15% as Hispanic of the approximately 20,000 individuals enrolled.47
Once a test is commercially available, underserved populations further face implicit bias from providers who fail to prescribe emerging technologies or are unaware of access barriers for their patients. Even when prescribed, social factors can dictate whether a patient takes a test.
Mistrust of the Medical Establishment
As is evident, patients' fears of unequal treatment and negative interactions are based upon previous and ongoing systemic discriminations, and constitute a significant barrier to accessing and using liquid biopsy. Multiple studies have established strong correlations between individual discrimination and patients' lack of trust in the health care system, avoidance or delay of care, and poor adherence to medical treatment.44,48-50
Mistrust of the medical system may be especially strong in the context of a new, experimental technology such as liquid biopsy, because of fears of being tested on and government misuse of personal health information.48 Liquid biopsy is especially susceptible to such concerns because of the nature of the genetic sequencing information collected, which may elicit worries around privacy. Although most liquid biopsies for cancer are focused on genomic findings from the tumor and not the patient's genetic material, this is poorly understood and can raise significant concerns.
Finally, continued evidence generation is important for equitable access and to demonstrate value in a generalizable population. Data from initial test development, validation, and clinical utility studies for liquid biopsy assays are often based on patient populations that do not represent the diversity of real-world practice settings, or for which demographic data are not known.51 This issue is further compounded by medical mistrust among underserved communities, negatively affecting clinical trial enrollment. Further evidence generation should focus on data that are more applicable to a diverse patient population to prevent further exacerbation of health inequalities in personalized medicine.20,52 This will help reassure providers and patients that all individuals experience the same benefit from tests.
Difficulty With Terminology
Ensuring that patients and consumers of all backgrounds understand the appropriate use and interpretation of liquid biopsies is important to their adoption. In the field of precision oncology, there is evidence that common shared language can improve patient understanding, communication between providers and patients, and facilitate shared decision making.53
Research from Cancer Support Community, a pan-cancer patient advocacy organization, shows that patients and caregivers have limited knowledge around liquid biopsy for treatment decisions.54 When testing participant familiarity and clarity of the term alone, 56% of respondents indicate they had heard of liquid biopsy and 25% say they have a basic understanding.55 Yet, when a plain-language definition of the term developed iteratively with feedback from patients and patient advocates is presented, 81% indicate a clear understanding.56
In the solid tissue biomarker testing space, inconsistent use of testing terminology has led to numerous testing terms to describe the same type of testing. The multitude of terms contributes to patient confusion over the role of this testing in their treatment decisions and care plans.53 Analysis by the Consistent Testing Terminology Working Group indicates that different testing terms are an obstacle to patient communication with providers.53
There is a need for the liquid biopsy community to work with patient-centric organizations to align on plain language for liquid biopsy to consistently communicate value to patients, policymakers, regulators, and other stakeholders within the health care delivery system who may be uncertain of the science, medical utility, or application of the technology. Creating patient-centric terminology around liquid biopsy is the responsibility of the entire health care ecosystem and must start with a shared lexicon among clinicians and payers, who are gatekeepers to patient access to new technologies. Subsequently, creating plain-language definitions of liquid biopsy, through focus groups, surveys, and testing of language with patients and consumers of all backgrounds, is critical.
OPPORTUNITIES AND RECOMMENDATIONS TO ADDRESS BARRIERS
Experience with previous technologies suggests that for liquid biopsies to break through these barriers and serve all communities, strong collaboration between test developers, payers, clinicians, and patient advocacy groups will be necessary. In addition, each stakeholder can take individual efforts to accelerate equitable liquid biopsy implementation.
To address general barriers to liquid biopsy adoption in all communities, stakeholders should consider the following actions:
1. Identify and raise awareness of the barriers to access as they are perceived by clinicians and payers.
2. Establish a common lexicon for liquid biopsy among clinicians, payers, and patients to improve understanding and appropriate use of testing.
3. Initiate further studies focusing on the clinical utility of liquid biopsy assays, to increase the likelihood of broad payer coverage and minimize the financial burden on patients.
4. Collaborate in the creation of consensus statements and evidence generation requirements to demonstrate value of liquid biopsies.35,57,58
5. Consider reporting test performance metrics, such as limit of detection and false-positive/false-negative rate, as part of an assay's results to increase transparency for providers.
6. Provide clinicians with decision-making support and educational materials to build trust and confidence in the technology.
7. Consider risk-based care reimbursement models to allow payers and clinicians to gain experience with liquid biopsy technology and reduce uncertainty around clinical value and financial impact, while also providing earlier access to patients. Illumina led two recent explorations into risk-based contracts with Harvard Pilgrim: one to expand patient access and reimbursement of noninvasive prenatal testing (NIPT) via open market access to NIPT for average-risk pregnancies, and another to leverage whole-genome sequencing (WGS) to support faster diagnoses of genetic diseases in children, and evaluate how insurance coverage of WGS affects patient care and healthcare costs.59,60
8. Ensure the requirements for test ordering and result delivery are not overly cumbersome or complicated.
To mitigate lack of awareness and education challenges specific to underserved populations, the following are recommended actions:
1. Align on patient-friendly terms for liquid biopsy and partner with community health centers, health departments, and patient advocacy organizations to capture insights on definitions.
2. Test patient-centric, plain-language terms with patients with cancer and the general public.
3. Throughout research on patient-centric language, share feedback with stakeholders responsible for educating providers and payers.
4. Emphasize consistent use of terms and definitions with all stakeholders and on test result reports.
5. Provide educational material for health care providers in underserved communities and for patients in the language they can understand, both in English and in translation.
As discussed above, it is critical to build trust in liquid biopsy technology and encourage adoption in underserved populations. Opportunities to reduce discrimination-related fears in the use of liquid biopsy tests are as follows:
1. Ensure broad representation of diverse types of individuals in clinical trial enrollment and the marketing of tests, including diversity and inclusion statements and information about the populations represented in related clinical trials.
2. Gather information from community leaders and health care centers about concerns related to blood-based testing and liquid biopsy.
3. Partner with these community leaders and organizations to address these concerns through educational content for both patients and providers, with a focus on educational materials for providers in underserved populations to alleviate privacy concerns.
4. Complement clinical trial data with real world evidence that shows utilization and outcomes in a more generalizable population.
5. Work toward the development of additional legislation to protect against misuse of genetic information, particularly in the context of insurance policies, both at the state and federal levels.
BLOODPAC'S ROLE IN THE ACCESSIBILITY DISCUSSION
Multistakeholder collaboration will be necessary to tackle all the barriers to equitable access of liquid biopsy technology, and the BLOODPAC Consortium is one of a number of organizations that are well positioned to engage in this work. Other collaborative groups such as the Foundation for the National Institute of Health (FNIH)—host of the International Liquid Biopsy Standardization Alliance—and Friends of Cancer Research, which is currently leading the ctMoniTR project, complement BLOODPAC's work in this space. A key step as the field begins to earnestly address the issues of access presented in this work will be to ensure that multistakeholder groups sufficiently represent all audiences. In particular, patient advocacy groups that work with underserved populations are critically important in the discussion and decision-making process.
Collaborative consortia have a unique role to play within the liquid biopsy field, allowing member organizations to consider a broad view that recognizes the full journey of new technologies. As liquid biopsy testing moves toward maturity, BLOODPAC and its sister not-for-profits are applying this holistic outlook to the upcoming challenge of making this technology accessible to all individuals.
CALL TO ACTION
Through the analysis of barriers to liquid biopsy use in both the general and underserved populations and the proposed solutions recommended above, we hope to create a useful roadmap for all stakeholders, including clinicians, payers, regulators, policymakers, and patient groups, to collaborate and enact change in this field.
To begin this process, BLOODPAC has launched the Accessibility Working Group to continue identifying key barriers to clinical use and access to liquid biopsy and accelerate clinical adoption. Specific goals of the BLOODPAC Accessibility Working Group are to
1. support the development of a standardized lexicon of terms for all use cases of liquid biopsy
2. develop educational content for providers on liquid biopsy technology and clinical applications, for dissemination by member organizations
3. work with BLOODPAC's reimbursement and data working groups to assess liquid biopsy value for each use case
4. explore avenues toward establishing the analytic validity of alternative, home-based collection of liquid biopsies.
We hope that the specific actions suggested here will ensure that the next decade will bring equitable adoption of this novel precision medicine technology to every community in need. Our success will be measured in our ability to prevent the introduction of new accessibility barriers into the continuum of cancer care and ensuring these technologies can be used to improve cancer outcomes for all.
ACKNOWLEDGMENT
The authors wish to acknowledge the BLOODPAC Executive Committee and Scientific Co-Chairs as of Fall 2023: Executive Committee: Philip G. Febbo—Senior Vice President and Chief Medical Officer, Illumina; Robert L. Grossman—Professor, University of Chicago CTDS & Founder/Director, Open Commons Consortium; Peter Kuhn—Professor, University of Southern California; Anne-Marie Martin—Senior Vice President and Global Head of the Experimental Medicine Unit in Oncology Research & Development, GlaxoSmithKline; Jake Vinson—Chief Executive Officer, Prostate Cancer Clinical Trials Consortium. Scientific Co-Chairs: Kelli Bramlett—Director of R&D, Thermo Fisher Scientific; Darya Chudova—SVP, Technology, Guardant Health; Jennifer Dickey—Head of Regulatory & Quality, Personal Genome Diagnostics; James H. Godsey—VP, Advanced Dx R&D, Quest Diagnostics; Jerry S.H. Lee—Chief Science and Innovation Officer, Lawrence J Ellison Institute for Transformative Medicine of USC; Howard I. Scher—Physician and Head, Biomarker Development Initiative at Memorial Sloan Kettering Cancer Center.
Phillip G. Febbo
Employment: Illumina
Leadership: Illumina, Varian Medical Systems
Stock and Other Ownership Interests: Illumina
Research Funding: Illumina
Travel, Accommodations, Expenses: Illumina
Mina Allo
Employment: Tempus, Henry Ford Health System
Emma B. Alme
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Gebra Cuyun Carter
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Robert Dumanois
Employment: Thermo Fisher Scientific, Carolina Radiology
Stock and Other Ownership Interests: Thermo Fisher Scientific
Estevan Kiernan
Employment: Illumina
Stock and Other Ownership Interests: Illumina
Travel, Accommodations, Expenses: Illumina
Nikki Martin
Stock and Other Ownership Interests: Roche
Lauren C. Leiman
Stock and Other Ownership Interests: Exact Sciences, Illumina, InVitae, Starr Surgical, Lilly
No other potential conflicts of interest were reported.
DISCLAIMER
The work described here was done through the BLOODPAC Consortium, which is a not-for-profit consortium consisting of members from industry, academia, not-for-profits, and US Government agencies, including companies that sell liquid biopsy assays, companies that use liquid biopsy assays as companion diagnostics, organizations that do research related to liquid biopsies, organizations that conduct clinical trials involving liquid biopsies, and agencies that develop policies and procedures related to liquid biopsies. In addition, some of the authors are employed by companies in the liquid biopsy field, have stock in companies in the liquid biopsy field, or consult with companies in the liquid biopsy field. The authors worked together collaboratively to develop consensus opinions and the authors do not have any particular or specific conflict with the work described in this paper, beyond those enumerated in the Conflict of Interest section.
SUPPORT
Supported by the BLOODPAC Consortium and its members.
AUTHOR CONTRIBUTIONS
Conception and design: Phillip G. Febbo, Mina Allo, Emma Alme, Robert Dumanois, Alessia Essig, Estevan Kiernan, Caitlin B. Kubler, Nikki Martin, Lauren C. Leiman
Financial support: Lauren C. Leiman
Administrative support: Robert Dumanois, Alessia Essig, Lauren C. Leiman
Collection and assembly of data: Emma Alme, Gebra Cuyun Carter, Robert Dumanois, Alessia Essig, Caitlin B. Kubler, Medeea C. Popescu, Lauren C. Leiman, Nikki Martin
Data analysis and interpretation: Emma Alme, Robert Dumanois, Caitlin B. Kubler
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Phillip G. Febbo
Employment: Illumina
Leadership: Illumina, Varian Medical Systems
Stock and Other Ownership Interests: Illumina
Research Funding: Illumina
Travel, Accommodations, Expenses: Illumina
Mina Allo
Employment: Tempus, Henry Ford Health System
Emma B. Alme
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Gebra Cuyun Carter
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Robert Dumanois
Employment: Thermo Fisher Scientific, Carolina Radiology
Stock and Other Ownership Interests: Thermo Fisher Scientific
Estevan Kiernan
Employment: Illumina
Stock and Other Ownership Interests: Illumina
Travel, Accommodations, Expenses: Illumina
Nikki Martin
Stock and Other Ownership Interests: Roche
Lauren C. Leiman
Stock and Other Ownership Interests: Exact Sciences, Illumina, InVitae, Starr Surgical, Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2. Williams PA, Zaidi SK, Sengupta R. AACR cancer disparities progress report 2022. Cancer Epidemiol Biomarkers Prev. 2022;31:1249–1250. doi: 10.1158/1055-9965.EPI-22-0542. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) National Comprehensive Cancer Network, Inc. 2024. https://www.nccn.org/ [Google Scholar]
- 4. Chen RC, Haynes K, Du S, et al. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7:878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 6.Goding Sauer A. Cancer Prevention and Early Detection 2022 Tables & Figures. Atlanta, GA, American Cancer Society; 2022. [Google Scholar]
- 7. Clarke CA, Lang K, Putcha G, et al. BLOODPAC: Collaborating to chart a path towards blood-based screening for early cancer detection. Clin Transl Sci. 2023;16:5–9. doi: 10.1111/cts.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vidula N, Niemierko A, Malvarosa G, et al. Tumor tissue- versus plasma-based genotyping for selection of matched therapy and impact on clinical outcomes in patients with metastatic breast cancer. Clin Cancer Res. 2021;27:3404–3413. doi: 10.1158/1078-0432.CCR-20-3444. [DOI] [PubMed] [Google Scholar]
- 9. Saarenheimo J, Eigeliene N, Andersen H, et al. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. doi: 10.3389/fonc.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benavides M, Alcaide-Garcia J, Torres E, et al. Clinical utility of comprehensive circulating tumor DNA genotyping compared with standard of care tissue testing in patients with newly diagnosed metastatic colorectal cancer. ESMO Open. 2022;7:100481. doi: 10.1016/j.esmoop.2022.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vellanki PJ, Ghosh S, Pathak A, et al. Regulatory implications of ctDNA in immuno-oncology for solid tumors. J Immunother Cancer. 2023;11:e005344. doi: 10.1136/jitc-2022-005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration . List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools) FDA; https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools [Google Scholar]
- 13. Thompson JC, Aggarwal C, Wong J, et al. Plasma genotyping at the time of diagnostic tissue biopsy decreases time-to-treatment in patients with advanced NSCLC-results from a prospective pilot study. JTO Clin Res Rep. 2022;3:100301. doi: 10.1016/j.jtocrr.2022.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25:4691–4700. doi: 10.1158/1078-0432.CCR-19-0624. [DOI] [PubMed] [Google Scholar]
- 15. Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1:873–881. doi: 10.1038/s43018-020-0096-5. [DOI] [PubMed] [Google Scholar]
- 16. Kotani D, Oki E, Nakamura Y, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29:127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasi PM, Fehringer G, Taniguchi H, et al. Impact of circulating tumor DNA–based detection of molecular residual disease on the conduct and design of clinical trials for solid tumors. JCO Precis Oncol. doi: 10.1200/PO.21.00181. 10.1200/PO.21.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signatera—Circulating Tumor DNA Blood Test. Natera; https://www.natera.com/oncology/signatera-advanced-cancer-detection/ [Google Scholar]
- 19. Cheung ATM, Palapattu EL, Pompa IR, et al. Racial and ethnic disparities in a real-world precision oncology data registry. NPJ Precis Oncol. 2023;7:7. doi: 10.1038/s41698-023-00351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geneviève LD, Martani A, Shaw D, et al. Structural racism in precision medicine: Leaving no one behind. BMC Med Ethics. 2020;21:17–13. doi: 10.1186/s12910-020-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruno DS, Hess LM, Li X, et al. Racial disparities in biomarker testing and clinical trial enrollment in non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39 suppl 15; abstr 9005. [Google Scholar]
- 22. Edwards TL, Breeyear J, Piekos JA, et al. Equity in health: Consideration of race and ethnicity in precision medicine. Trends Genet. 2020;36:807–809. doi: 10.1016/j.tig.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griffith DM. Precision medicine approaches to health disparities research. Ethn Dis. 2020;30(suppl 1):129–134. doi: 10.18865/ed.30.S1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cykert S, Eng E, Manning MA, et al. A multi-faceted intervention aimed at Black-White disparities in the treatment of early stage cancers: The ACCURE pragmatic quality improvement trial. J Natl Med Assoc. 2020;112:468–477. doi: 10.1016/j.jnma.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 25. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 26.Tong M, Hill L, Artiga S. Racial Disparities in Cancer Outcomes, Screening, and Treatment. Kaiser Family Foundation; https://www.kff.org/racial-equity-and-health-policy/issue-brief/racial-disparities-in-cancer-outcomes-screening-and-treatment/ [Google Scholar]
- 27. Syed ST, Gerber BS, Sharp LK. Traveling towards disease: Transportation barriers to health care access. J Community Health. 2013;38:976–993. doi: 10.1007/s10900-013-9681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goding Sauer A, Siegel RL, Jemal A, et al. Current prevalence of major cancer risk factors and screening test use in the United States: Disparities by education and race/ethnicity. Cancer Epidemiol biomarkers Prev. 2019;28:629–642. doi: 10.1158/1055-9965.EPI-18-1169. [DOI] [PubMed] [Google Scholar]
- 29. Downey LH. Rural populations and health: Determinants, disparities, and solutions. Prev Chronic Dis. 2013;10:130097. [Google Scholar]
- 30. Napolitano S, Caputo V, Ventriglia A, et al. Liquid biopsy at home: Delivering precision medicine for patients with cancer during the COVID-19 pandemic. Oncologist. 2022;27:e633–e641. doi: 10.1093/oncolo/oyac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy SR, Broder MS, Chang E, et al. Cost of cancer management by stage at diagnosis among Medicare beneficiaries. Curr Med Res Opin. 2022;38:1285–1294. doi: 10.1080/03007995.2022.2047536. [DOI] [PubMed] [Google Scholar]
- 32. McGarvey N, Gitlin M, Fadli E, et al. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv Res. 2022;22:1155. doi: 10.1186/s12913-022-08457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol. 2018;36:1631–1641. doi: 10.1200/JCO.2017.76.8671. [DOI] [PubMed] [Google Scholar]
- 34. Hasenleithner SO, Speicher MR. A clinician’s handbook for using ctDNA throughout the patient journey. Mol Cancer. 2022;21:81–29. doi: 10.1186/s12943-022-01551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ijzerman MJ, de Boer J, Azad A, et al. Towards routine implementation of liquid biopsies in cancer management: It is always too early, until suddenly it is too late. Diagnostics. 2021;11:103. doi: 10.3390/diagnostics11010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang M, Forbes ME, Bitting RL, et al. Incorporating blood-based liquid biopsy information into cancer staging: Time for a TNMB system? Ann Oncol. 2018;29:311–323. doi: 10.1093/annonc/mdx766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dieguez G, Carioto J. The Landscape of Biomarker Testing Coverage in the United States. Milliman White Paper; https://www.milliman.com/-/media/milliman/pdfs/2022-articles/2-16-22_the_landscape_of_biomarker_testing_coverage_in_the_us.ashx [Google Scholar]
- 38. Douglas MP, Ragavan MV, Chen C, et al. Private payer and medicare coverage policies for use of circulating tumor DNA tests in cancer diagnostics and treatment. J Natl Compr Canc Netw. 2023;21:609–616.e4. doi: 10.6004/jnccn.2023.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Cancer Society Cancer Action Network . Access to Biomarker Testing. https://www.fightcancer.org/what-we-do/access-biomarker-testing [Google Scholar]
- 40. Douglas MP, Gray SW, Phillips KA. Private payer and medicare coverage for circulating tumor DNA testing: A historical analysis of coverage policies from 2015 to 2019. J Natl Compr Cancer Netw. 2020;18:866–872. doi: 10.6004/jnccn.2020.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bayle A, Bonastre J, Chaltiel D, et al. ESMO study on the availability and accessibility of biomolecular technologies in oncology in Europe. Ann Oncol. 2023;34:934–945. doi: 10.1016/j.annonc.2023.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Ndugga N, Artiga S. Disparities in Health and Health Care: 5 Key Questions and Answers. Kaiser Family Foundation; 2021. pp. 1–12. [Google Scholar]
- 43.Togioka BM, Duvivier D, Young E. Diversity and Discrimination in Healthcare. St Petersburg, FL, StatPearls; 2021. [Google Scholar]
- 44. Nong P, Raj M, Creary M, et al. Patient-reported experiences of discrimination in the US health care system. JAMA Netw Open. 2020;3:e2029650. doi: 10.1001/jamanetworkopen.2020.29650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedman JY, Anstrom KJ, Weinfurt KP, et al. Perceived racial/ethnic bias in healthcare in Durham County, North Carolina: A comparison of community and national samples. N C Med J. 2005;66:267–275. [PubMed] [Google Scholar]
- 46. Rivenbark JG, Ichou M. Discrimination in healthcare as a barrier to care: Experiences of socially disadvantaged populations in France from a nationally representative survey. BMC Public Health. 2020;20:31. doi: 10.1186/s12889-019-8124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guardant Health announces positive results from pivotal ECLIPSE study evaluating a blood test for the detection of colorectal cancer. https://investors.guardanthealth.com/press-releases/press-releases/2022/Guardant-Health-announces-positive-results-from-pivotal-ECLIPSE-study-evaluating-a-blood-test-for-the-detection-of-colorectal-cancer/default.aspx
- 48. Smith AC, Woerner J, Perera R, et al. An investigation of associations between race, ethnicity, and past experiences of discrimination with medical mistrust and COVID-19 protective strategies. J Racial Ethn Health Disparities. 2022;9:1430–1442. doi: 10.1007/s40615-021-01080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bird ST, Bogart LM. Perceived race-based and socioeconomic status (SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11:554–563. [PubMed] [Google Scholar]
- 50. Harris R, Cormack D, Tobias M, et al. Self-reported experience of racial discrimination and health care use in New Zealand: Results from the 2006/07 New Zealand Health Survey. Am J Public Health. 2012;102:1012–1019. doi: 10.2105/AJPH.2011.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Memorial Sloan Kettering Cancer Center . At AACR, MSK Researchers Spotlight Health Disparities and Propose Solutions. 2022. https://www.mskcc.org/news/aacr-msk-researchers-spotlight-health-disparities-and-propose-solutions [Google Scholar]
- 52. Cohn EG, Henderson GE, Appelbaum PS. Distributive justice, diversity, and inclusion in precision medicine: What will success look like? Genet Med. 2017;19:157–159. doi: 10.1038/gim.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin NA, Tepper JE, Giri VN, et al. Adopting consensus terms for testing in precision medicine. JCO Precis Oncol. doi: 10.1200/PO.21.00027. 10.1200/PO.21.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danelle Johnston MRH-OO. Relevance of Health Literacy to Precision Medicine. National Academies Press; 2016. Patient preferences and understanding of a precision medicine lexicon—Toward the development of patient-friendly, consistent terminology. [Google Scholar]
- 55.Saxton C. Developing a Patient-Friendly, Plain Language Precision Medicine Lexicon, Journal of Oncology Navigation & Survivorship (JONS) 12. 2021. [Google Scholar]
- 56. Claire Saxton M, Gonzalo MB, Karubian J, et al. Patient preferences and understanding of a precision medicine lexicon-towards the development of patient-friendly, consistent terminology. Acad Oncol Nurse Patient Navigators Conf. 2021;12:390–391. [Google Scholar]
- 57. Faulkner E, Holtorf AP, Walton S, et al. Being precise about precision medicine: What should value frameworks incorporate to address precision medicine? A report of the personalized precision medicine special interest group. Value Health. 2020;23:529–539. doi: 10.1016/j.jval.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 58. Garfield S, Polisena J, S Spinner D, et al. Health technology assessment for molecular diagnostics: Practices, challenges, and recommendations from the medical Devices and diagnostics special interest group. Value Health. 2016;19:577–587. doi: 10.1016/j.jval.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Illumina, Harvard Pilgrim Partner to Improve NIPT Access for Women Under 35. myADLM.org; https://www.aacc.org/cln/articles/2018/april/illumina-harvard-pilgrim-partner-to-improve-nipt-access-for-women-under-35 [Google Scholar]
- 60.Illumina and Harvard Pilgrim Health Care Expand Access to Whole-Genome Sequencing for Genetic Disease Testing. Nasdaq; https://www.nasdaq.com/press-release/illumina-and-harvard-pilgrim-health-care-expand-access-to-whole-genome-sequencing-for [Google Scholar]