Abstract
Risk of cardiovascular disease (CVD) may be changing in people with cystic fibrosis (pwCF) with widespread use of highly effective modulator therapy (HEMT). We performed a retrospective analysis of patients who had lipids checked before and after initiation of ivacaftor or elexacaftor/tezacaftor/ivacaftor. We hypothesized that HEMT negatively impacts lipids (total cholesterol [TC], low-density lipoprotein [LDL], high-density lipoprotein [HDL], TC/HDL ratio). 41 adult patients were included. Paired t-tests showed statistically significant increases in TC (mean difference 16.3 mg/dL, p=0.007, n=40), LDL (mean difference 17.1 mg/dL, p<0.001, n=35), and TC/HDL ratio (mean difference 0.40, p=0.014, n=39) after HEMT initiation. HDL was unchanged (mean difference −1.5 mg/dL, p=0.69, n=39). Linear mixed models showed CF liver disease was associated with significantly blunted changes in TC and LDL. Family history of CVD risk factors was associated with significantly accentuated increases in TC and LDL. These data suggest a role for more lipid screening in pwCF.
Keywords: Cystic fibrosis, CFTR modulators, Cholesterol, Cardiovascular disease
1. Background
Historically, cardiovascular disease (CVD) has been rare in people with cystic fibrosis (pwCF) [1–3]. However, with advances in CF treatments, pwCF are living longer, experiencing increased rates of being overweight or obese, and facing a higher lifetime risk of cystic fibrosis related diabetes (CFRD) related to aging [4, 5]. Recent case reports of myocardial infarction, combined with prior evidence of pre-clinical atherosclerosis, suggest that the risk of CVD in pwCF may be changing. [6–8].
Hyperlipidemia is an important risk factor for CVD. Prior to widespread HEMT use, lipid studies in pwCF often demonstrated low total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), whereas triglycerides were typically elevated compared to age-matched controls [9–14]. It is unknown, however, whether HEMT like ivacaftor (IVA) or elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) influences serum lipids in pwCF due to altered absorption or metabolism.
2. Methods
We conducted a retrospective observational study to test the hypothesis that initiation of HEMT would significantly alter lipid profiles in pwCF. The study was approved by the University of North Carolina Institutional Review Board (IRB). Included pwCF were ≥18 years of age at time of chart review and had lipids checked within 5 years of starting HEMT and at any timepoint thereafter. Lipids were not consistently collected in fasting state. Testing at UNC was performed by the Atellica CH Cholesterol_2 (Chol_2) assay via enzymatic testing with the Atellica CH Analyzer (by Siemens). Calculated LDL was determined by the Friedewald equation [15]. Testing performed at outside lab facilities (27/82 samples, or 32.9%) may have used other methods, which were not documented. Exclusion criteria included solid organ transplantation and type 1 diabetes. Primary endpoints were absolute values and changes in TC, LDL, HDL, and TC/HDL ratio from baseline after HEMT initiation. Demographic and clinical characteristics were assessed at HEMT initiation and at the time of the post-HEMT lipid panel. The presence of pancreatic insufficiency, CFRD, and CF liver disease (CFLD) were determined by careful chart review of history, imaging, lab, and prescription data. Other CVD risk factors were similarly ascertained, including hypertension (by diagnosis code), any recorded family history of CVD or CVD risk factors (including hypertension, hyperlipidemia, heart disease, stroke), tobacco use, and body mass index (BMI).
Pre/Post-HEMT TC, HDL, LDL, and TC/HDL values were compared via paired t-tests. The significance of findings did not change when potential outliers were excluded; therefore, all data were used. Secondary analyses using linear mixed effect models were performed to assess the effect of selected individual covariates (age, sex, pancreatic status, CFRD, CFLD, statin therapy, prior modulator therapy, duration on HEMT, BMI, family history) on the size and significance of lipid parameter changes using GraphPad Prism version 9.5.0.
3. Results
41 patients met inclusion criteria (Table 1). An average of 37 months (range 5-91 months) separated lipid panel measurements. Patients were on HEMT an average of 18 months when the post-HEMT lipid panel was performed. 23 patients were on lumacaftor/ivacaftor (LUM/IVA) or tezacaftor/ivacaftor (TEZ/IVA) prior to HEMT. HEMT consisted of IVA (n=3), or ELX/TEZ/IVA (n=38).
Table 1.
Study population characteristics (n=41)
| Mean Age at HEMT start, years (range) | 40 (16 – 73) |
| Mean Duration HEMT, months (range) | 18 (3 – 49) |
| Mean Baseline FEV1pp, % (range) | 66% (19 - 125%) |
| Mean Baseline BMI (kg/m2) (range) | 25.0 (17.4 – 33.6) |
| Female (%) | 27 (66%) |
| Pancreatic Insufficient (%) | 35 (85%) |
| CFRD (%) | 23 (56%) |
| CF liver disease (%) | 12 (29%) |
| Chronic pseudomonas infection (%) | 27 (66%) |
| Prior modulator therapy (%) | 23 (56%) |
HEMT = highly effective modulator therapy; FEV1pp = forced expiratory volume in 1 second, percent predicted; BMI = body mass index; CFRD = cystic fibrosis related diabetes; CF = cystic fibrosis.
Baseline CVD risk factors were assessed in the study cohort. Tobacco use was low (3 patients). Nine patients (22%) had hypertension. Five patients had TC >200 mg/dL and seven patients were prescribed statins. Fifteen patients (37%) were overweight (BMI≥25 kg/m2), four additional patients (10%) were obese (BMI≥30 kg/m2). Twenty patients (49%) had a documented family history of CVD or CVD risk factors.
After initiation of HEMT, significant changes in lipid profiles were observed. TC, LDL, and the TC/HDL ratio significantly increased, while HDL did not change significantly (Table 2). BMI also significantly rose from 25.0 kg/m2 to 26.0 kg/m2 and 3 additional patients were diagnosed with hypertension. Five additional patients had TC >200 mg/dL post-HEMT initiation, and 16 had an increase in TC of ≥20 mg/dL. Three patients were newly prescribed a statin medication.
Table 2.
Lipid measurements before and after modulator initiation
| Pre-HEMT mean | Post-HEMT mean | Difference mean (95%CI) | p value | |
|---|---|---|---|---|
|
TC (mg/dL) n = 40 (ref < 200 mg/dL) |
152.5 | 168.8 | 16.3 (4.8, 27.7) | 0.007 |
|
HDL (mg/dL) n = 39 (ref: 40 - 60 mg/dL) |
53.2 | 52.2 | −1.5 (−6.4, 4.3) | 0.69 |
|
LDL (mg/dL) n = 35 (ref: 40 - 99 mg/dL) |
73.5 | 90.6 | 17.1 (8.4, 25.8) | <0.001 |
|
TC/HDL n = 39 (ref: 1.0 – 4.5) |
3.07 | 3.49 | 0.40 (0.09, 0.74) | 0.014 |
Results from paired t-tests are shown. Reference normal ranges are italicized for context, though elevations beyond this range do incorporate additional stratification of risk not shown here. HEMT = highly effective modulator therapy; TC = total cholesterol; HDL = high-density lipoprotein; LDL = low density lipoprotein. CI = confidence interval.
Linear models evaluated potential factors associated with changes in TC and LDL. The presence of CFLD was associated with a significantly blunted change in TC after HEMT initiation (p=0.034; TC mean difference pre/post HEMT −3.0 mg/dL in those with CFLD vs. 23.6 mg/dL in those without CFLD). Patients with CFLD also had a significantly blunted change in LDL after HEMT initiation (p=0.009; LDL mean difference −2.7 mg/dL in those with CFLD vs. 23.5 mg/dL in those without CFLD). In contrast, family history of CVD risk factors was associated with a significantly larger increase in TC after HEMT initiation (p=0.002; TC mean difference 33.8 mg/dL in those with family history vs. 0.3 mg/dL in those without documented family history). Family history was also associated with a significantly larger increase in LDL after HEMT initiation (p=0.023; LDL mean difference 25.9 mg/dL in those with family history vs. 6.6 mg/dL in those without family history) (Figure 1). Neither baseline nor change in BMI was correlated with magnitude of change in TC or LDL, nor was pancreatic status, CFRD, age, sex, statin use, prior modulator therapy, or duration on HEMT.
Figure 1.
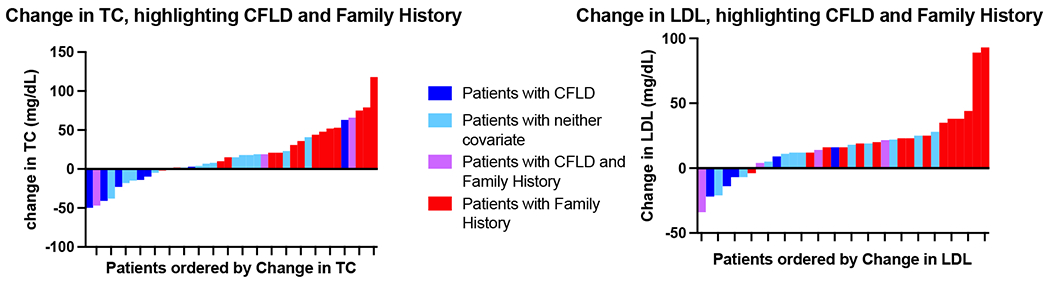
Waterfall plots of change in TC and change in LDL. TC = total cholesterol; CFLD = CF liver disease; LDL = low-density lipoprotein.
4. Discussion and Conclusions
This study provides valuable insights into the changes in lipid profiles and CVD risk factors in pwCF after initiating HEMT. Statistically significant increases in TC and LDL occurred in pwCF started on HEMT over a relatively short period of time, whereas cardioprotective HDL did not change.
In a study by Petersen et al., lipid changes were assessed in patients with CFRD after initiation of ELX/TEZ/IVA. They noted similar increases in TC and LDL as observed in our study, though HDL also significantly increased [16]. In our study, CFRD was not associated with the magnitude of change in TC or LDL, indicating that CFRD may not be the only criteria that should be used for lipid screening in pwCF. However, a family history of CVD risk factors was associated with larger increases in TC and LDL, highlighting the importance of assessing family history in pwCF. Although our cohort had few pancreatic sufficient patients (n=6), prior studies pre-HEMT have demonstrated that TC, LDL, and triglycerides are higher in these patients [13, 14]. Three PS patients were on statin therapy, potentially blunting significant increases in TC and LDL.
These results have potential implications for future screening and treatment practices. Current Cystic Fibrosis Foundation guidelines recommend annual lipid screening for patients with CFRD plus other CVD risk factors [17]. However, our data suggest that a broader population of pwCF may require lipid screening, particularly considering ~90% of patients are eligible for HEMT. Our data also support previous reports of increasing hypertension in pwCF, especially after ELX/TEZ/IVA initiation [4, 18]. When combined with other CVD risk factors, lipid lowering therapy for hyperlipidemia should be considered in more pwCF on HEMT.
Our study’s limitations include its retrospective nature and small sample size. Samples for lipid measurement were not consistently collected in the fasting state, preventing meaningful triglyceride evaluation. Testing methods may have varied at outside lab facilities. Our study population was also older, with higher rates of hypertension, CFRD, and obesity than reported in the US Registry, indicating a selection bias in this sample related to perceived need of measuring a lipid profile [5]. Family history of CVD was assessed inconsistently, however while details were limited (such as which relatives were affected), broadly inclusive use of family history appears to be a risk factor for changes in lipids at least in this higher risk group. These limitations reduce our ability to understand the effects of HEMT on a lower-risk population. Further data is needed, therefore, to refine risks in the general CF population and in subpopulations of interest. Prospective collection of samples at timed intervals related to HEMT initiation would also be useful.
Future investigations include understanding how HEMT affects lipid absorption and synthesis. In our population, CFLD was associated with minimal change in TC and LDL, and lower cholesterol levels have also been noted in patients with cirrhosis from other causes [19, 20]. This raises the question whether HEMT increases lipid levels via increased hepatic synthesis. However, untangling potential effects of HEMT on serum cholesterol levels, absorption, and synthesis requires additional study. We must also determine whether HEMT-induced changes in lipid profiles and other risk factors will translate into clinically apparent CVD. Our preliminary conclusions include emphasizing lipid screening in pwCF with family history of CVD and other CVD risk factors.
Highlights:
Risk of cardiovascular disease may be changing in people with cystic fibrosis
Total cholesterol and low-density lipoprotein increased after starting modulators
High-density lipoprotein did not change after modulator initiation
Family history was associated with greater increases in total cholesterol
Increased cholesterol screening may be warranted in cystic fibrosis
Acknowledgments:
We acknowledge the assistance of the NC Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489.
Funding:
This work was supported by DESPOT20B0 and P30 DK065988.
Abbreviations
- CVD
cardiovascular disease
- pwCF
people with cystic fibrosis
- HEMT
highly effective modulator therapy
- CFRD
cystic fibrosis related diabetes
- TC
total cholesterol
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- IVA
ivacaftor
- ELX/TEZ/IVA
elexacaftor-tezacaftor-ivacaftor
- IRB
Institutional Review Board
- CFLD
cystic fibrosis liver disease
- BMI
body mass index
- LUM/IVA
lumacaftor-ivacaftor
- TEZ/IVA
tezacaftor-ivacaftor
- FEV1pp
forced expiratory volume in 1 second, percent predicted
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
Conflict of interest statement: none.
References
- 1.Skolnik K, et al. , Coronary artery disease in cystic fibrosis: An emerging concern? J Cyst Fibros, 2016. 15(6): p. e70–e71. [DOI] [PubMed] [Google Scholar]
- 2.Poore TS, Taylor-Cousar JL, and Zemanick ET, Cardiovascular complications in cystic fibrosis: A review of the literature. J Cyst Fibros, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Perrin FM and Serino W, Ischaemic heart disease--a new issue in cystic fibrosis? J R Soc Med, 2010. 103 Suppl 1: p. S44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harindhanavudhi T, et al. , Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: A single-center analysis. Journal of Cystic Fibrosis, 2020. 19(1): p. 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2021 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2021. [accessed 2022 Nov 23]. Available from: https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf. [Google Scholar]
- 6.Thambuluru SR, et al. , Acute ST-elevation myocardial infarction in two young women with cystic fibrosis and cystic fibrosis-related diabetes. J Cyst Fibros, 2021. [DOI] [PubMed] [Google Scholar]
- 7.Buehler T, et al. , Increased arterial stiffness in children with cystic fibrosis: Table 1–. European Respiratory Journal, 2012. 39(6): p. 1536–1537. [DOI] [PubMed] [Google Scholar]
- 8.Hull JH, et al. , Increased augmentation index in patients with cystic fibrosis. European Respiratory Journal, 2009. 34(6): p. 1322–1328. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa V, et al. , Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr, 2002. 75(6): p. 1005–11. [DOI] [PubMed] [Google Scholar]
- 10.Georgiopoulou VV, et al. , Metabolic abnormalities in adults with cystic fibrosis. Respirology, 2010. 15(5): p. 823–9. [DOI] [PubMed] [Google Scholar]
- 11.Woestenenk JW, et al. , Dietary intake and lipid profile in children and adolescents with cystic fibrosis. J Cyst Fibros, 2017. 16(3): p. 410–417. [DOI] [PubMed] [Google Scholar]
- 12.Ishimo MC, et al. , Hypertriglyceridemia is associated with insulin levels in adult cystic fibrosis patients. J Cyst Fibros, 2013. 12(3): p. 271–6. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes B, et al. , Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros, 2010. 9(1): p. 24–8. [DOI] [PubMed] [Google Scholar]
- 14.Nowak JK, et al. , Cystic fibrosis dyslipidaemia: A cross-sectional study. J Cyst Fibros, 2019. 18(4): p. 566–571. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, and Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972. 18(6): p. 499–502. [PubMed] [Google Scholar]
- 16.Petersen MC, et al. , Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J Cyst Fibros, 2022. 21(2): p. 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran A, et al. , Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care, 2010. 33(12): p. 2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramegna A, et al. , Onset of systemic arterial hypertension after initiation of elexacaftor/tezacaftor/ivacaftor in adults with cystic fibrosis: A case series. J Cyst Fibros, 2022. 21(5): p. 885–887. [DOI] [PubMed] [Google Scholar]
- 19.Ghadir MR, et al. , The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat Mon, 2010. 10(4): p. 285–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Farooque U, et al. , The Pattern of Dyslipidemia in Chronic Liver Disease Patients. Cureus, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


