Abstract
CD14 is a glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein which functions as a receptor on myeloid cells for ligands derived from microbial pathogens such as lipopolysaccharide (LPS). We have studied the importance of the GPI tail of CD14 in signalling with the promonocytic cell line THP-1 expressing recombinant CD14 in a GPI-anchored form (THP1-wtCD14 cells) or in a transmembrane form (THP1-tmCD14). We found that, like other GPI-anchored molecules, GPI-anchored CD14 was recovered mainly from a Triton X-100-insoluble fraction, whereas transmembrane CD14 was fully soluble in Triton X-100. LPS induced cell activation of THP1-wtCD14 and of THP1-tmCD14 (protein tyrosine kinase phosphorylation, NF-κB activation, and cytokine production) in a very similar manner. However, anti-CD14 antibody-induced cross-linking caused a rapid calcium mobilization signal only in GPI-anchored CD14 cells. Studies with pharmacologic inhibitors of intracellular signalling events implicate phospholipase C and protein tyrosine kinases in the genesis of this antibody-induced calcium signal. Our results suggest that GPI anchoring and CD14 targeting to glycolipid-rich membrane microdomains are not required for LPS-mediated myeloid cell activation. GPI anchoring may however be important for other signalling functions, such as those events reflected by antibody cross-linking.
Proteins attached to the outer leaflet of the cell membrane via glycosylphosphatidylinositol (GPI) anchors are often receptors that mediate cell activation and/or ligand uptake (2, 9, 18). Because such proteins lack transmembrane domains and cytoplasmic tails and therefore do not directly communicate with the cell interior, there has been considerable interest in determining how this group of membrane proteins function. A number of GPI-anchored proteins are also found in soluble form in plasma, most likely without the GPI anchor. In some cases, after combining with ligand, these soluble receptors acquire agonist activity after contacting cellular targets (3, 6, 12, 26).
One GPI-anchored protein, CD14, has been the focus of considerable study, since it has a key role in host defense responses to microbial pathogens (15, 25). CD14 is found in two distinct forms; a 50- to 55-kDa glycoprotein present as a GPI-anchored membrane protein on myeloid lineage cells (MO) and a soluble serum protein lacking the GPI anchor (37). Both forms of CD14 bind the endotoxin (lipopolysaccharide [LPS]) of gram-negative bacteria, lipoarabinomannan from mycobacteria, and other substances from microbial pathogens that include gram-positive bacteria and yeast (25). A substantial body of data that clearly implicates GPI-anchored CD14 in the activation of myeloid cells and soluble CD14 in the activation of nonmyeloid cells, such as endothelial and epithelial cells, has emerged (37).
Our previous studies demonstrated that expression of recombinant, GPI-anchored CD14 in cell lines such as 70Z/3 that normally do not express CD14 markedly enhances cellular responses to LPS and other ligands such as lipoarabinomannan (17, 25). Such transfected cell lines have provided an opportunity to use CD14 as a model protein to investigate how GPI-anchored proteins function in cell activation. Our findings suggested that for LPS the primary function of CD14 is to bind ligand and then facilitate interaction with an additional protein(s) that initiates transmembrane signalling (16, 37).
By using primary isolates of myeloid lineage cells, it was shown by others that cross-linking of CD14 with antibody caused elevations in intracellular Ca2+ (19). In contrast, we have shown that LPS stimulation of MO via CD14-dependent mechanisms does not induce increased intracellular Ca2+ (20). Despite the fact that the physiologic counterpart of antibody cross-linking of CD14 is not known, we felt this event might provide an additional signalling pathway to investigate how GPI-anchored proteins signal.
Here we use stably transfected cell lines derived from THP-1 cells, a human monocytic cell line (10), expressing GPI-anchored CD14 (THP1-wtCD14) or transmembrane CD14 (THP1-tmCD14). We show that GPI-anchored CD14 is mostly localized to a Triton X-100 (TX100)-insoluble fraction of the plasma membrane, while the transmembrane form of CD14 is completely soluble in TX100. CD14 expression markedly enhances LPS responsiveness, and both forms of CD14 supported nearly equivalent LPS-induced cell activation. In contrast, elevation of intracellular Ca2+ by antibody cross-linking of CD14 was observed only with THP1-wtCD14 cells.
MATERIALS AND METHODS
Cells and transfections.
The monocytic THP-1 cell line was provided by D. Altieri (The Scripps Research Institute, La Jolla, Calif.) and maintained in low-endotoxin RPMI 1640 supplemented with 10% fetal bovine serum (Sigma), 10 mM HEPES, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml (complete RPMI). The human CD14 (hCD14) cDNA was cloned in pRc/RSV vector for expression of hCD14 with a GPI membrane anchor. A construct encoding a transmembrane form of hCD14 containing the transmembrane domain and cytoplasmic tail of tissue factor was used as previously described (16). To establish stably transfected THP-1 cell lines, the following procedure was followed. Cells were pelleted by low-speed centrifugation, washed at room temperature, and resuspended (106 cells/ml) in serum-free RPMI 1640 containing 1 mg of human serum albumin (Miles) per ml (RPMI/HSA). A cell suspension (700 μl) was added to a 0.4-cm-path-length sterile Bio-Rad electroporation cuvette and mixed with 10 μg of vector only or 10 μg of vector containing a cDNA encoding GPI or transmembrane form of hCD14. Transfections by electroporation were performed with the Gene Pulser apparatus (Bio-Rad) at 200 V and 960 μF capacitance. Immediately after electroporation, 700 μl of complete RPMI (37°C) was added to the cuvette. These conditions resulted in the death of >90% of the cells. Electroporated cells were then transferred to 10 ml of complete RPMI in a T25 flask kept upright. After 48 h, cells were pelleted and resuspended in complete RPMI containing 0.5 mg of G418 sulfate (Gibco) per ml. About 4 weeks was necessary for stably transfected G418-resistant clones to grow; subsequently cells were maintained in complete RPMI supplemented with 0.5 mg of G418 per ml. Cells transfected with the vector only were named THP1-RSV, cells expressing GPI-anchored CD14 were named THP1-wtCD14, and cells expressing transmembrane CD14 were named THP1-tmCD14. CD14 expression was assessed by flow cytometry (Becton Dickinson) as previously described (17).
Detergent solubility of CD14.
Unless otherwise noted, all steps were performed at 4°C. Cells (5 × 106) were removed from tissue culture flasks and washed twice with serum-free RPMI. The washed cell pellet was maintained for 30 min in 1 ml of sulfo–N-hydroxysuccinimide–biotin (0.5 mg/ml; Pierce, Rockford, Ill.) freshly dissolved in phosphate-buffered saline (PBS) containing 0.1 mM CaCl2 and 0.1 mM MgCl2. The cells were recovered by centrifugation and mixed with Dulbecco’s modified Eagle medium for 15 min followed by centrifugation and two washes in PBS. The washed cells were then treated with 1 ml of TBST (10 mM Tris-HCl, 0.15 M NaCl, 1% TX100, 1 mM phenylmethylsulfonyl fluoride; pH 8.0) for 30 min, and the soluble and insoluble fractions were recovered by centrifugation. The insoluble pellet was further treated with TBST-OG (TBST plus 60 mM octylglucoside) for 30 min, and the soluble and insoluble fractions were recovered by centrifugation. The soluble fractions were stored at −20°C until further use. After thawing, each sample was precleared by mixing with 25 μl of protein A-Sepharose beads (PrAS) previously washed four times with TBST. After a 24-h treatment, the precleared lysates were collected by centrifugation and mixed with PrAS that had been mixed with an immunoglobulin G (IgG) fraction of goat anti-hCD14 serum. The precleared lysates were then mixed with the PrAS-antibody conjugates and mixed for 3 h followed by six washes with TBST. After these washes, the PrAS were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and placed in a boiling water bath for 10 min, and after removal of the PrAS by centrifugation, 50 μl of the supernatant was subjected to SDS-PAGE followed by electrotransfer to a nitrocellulose membrane. The presence of biotinylated hCD14 was detected by using a peroxidase-streptavidin conjugate and an enhanced chemiluminescence (ECL) kit (Amersham). The resultant film was analyzed by scanning densitometry.
Functional assays. (i) Cytokine production.
The morning of the experiment, cell lines were washed, resuspended in fresh RPMI 1640, and distributed in microtiter plates (Costar) to obtain a final concentration of 1 × 105 to 2 × 105 cells/well (200 μl of endotoxin-free RPMI with 5% fetal bovine serum). Various concentrations of Escherichia coli O111:B4 LPS (List Biological, Campbell, Calif.) in RPMI/HSA were added to the wells, and cells were incubated for 6.5 h at 37°C. At the end of incubation, conditioned media were sampled and kept frozen at −20°C until the day of cytokine assay. Human tumor necrosis factor alpha (TNF-α) antigen levels in conditioned media were assessed with an enzyme-linked immunosorbent assay (ELISA) kit (Dupont-NEN, Cambridge, Mass.) and human interleukin 8 (IL-8) concentrations were assayed by a sandwich ELISA as previously described (26).
(ii) NF-κB activation.
THP1-RSV, THP1-wtCD14, and THP1-tmCD14 cells were washed, resuspended in fresh RPMI 1640 containing 5% fetal bovine serum without G418, distributed in 48-well plates (Costar) at 1 × 106 to 1.4 × 106 cells/ml, and maintained in the incubator for 4 to 5 h prior to the experiment. Cells were then stimulated for various times with different concentrations of Re595 LPS (List). In some experiments, the anti-CD14 monoclonal antibody (MAb) 28C5 (provided by D. Leturcq, R. W. Johnson Pharmaceutical Research Institute, San Diego, Calif.) or the anti-CD18 MAb IB4 (provided by K. Arfors, Pharmacia, La Jolla, Calif.) were premixed with cells 30 min prior to addition of LPS. At the conclusion of the experiment, cells were rapidly chilled on ice, transferred to Eppendorf tubes, pelleted, and washed two times with ice-cold PBS, pH 7.3. Nuclear extracts were then prepared as described elsewhere (17). Briefly, cells were lysed in a TX100-containing buffer in the presence of protease inhibitors, and nuclei were prepared by centrifugation at 13,000 × g for 30 s. Nuclear proteins were extracted in a high-salt buffer (0.4 M NaCl) and used for electrophoretic mobility shift assay (EMSA). Nuclear proteins (2.5 μg) were added to 12 μl of a binding buffer containing 5 mM HEPES (pH 8.5), 5 mM MgCl2, 50 mM dithiothreitol, 0.4 mg of poly(dI-dC) per ml, 0.1 mg of sonicated double-stranded salmon sperm DNA per ml, and 10% glycerol, and 20 to 50 fmol of 32P-labeled NF-κB oligonucleotide probe (30,000 to 50,000 cpm) (Promega) (5′-AGTTGAGGGGACTTTCCAGG-3′) and incubated for 10 min at room temperature (16). Samples were analyzed on 5% acrylamide gels made in Tris-glycine-EDTA buffer. Electrophoresed gels were then transferred onto Whatman filter paper, dried, and subjected to autoradiography.
(iii) Calcium mobilization.
The day of the assay, cells were washed, resuspended in RPMI/HSA at 5 × 107 cells/ml, and incubated with 5 μl of Indo-1 AM (1 mM in dimethyl sulfoxide [Molecular Probes]) per ml of cells at 37°C for 30 min. Cells were then pelleted, resuspended in PBS containing 2 mg of HSA per ml, and kept at room temperature until use. Continuous fluorescence measurements of bound and free Indo-1 were made with an SLM 8000 photon-counting spectrofluorometer (SLM-Aminco) or a CAF-110 spectrofluorometer (Jasco, Tokyo, Japan) detecting at 400 and 490 nm, respectively, with an excitation wavelength of 340 nm. Calcium mobilization was measured at 37°C as the ratio of fluorescence intensities of emission at 400 and 490 nm. For the assay, 5 × 105 cells were suspended in 250 μl of PBS, pH 7.3, and transferred to the cuvette. To study cross-linking of CD14, murine anti-CD14 MAb 63D3 (American Type Culture Collection) was added to the cells at different concentrations ranging from 1.5 to 100 μg/ml, followed by the addition of an affinity-purified F(ab′)2 fraction of a goat anti-murine IgG, specific for the Fc moiety of murine IgG (Jackson Laboratories) at a concentration of 250 μg/ml. Other MAbs used in this assay included anti-CD14 MAbs 28C5, 18E12 (both provided by D. Leturcq, R. W. Johnson Pharmaceutical Research Institute, San Diego, Calif.), and MY4 (Coulter). An IgG and an F(ab′)2 fraction of goat anti-hCD14 polyclonal antibody were also used without a second antibody. The calcium ionophore ionomycin (Calbiochem, San Diego, Calif.) was used at 10 μg/ml. In some experiments, LPS (up to 1 μg/ml) in the presence or absence of 1 μg of purified rabbit LPS-binding protein per ml was also added to the cells. In some experiments, cells were pretreated with pharmacologic inhibitors under the following conditions; the phospholipase C inhibitor U73122 or its inactive analog U73343 (Calbiochem) was added to cells 2 min prior to addition of anti-CD14 MAb. In other experiments, the protein tyrosine kinase inhibitor genistein or herbimycin A (20 μg/ml) was added 30 min prior to addition of anti-CD14 MAb.
(iv) Protein tyrosine phosphorylation.
Cell lines were resuspended at 4 × 106 cells/ml in RPMI 1640 containing 5% fetal calf serum and kept for 2 h at 37°C in 24-well plates (Costar) before stimulation. E. coli O111:B4 LPS (100 ng/ml) or a hyperosmolar solution of NaCl (final concentration of 150 mosM above the RPMI concentration) was then added to the cell suspensions and incubated for 30 min at 37°C in a CO2-enriched atmosphere. At the end of the incubation, the mixtures of agonists plus cells were put on ice and 0.5 ml of ice-cold Tris-buffered saline (TBS) containing 1 mM sodium orthovanadate (Na3 VO4) was added to each well. After centrifugation at 4°C, cells were washed once with TBS-Na3 VO4 at 4°C. Cells were then resuspended in 65 μl of a lysis buffer containing TBS (pH 7.5), 2 mM EDTA, 10% glycerol, 1% TX100, and protease inhibitors and incubated on ice for 15 min. Subsequently, insoluble material was removed by microcentrifuge centrifugation at high speed for 20 min at 4°C. Proteins contained in 10-μl samples of the soluble cell lysates were separated on an SDS–12% polyacrylamide gel, transferred onto a nitrocellulose membrane (Bio-Rad), immunoblotted with an antiphosphotyrosine MAb (4G10 [UBI]), followed by a goat anti-mouse IgG peroxidase conjugate antibody (Santa Cruz), and revealed by ECL. Membranes were then treated to strip the 4G10 at 60°C for 2 h in a solution containing 2% SDS and 3% dithioerythritol, reprobed with an anti-p38 peptide antibody followed by protein G-horseradish peroxidase (Bio-Rad), and analyzed with the ECL kit. The remaining 55-μl samples of the cell lysates were immunoprecipitated with anti-p38 antibody and protein G agarose beads (Pierce Chemicals) in TBS (pH 7.5) containing 1% bovine serum albumin (Sigma) plus 0.05% Tween 20. Immunoprecipitates were then boiled in SDS sample buffer under reducing conditions and analyzed by SDS-PAGE and Western blotting using antiphosphotyrosine MAb 4G10 as described above for crude cell extracts.
RESULTS
Expression of GPI-anchored or transmembrane CD14 in THP-1 cells.
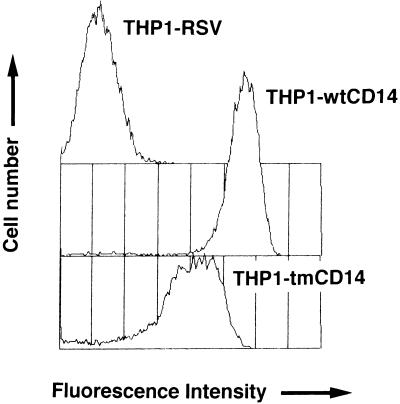
THP-1 cells were transfected with empty vector or vector containing DNA encoding either GPI-anchored or transmembrane CD14; selection of stably transfected lines was accomplished with G418. CD14 expression measured by flow cytometry with fluoresceinated anti-CD14 MY4 MAb revealed expression of both GPI-anchored and transmembrane CD14 (Fig. 1). Flow cytometry histograms were superimposable when anti-CD14 MAb 63D3, instead of MY4, was used. The number of CD14 molecules per cell was quantified by using 125I-labeled MAb 28C5 Fab fragments; THP1-wtCD14 and THP1-tmCD14 cells were found to express 2 × 106 and 2 × 105 CD14 molecules/cell, respectively (35a). CD14 expression was not detected in THP-1 cells transfected with empty vector (THP1-RSV cells) either by fluorescence-activated cell sorting or by using radiolabeled antibodies; cell fluorescence resulting from staining THP1-RSV cells with MY4 or an isotype control was indistinguishable (data not shown). If THP1-RSV cells express CD14, it is below the level detected by flow cytometry analysis or binding of radiolabeled antibody; we estimate this to be <1,000 molecules of CD14/cell.
FIG. 1.
Surface CD14 expression in THP-1 cells that were transfected with an empty vector (THP1-RSV) or that express a GPI-anchored form of CD14 (THP1-wtCD14) or a transmembrane form of CD14 (THP1-tmCD14). Flow cytometry of cells incubated with fluoresceinated anti-CD14 MAb MY4 was done. Fluorescence intensity is shown on a log scale.
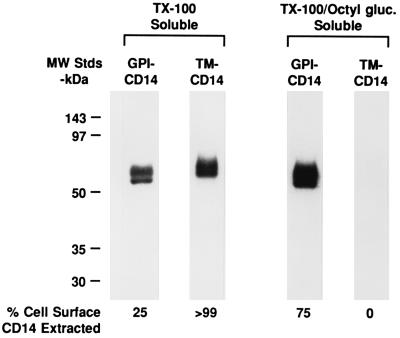
Others have shown that GPI-anchored proteins are insoluble in TX100, while most integral membrane proteins are fully soluble in this detergent (5, 29). To characterize the two forms of CD14 expressed by THP-1 cells, we biotinylated the surface proteins of THP1-wtCD14 and THP1-tmCD14 cell lines, treated the cells at 4°C with a buffer containing TX100, and prepared a TX100-soluble (TX100s) fraction and a TX100-insoluble (TX100i) fraction. The TX100i portion was further extracted with a buffer containing TX100 and octylglucoside. Aliquots of each sample were treated with protein A-Sepharose containing goat anti-CD14 IgG, and the resultant immunocomplexes were subjected to SDS-PAGE followed by electrotransfer to a nitrocellulose membrane. The presence of biotinylated CD14 in these fractions was visualized by staining with peroxidase-streptavidin, and the amount of biotinylated CD14 present in each lane was quantified by scanning densitometry. Results shown in Fig. 2 indicate that the majority of the GPI-anchored CD14 is present in a TX100i fraction of the plasma membrane and that an additional treatment with TX100-octylglucoside was needed to solubilize the remaining CD14. In contrast, the integral membrane form of CD14 was fully soluble in TX100.
FIG. 2.
Different detergent solubilities of GPI-anchored CD14 (GPI-CD14) or transmembrane-anchored CD14 (TM-CD14). After surface biotinylation, THP1-wtCD14 and THP1-tmCD14 cells were treated with detergents (TX100 and octylglucoside [Octyl gluc.]) and centrifuged as indicated in the text. CD14 was immunoprecipitated, subjected to SDS-PAGE, electrotransferred to a nitrocellulose membrane, and detected by using streptavidin-peroxidase conjugate and an ECL kit. The percent cell surface CD14 extracted was determined by scanning densitometry. The positions of molecular mass standards (MW Stds) are indicated to the left.
Functional responses of THP1-wtCD14 and THP1-tmCD14 to LPS.
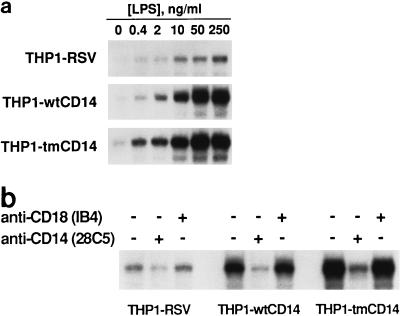
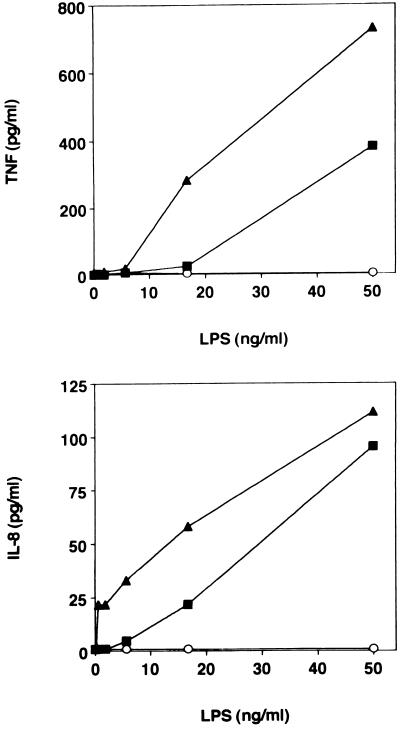
We next evaluated LPS-induced NF-κB activation and cytokine production by the three THP-1 cell lines. Various concentrations of Re595 LPS were added to THP1-RSV, -wtCD14, and -tmCD14 cell lines, and after 60 min, nuclear extracts were examined for the presence of NF-κB in an EMSA. We ascertained that maximal NF-κB activation was observed 45 to 60 min after LPS addition (data not shown). The expression of CD14 markedly enhanced the ability of LPS to activate NF-κB; addition of up to 250 ng of LPS per ml resulted in weak activation of NF-κB in THP1-RSV cells, while as little as 10 ng of LPS per ml induced a strong NF-κB response in both lines expressing CD14 (Fig. 3a). Inclusion of the anti-hCD14 MAb 28C5 markedly inhibited the enhanced NF-κB activation, while the addition of IB4, an anti-CD18 MAb, was without effect (Fig. 3b). In a separate series of experiments, various amounts of LPS were added to the three THP-1 cell lines and after 6.5 h of culture, the supernatants were assayed for either TNF-α or IL-8. Results shown in Fig. 4 demonstrate that expression of either form of CD14 markedly enhances LPS-induced cytokine release. The IL-8 response was consistently detectable at a lower LPS concentration than the TNF response.
FIG. 3.
LPS-induced NF-κB activation in THP1-RSV, THP1-wtCD14, and THP1-tmCD14 cells. NF-κB activation was assessed by EMSA as indicated in the text. (a) LPS dose response. (b) Effects of anti-CD14 and anti-CD18 MAb treatments. The presence (+) or absence (−) or MAbs is indicated.
FIG. 4.
LPS-induced TNF-α and IL-8 production by THP1-RSV (○), THP1-wtCD14 (▪), and THP1-tmCD14 (▴).
Protein tyrosine phosphorylation after LPS stimulation.
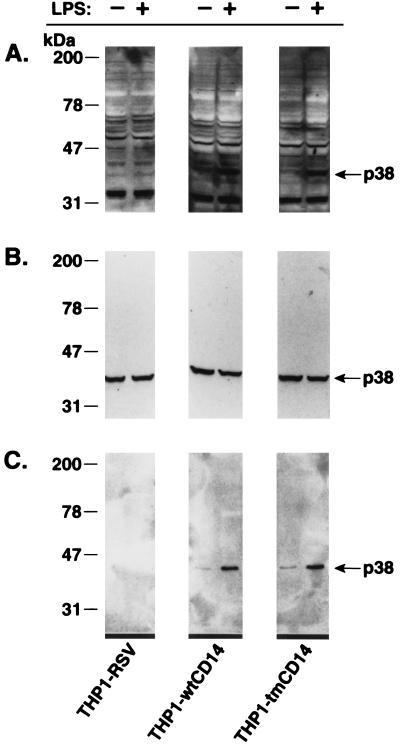
Activation of intracellular kinase cascades occurs after ligation of many cell surface receptors (4, 8, 21). We and others have shown that LPS induces a rapid increase in tyrosine phosphorylation of a number of proteins including members of the MAP kinase family such as ERK, JNK, and p38 (7, 13, 24). Thus, we first compared protein tyrosine phosphorylation patterns in whole-cell extracts of unstimulated and LPS-treated THP-1 cell lines (Fig. 5A). These data show that LPS induced increased tyrosine phosphorylation of a 38-kDa protein in THP1-wtCD14 or THP1-tmCD14 cells but under identical experimental conditions failed to induce this event in THP1-RSV cells. In contrast, when all three transfected THP-1 cell lines were exposed to increased extracellular osmolarity, protein tyrosine phosphorylation of a 38-kDa protein was increased (data not shown). Because of our previous studies (13) we suspected that this 38-kDa protein was p38, a member of the MAP kinase family. The identity of the 38-kDa protein was determined with the use of an antiserum raised against a carboxy-terminal peptide of p38 (13). Both Western blots (Fig. 5B) and immunoprecipitation experiments (Fig. 5C) revealed that the 38-kDa protein was recognized by the anti-p38 serum and that LPS induced the tyrosine phosphorylation of p38. LPS failed to induce tyrosine phosphorylation of proteins corresponding to ERK1 and ERK2 in these experiments. However, we did detect LPS-induced phosphorylation of proteins corresponding to ERK (data not shown) when we used THP-1 cells expressing CD14 after treatment with calcitriol (10).
FIG. 5.
LPS induced p38 tyrosine phosphorylation in THP1-wtCD14 and THP1-tmCD14 cells. The presence (+) or absence (−) of LPS is indicated at the top of the figure. (A) Tyrosine phosphorylation of a 38-kDa protein after LPS treatment as assessed by phosphotyrosine immunoblotting (MAb 4G10) of cell lysates separated by SDS-PAGE is shown. (B) The membrane shown in panel A was treated to strip off antibodies and was reprobed with anti-p38 antipeptide antibody (13). (C) Immunoprecipitated p38 from cell lysates is phosphorylated in response to LPS. Phosphorylation of p38 was assessed by phosphotyrosine immunoblotting of the immunoprecipitated p38 as described for panel A for crude cell lysates.
Functional responses of THP1-wtCD14 and THP1-tmCD14 cells resulting from CD14 cross-linking.
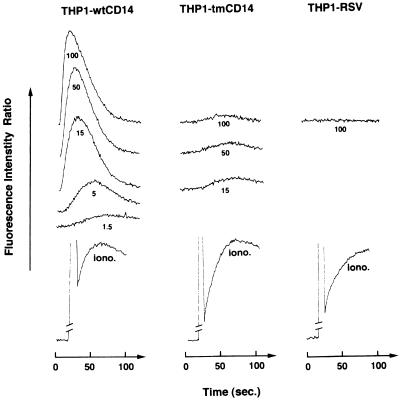
It has been shown by others that antibody-induced cross-linking of CD14 on myeloid lineage cells resulted in an elevation of intracellular Ca2+ (19). We attempted to induce similar changes in 70Z/3 cells expressing CD14, but cross-linking of CD14 on such cell lines failed to induce elevations of intracellular Ca2+ (data not shown). In contrast, a preliminary study with THP-1 cells expressing high levels of GPI-anchored CD14 after treatment with calcitriol indicated that CD14 cross-linking induced an elevation of intracellular Ca2+ (26a). This observation provided the basis for the following experiments using THP1-wtCD14 and THP1-tmCD14 cell lines. THP1-wtCD14, -tmCD14, and -RSV cell lines were treated with various amounts of the anti-hCD14 MAb 63D3 (primary) followed by the addition of an affinity-purified F(ab′)2 fraction of goat anti-murine IgG (secondary) specific for the Fc portion of murine MAb. Addition of various amounts of the primary antibody followed by the secondary antibody to THP1-wtCD14, but not to THP1-tmCD14 or THP1-RSV cells, resulted in a marked elevation of intracellular Ca2+ (Fig. 6). Control experiments revealed that addition of 63D3 or the F(ab′)2 antibody separately did not induce any changes in intracellular Ca2+ (data not shown). We also determined that one other anti-hCD14 MAb, 18E12, and an F(Ab′)2 fragment of goat anti-hCD14 IgG could be substituted for 63D3 (data not shown). This effect is not observed with all MAbs to human CD14, since the anti-hCD14 MAbs MY4 and 28C5 do not act as agonists. All of the transfected lines responded equally well to ionomycin.
FIG. 6.
CD14 cross-linking induces calcium mobilization only in cells expressing the GPI-anchored form of CD14. Calcium mobilization was measured in calcium-free buffer with cells loaded with Indo-1 and expressed as the ratio of fluorescence intensities of emission at 400 and 490 nm. Anti-CD14 MAb 63D3 (doses ranging from 1.5 to 100 μg/ml) and a secondary goat anti-mouse F(ab′)2 IgG antibody (250 μg/ml) were added to Indo-1-loaded THP1 transfectants. Similar loading of the different cell lines was assessed by fluorescence levels induced by treatment with ionomycin.
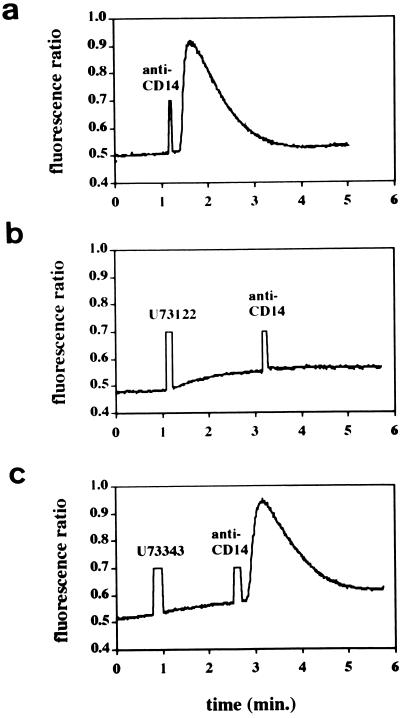
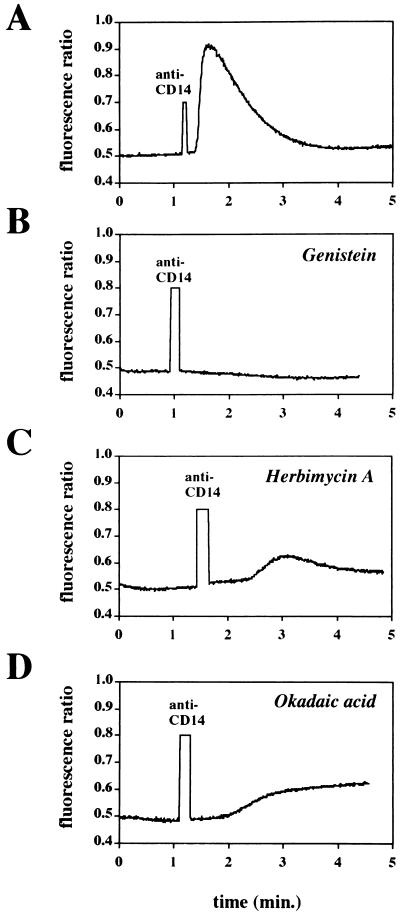
We next performed experiments with selected pharmacologic inhibitors of intracellular kinases, phospholipases, or phosphatases to investigate signalling mechanisms that might be associated with the increase in intracellular Ca2+. An inhibitor of phospholipase C (U73122) and its inactive analog (U73343 [38]) and two inhibitors of protein tyrosine kinases (genistein [1] and herbimycin [36]) were used in this series of experiments. Pretreatment of cells with U73122 (2.5 μM) completely blocked the increase in Ca2+ induced by antibody-induced cross-linking of CD14, while an equivalent amount of the inactive analog was without effect (Fig. 7). Likewise, pretreatment with the protein tyrosine kinase inhibitors genistein (1) and herbimycin A (36) inhibited the rise in Ca2+ (Fig. 8). When we evaluated the effects of the same inhibitors on LPS-induced NF-κB activation in THP1-wt cells, we observed that both U73122 and herbimycin blocked LPS-induced NF-κB activation while genistein was without effect (data not shown). A phosphoprotein phosphatase inhibitor, okadaic acid (20 μg/ml) (30), markedly slowed the onset and reduced the magnitude of cross-linking-induced Ca2+ (Fig. 8). In contrast, okadaic acid enhanced LPS-induced NF-κB activation (data not shown).
FIG. 7.
Calcium mobilization induced by anti-CD14 antibody cross-linking in THP1-wtCD14 cells. No inhibitor (A) or phospholipase C inhibitor U73122 (2.5 μM) (B) or inactive chemical analog U73343 (2.5 μM) (C) was added 2 min prior to cross-linking.
FIG. 8.
Effects of inhibitors on calcium mobilization induced by anti-CD14 antibody cross-linking in THP1-wtCD14 cells. No inhibitor (A) or genistein (20 μg/ml) (B), herbimycin A (20 μg/ml) (C), or okadaic acid (20 μg/ml) (D) was added 30 min prior to anti-CD14 MAb.
DISCUSSION
In this report, we describe experiments with stably transfected cell lines derived from the human monocytic cell line THP-1 that express a GPI-anchored or transmembrane form of hCD14. GPI-anchored CD14 is localized in a TX100-insoluble domain, while the transmembrane form of CD14 is completely soluble in this detergent. Nevertheless, both forms of CD14 support essentially identical levels of LPS-induced cell activation, as evaluated by NF-κB activation, protein tyrosine phosphorylation, and cytokine production. In contrast, increased intracellular Ca2+ induced by antibody cross-linking of CD14 is favored in cells bearing GPI-anchored CD14. The present results distinguish functions of CD14 that require the presence of its GPI anchor from those that can be carried out equally well with GPI-anchored or transmembrane forms of the protein.
This study provides new information about the function of GPI-anchored proteins using CD14 as a model. Previously a number of independent studies have shown that GPI-anchored proteins are localized to subdomains of the plasma membrane enriched in glycosphingolipids and cholesterol (5, 14, 32). Characteristically, proteins localized to these domains are insoluble in TX100, while in contrast, integral membrane proteins are fully TX100 soluble (5). Our findings are consistent with this; GPI-anchored CD14 was found to be largely TX100 insoluble, while the transmembrane form of CD14 was completely solubilized by treatment under identical extraction conditions. Until recently, detergent insolubility was used as evidence to support localization in plasma membrane invaginations named caveolae (18). However, recent evidence suggests that this is not the case (22) but that following antibody-induced cross-linking, GPI-anchored proteins can move to caveola-like structures. Whether ligand binding to a GPI-anchored protein induces a similar redistribution has not yet been determined. Moreover, some cell types appear to lack caveolae but still express GPI-anchored proteins (11). Other studies have compared the function of proteins that are normally GPI anchored by converting such proteins to integral membrane proteins and expressing both forms following transfection or by using transgenic mice (27, 28, 31, 34, 35). These studies have utilized T cells or T-cell lines. In each case, cell activation was measured after antibody-induced cross-linking, and in all the previous studies but one (27), it was found that only the GPI-anchored form of the protein supported cell activation. The transmembrane forms of proteins investigated in these studies failed to support early cell activation events such as increased protein tyrosine phosphorylation as well as later events such as IL-2 production. It has been shown that tyrosine kinases of the Src family appear to be associated with detergent-insoluble domains, suggesting that these subdomains of the membrane may be involved in transmembrane signalling or other specialized cell functions (29, 31, 33), although this involvement is by no means certain (23). The loss of function associated with conversion to a transmembrane protein may result from uncoupling of such interactions.
In contrast, our previous study with CD14 using the murine pre-B cell line 70Z/3 showed that GPI-anchored CD14 and transmembrane CD14 support essentially equal levels of cell activation including protein tyrosine phosphorylation (16). However, we were unable to use a 70Z/3 cell line expressing CD14 to study the effects of antibody cross-linking, since we could not detect signals generated following cross-linking of CD14. Of interest are observations that GPI-anchored CD14 in 70Z/3 cell lines is associated with TX100-insoluble membrane fractions (data not shown). The THP-1 cell line proved to be a useful line to study cross-linking-induced changes in intracellular Ca2+. At this time, we cannot provide an explanation for differences between CD14-positive THP-1 and 70Z/3 cell lines. Presumably, they result from differences in the availability of important accessory molecules required to generate the increased intracellular Ca2+ signal following CD14 cross-linking. In order to generate increased intracellular Ca2+ following cross-linking of GPI-anchored CD14, interactions of a variety of intracellular proteins may be required. However, we cannot rule out the possibility that the lower CD14 expression in THP1-tmCD14 cells was not responsible for the lack of Ca2+ mobilization. In this study, using pharmacologic inhibitors, we have implicated several potential classes of intracellular signalling molecules: protein tyrosine kinase(s), phosphoprotein phosphatases, and phospholipase(s) C. Whether such proteins are associated with detergent-insoluble microdomains together with CD14 remains to be determined. Finally, our data indicate that GPI and transmembrane forms of CD14 are targeted to different membrane subdomains (GPI and transmembrane domains, respectively). This might be irrelevant for CD14 signalling function but may profoundly affect CD14 cross-linking-induced Ca2+ mobilization.
ACKNOWLEDGMENTS
We acknowledge Marie-Claude Widmer for excellent technical assistance.
This work was supported in part by the Swiss National Science Foundation grant 32-040344 to J.P. J.P. is also the recipient of grants from the Prof. Dr. Max Cloetta Foundation and the 3R Foundation. This work was also supported by National Institutes of Health grants AI15136 (R.J.U.), GM28485 (R.J.U.), GM37696 (R.J.U. and P.S.T.), and HL23584 (P.S.T.).
Footnotes
Publication 9661-IMM of the Department of Immunology, The Scripps Research Institute.
REFERENCES
- 1.Aldyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Anderson R G W, Kamen B A, Rothberg K G, Lacey S W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 3.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 4.Blumer K J, Johnson G L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Davis S, Aldrich T H, Ip N Y, Stahl N, Scherer S, Farruggella T, DiStefano P S, Curtis R, Panayotatos N, Gascan H, Chevalier S, Yancopoulos G D. Released form of CNTF receptor α component as a soluble mediator of CNTF responses. Science. 1993;259:1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- 7.Derijard B, Hibi M, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 8.Derijard B, Raingeaud J, Barrett T, Wu I, Han J, Ulevitch R J, Davis R J. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson M A J. What can GPI do for you? Parasitol Today. 1994;10:48–52. doi: 10.1016/0169-4758(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 10.Fleit H B, Kobasiuk C D. The human monocyte-like cell line THP-1 expresses Fc-γ-RI and Fc-γ-RII. J Leukocyte Biol. 1991;49:556–565. doi: 10.1002/jlb.49.6.556. [DOI] [PubMed] [Google Scholar]
- 11.Fra A M, Williamson E, Simons K, Parton R G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 12.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 14.Hanada K, Nishijima M, Akamatsu Y, Pagano R E. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J Biol Chem. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- 15.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee J-D, Kravchenko V, Kirkland T N, Han J, Mackman N, Moriarty A, Leturcq D, Tobias P S, Ulevitch R J. GPI-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci USA. 1993;90:9930–9934. doi: 10.1073/pnas.90.21.9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J-D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisanti M P, Scherer P E, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domain: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 19.Lund-Johansen F, Olweus J, Aarli A, Bjerknes R. Signal transduction in human monocytes and granulocytes through the PI-linked antigen CD14. FEBS Lett. 1990;273:55–58. doi: 10.1016/0014-5793(90)81049-t. [DOI] [PubMed] [Google Scholar]
- 20.Martin T R, Mathison J C, Tobias P S, Maunder R J, Ulevitch R J. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide: implications for cytokine production in normal and injured lungs. J Clin Invest. 1992;90:2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda S, Gotoh Y, Nishida E. Signaling pathways mediated by the mitogen-activated protein (MAP) kinase kinase/MAP kinase cascade. J Leukocyte Biol. 1994;56:548–553. doi: 10.1002/jlb.56.5.548. [DOI] [PubMed] [Google Scholar]
- 22.Mayor S, Rothberg K G, Maxfield F R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 23.Meng F, Lowell C. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 25.Pugin J, Heumann D, Tomasz A, Kravchenko V, Akamatsu Y, Nishijimi M, Glauser M-P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 26.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide (LPS) activation of human endothelial and epithelial cells is mediated by LPS binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Pugin, J., and R. J. Ulevitch. Unpublished data.
- 27.Resta R, Hooker S W, Laurent A B, Shuck J K, Misumi Y, Ikehara Y, Koretzky G A, Thompson L F. Glycosyl phosphatidylinositol membrane anchor is not required for T cell activation through CD73. J Immunol. 1994;153:1046–1053. [PubMed] [Google Scholar]
- 28.Robinson P J, Millrain M, Antoniou J, Simpson E, Mellor A L. A glycophospholipid anchor is required for Qa-2-mediated T cell activation. Nature. 1989;342:85–87. doi: 10.1038/342085a0. [DOI] [PubMed] [Google Scholar]
- 29.Sargiacomo M, Sudol M, Tang Z L, Lisanti M P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonthal A. Okadaic acid. A valuable new tool for the study of signal transduction and cell cycle regulation? New Biol. 1992;4:16–21. [PubMed] [Google Scholar]
- 31.Shenoy-Scaria A M, Kwong J, Fujita T, Olszowy M W, Shaw A S, Lublin D M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 32.Smart E J, Foster D C, Yin Y-S, Kamen B A, Anderson R G W. Protein kinase C activators inhibit receptor-mediated potocytosis by preventing internalization of caveolae. J Cell Biol. 1994;124:307–313. doi: 10.1083/jcb.124.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanova I, Horejsi V, Ansotegui I J, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 34.Su B, Waneck G L, Flavell R A, Bothwell A L M. The glycosyl phosphatidylinositol anchor is critical for Ly-6A/E-mediated T cell activation. J Cell Biol. 1991;112:377–384. doi: 10.1083/jcb.112.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas P M, Samuelson L E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- 35a.Tobias, P. S. Unpublished data.
- 36.Uehara Y, Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- 37.Ulevitch R J, Tobias P S. Recognition of endotoxin by cells leading to transmembrane signalling. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L, Paik W Y, Cesnjaj M, Balla T, Tornic M, Calt K J, Slojlkovic S S. Effects of the phospholipase-C inhibitor, U73122, on signaling and secretion in pituitary gonadotrophs. Endocrinology. 1995;136:1079–1088. doi: 10.1210/endo.136.3.7867562. [DOI] [PubMed] [Google Scholar]