Abstract
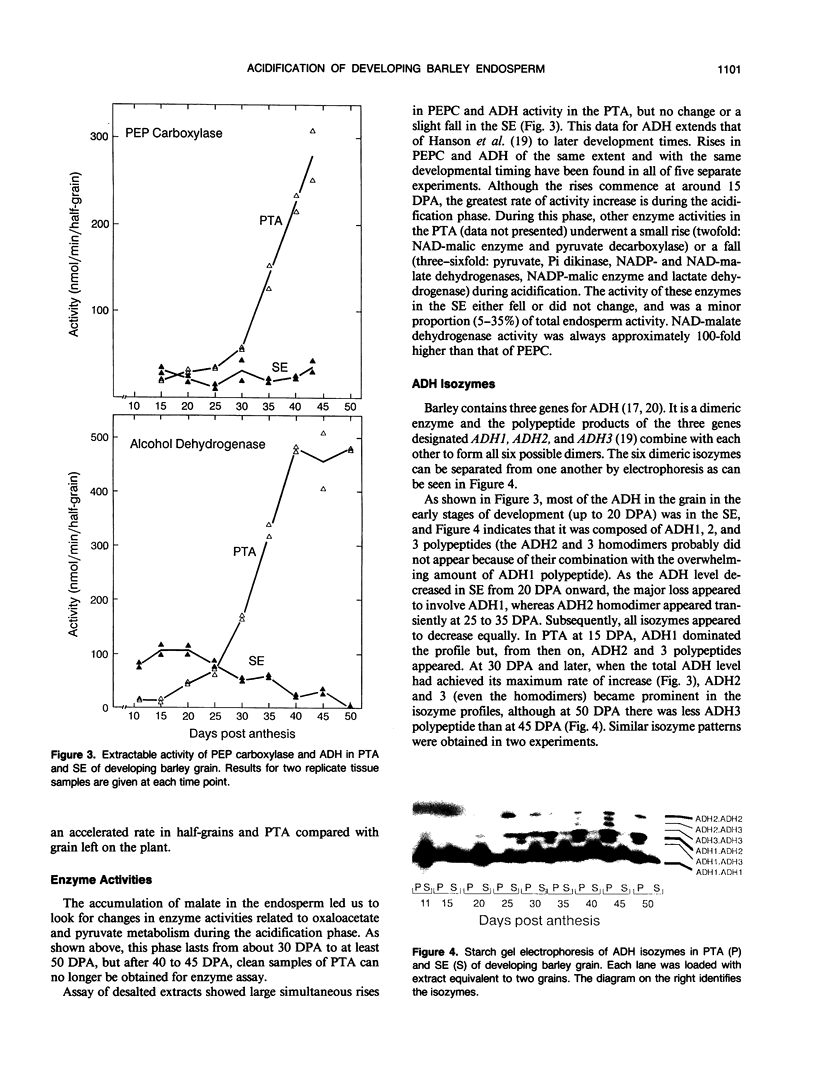
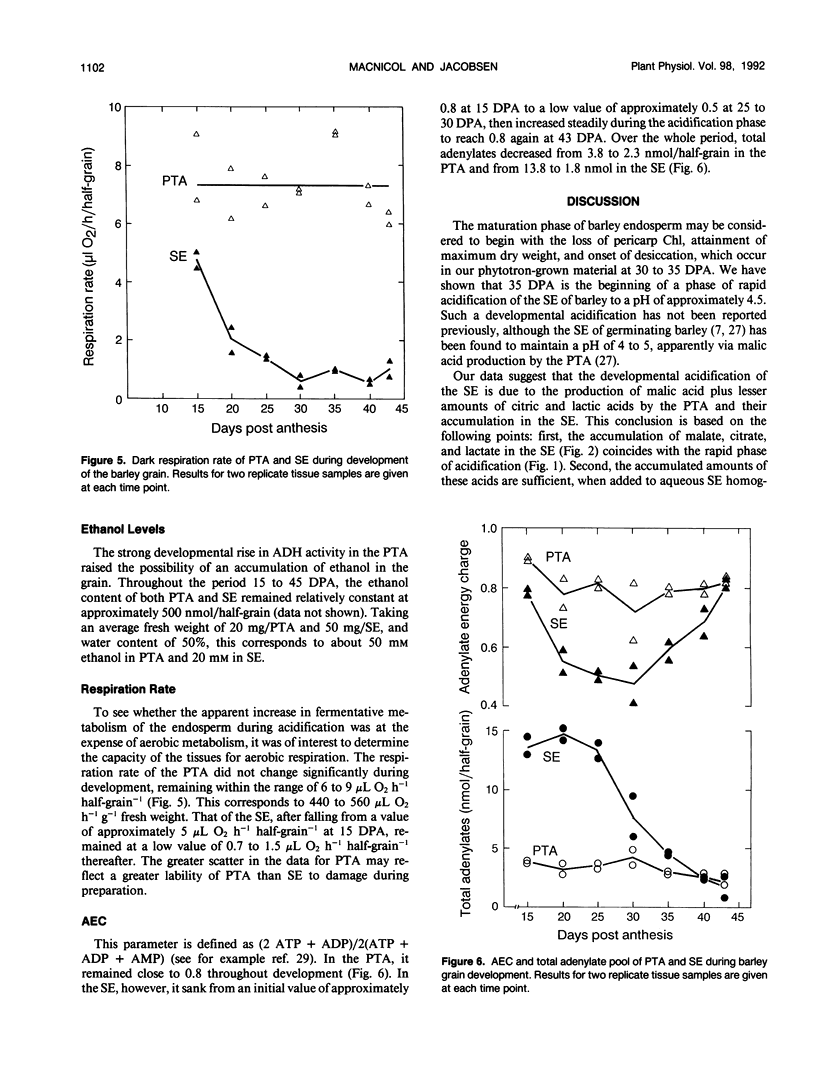
The starchy endosperm (SE) of the developing grain (caryopsis) of barley (Hordeum vulgare L.) cv Himalaya, as well as that of other barley cultivars examined, acidifies during maturation. The major decrease in pH begins with the attainment of maximum grain dry weight, onset of dehydration, and completion of chlorophyll loss. Acidification is correlated with the accumulation of malate and lesser amounts of citrate and lactate, produced and probably secreted by the pericarp/testa/aleurone (PTA). It is accompanied by large concurrent rises in phosphoeno/pyruvate carboxylase and alcohol dehydrogenase (ADH) activity in the PTA. The activity of seven other enzymes of oxaloacetate and pyruvate metabolism was found to fall or rise only slightly during acidification. Sequential changes in relative amount of ADH isozymes were found in both PTA and SE. The PTA maintained a high respiration rate and adenylate energy charge (AEC) throughout acidification, whereas the SE showed a low respiration rate and rising AEC. The data are consistent with the occurrence of hypoxia in the SE. It is suggested that the above enzyme changes are required for the development of a malate/ethanol fermentation (i.e. a mixed metabolism) in the aleurone layer during maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers R. A., Watts R. W. The quantitative extraction and gas-liquid chromatographic determination of organic acids in urine. Analyst. 1972 Dec;97(161):958–967. doi: 10.1039/an9729700958. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Ching K. K. Content of adenosine phosphates and adenylate energy charge in germinating ponderosa pine seeds. Plant Physiol. 1972 Nov;50(5):536–540. doi: 10.1104/pp.50.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Brown A. H. Three alcohol dehydrogenase genes in wild and cultivated barley: characterization of the products of variant alleles. Biochem Genet. 1984 Jun;22(5-6):495–515. doi: 10.1007/BF00484519. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V., Zwar J. A. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Association of NADP- and NAD-linked malic enzyme acitivities in Zea mays: relation to C4 pathway photosynthesis. Arch Biochem Biophys. 1977 Mar;179(2):361–369. doi: 10.1016/0003-9861(77)90123-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]



