Abstract
BACKGROUND:
Imaging evaluation of arrhythmogenic right ventricular cardiomyopathy (ARVC) remains challenging. Myocardial strain assessment by echocardiography is an increasingly utilized technique for detecting subclinical left ventricular (LV) and right ventricular (RV) dysfunction. We aimed to evaluate the diagnostic and prognostic utility of LV and RV strain in ARVC.
METHODS:
Patients with suspected ARVC (n = 109) from a multicenter registry were clinically phenotyped using the 2010 ARVC Revised Task Force Criteria and underwent baseline strain echocardiography. Diagnostic performance of LV and RV strain was evaluated using the area under the receiver operating characteristic curve analysis against the 2010 ARVC Revised Task Force Criteria, and the prognostic value was assessed using the Kaplan-Meier analysis.
RESULTS:
Mean age was 45.3±14.7 years, and 48% of patients were female. Estimation of RV strain was feasible in 99/109 (91%), and LV strain was feasible in 85/109 (78%) patients. ARVC prevalence by 2010 ARVC Revised Task Force Criteria is 91/109 (83%) and 83/99 (84%) in those with RV strain measurements. RV global longitudinal strain and RV free wall strain had diagnostic area under the receiver operating characteristic curve of 0.76 and 0.77, respectively (both P<0.001; difference NS). Abnormal RV global longitudinal strain phenotype (RV global longitudinal strain > −17.9%) and RV free wall strain phenotype (RV free wall strain > −21.2%) were identified in 41/69 (59%) and 56/69 (81%) of subjects, respectively, who were not identified by conventional echocardiographic criteria but still met the overall 2010 ARVC Revised Task Force Criteria for ARVC. LV global longitudinal strain did not add diagnostic value but was prognostic for composite end points of death, heart transplantation, or ventricular arrhythmia (log-rank P=0.04).
CONCLUSIONS:
In a prospective, multicenter registry of ARVC, RV strain assessment added diagnostic value to current echocardiographic criteria by identifying patients who are missed by current echocardiographic criteria yet still fulfill the diagnosis of ARVC. LV strain, by contrast, did not add incremental diagnostic value but was prognostic for identification of high-risk patients.
Keywords: arrhythmogenic right ventricular dysplasia, diagnosis, echocardiography, prognosis, ventricular dysfunction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited life-threatening disease primarily affecting young people.1 Diagnosis is challenging and often involves multimodal investigation integrated with clinical evaluation.2 While echocardiography forms part of the 2010 ARVC Revised Task Force Criteria (TFC) for diagnosis,3,4 there are limitations to conventional echocardiographic approaches and, hence, increasing focus on alternative imaging modalities, including advanced echocardiographic analysis and magnetic resonance imaging.5 Strain assessment, which can identify ventricular dysfunction before conventional echocardiographic approaches by detection of subtle tissue deformation abnormalities, provides a promising avenue for improved clinical assessment of ARVC using echocardiography.6,7 Increasingly, there is greater awareness of pathology of the left ventricle (LV) in ARVC in addition to right ventricular (RV) pathology.8
To date, there have been no prospective, multicenter studies evaluating the diagnostic and prognostic value of LV and RV strain in ARVC. We aimed to study the diagnostic and prognostic utility of LV and RV strain in the National Institutes of Health–sponsored North American ARVC Registry and compare performance with conventional echocardiographic criteria. We hypothesized that RV and LV strains would have diagnostic and prognostic utility in ARVC.
METHODS
Subject Recruitment
We evaluated 109 patients with suspected ARVC who were enrolled in a registry sponsored by the National Institutes of Health across 11 centers in the United States between 2014 and 2018. Echocardiogram were submitted at the time of enrollment and analyzed by a core laboratory (see below). Patients also underwent 12-lead electrocardiography, signal-averaged electrocardiography, and 24-hour Holter electrocardiography. Echocardiography, signal-averaged electrocardiography, and imaging were stored and reviewed at centralized cores. After enrollment, patients were classified by independent review as definite, borderline, and possible ARVC by TFC. Patients not fulfilling these ARVC criteria were excluded from the current analysis. Studies had local institutional review board approvals, and subjects gave informed consent to participate. The data that support the findings of this study are available from D.Y.S. (dysanborn@mgh.harvard.edu), upon reasonable request.
Clinical Assessment
Patients underwent clinical phenotyping according to the 2010 ARVC TFC that incorporated a combination of structural abnormalities (by imaging), tissue abnormality by endomyocardial biopsy, electrocardiographic criteria, history of arrhythmia, and family history (full description of TFC is given in Table S1).3
Echocardiographic Assessment
Echocardiographic acquisition was performed locally across the 11 recruiting sites. However, echocardiographic analysis, including conventional ARVC assessment4 and RV strain analysis, was performed (blinded to ARVC TFC status) at an independent echocardiography core laboratory based at Massachusetts General Hospital, Boston, using Syngo Dynamics, version VA20 (Siemens Healthineers, Erlangen, Germany), and TomTec Arena TTA2.50 (Philips Healthcare, Andover, MA) software. Syngo Dynamics was used for 2-dimensional measurements, while TOMTEC Arena was used for strain measurements. RV analysis was performed from apical RV-focused views, per recommendations.9 RV strain assessment was performed if there was adequate visualization of the RV endocardium throughout at least 1 complete cardiac cycle.
RV global longitudinal strain (RV-GLS) was calculated as the average end-systolic strain of the 6 segments of the RV (basal septal, mid septal, apical septal, apical free wall, mid free wall, and basal free wall). RV free wall strain (RV-FWS) was calculated as the average of the 3 free wall segments. RV mechanical dispersion was calculated as the SD of the time to peak strain in the 6 endocardial segments, and RV free wall mechanical dispersion was calculated as the SD of the time to peak strain in the 3 free wall segments. LV strain was measured as endocardial global longitudinal strain (LV global longitudinal strain [LV-GLS]), the average of end-systolic strain in all LV segments, using a combination of apical 2-, 3-, and 4-chamber views.
Clinical Outcomes
Patients in the registry were prospectively followed. We analyzed a composite outcome of death (all-cause mortality), heart transplantation, or life-threatening ventricular arrhythmia. The composite end-point time was determined by the first event where multiple events were registered. Life-threatening ventricular arrhythmias in this cohort were defined as sudden cardiac death, sudden cardiac arrest, sustained ventricular tachycardia, or appropriate implantable cardioverter defibrillator therapy (therapy defined as antitachycardia pacing or shock).
Statistical Analysis
Diagnostic performance of RV strain metrics was evaluated using receiver operating curve analysis against the reference diagnosis by TFC. Metrics of diagnostic performance included area under the receiver operating characteristic curve, sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, overall test accuracy, and net reclassification index, at optimal cutoff determined by the Youden index, when sensitivity and specificity are equally treated. Comparative performance was evaluated using the area under the receiver operating characteristic curve comparison. Diagnostic performance was also evaluated in the subset of patients not fulfilling major or minor echocardiographic TFC (minor criteria include the presence of regional akinesis, dyskinesis, or aneurysm, with RV outflow tract dimension in the short axis ≥32 but <36 mm or long axis ≥29 but <32 mm, or fractional area change ≤40 but >33%; major criteria include the presence of regional akinesis, dyskinesis, or aneurysm, with RV outflow tract dimension in the short axis ≥36 mm or long axis ≥32 mm, or fractional area change ≤33%).3
The prognostic value of LV and RV strain metrics was evaluated using Kaplan-Meier analysis. The prognostic value of left ventricular ejection fraction was also assessed.
Statistical analysis was performed using IBM SPSS 26 (IBM Corporation, Armonk, NY), and net reclassification analyses were computed using R, v4.0 (package nricens).10
All studies were approved by local institutional review boards, and patients provided written informed consent.
RESULTS
The study cohort comprised 109 registry patients with suspected ARVC (83 definite, 8 borderline, and 14 possible by TFC; 87 probands and 22 family members). The mean age was 45.3±14.7 years; the cohort was 48% female and 95% White race. Baseline characteristics are outlined in Table 1. The prevalence of definite or borderline ARVC by TFC was 91/109 (83%).
Table 1.
Cohort Characteristics
| Variable | Mean±SD, median (IQR) or n/total (%) |
|---|---|
| Age, y | 45.3±14.7 |
| Female | 52/109 (48%) |
| Height, cm | 172.4±9.8 |
| Weight, kg | 82.5 (65.8–101.3) |
| History of syncope | 31/97 (28%) |
| Previous cardiac arrest | 11/98 (10%) |
| Major ECG repolarization TFC | 68/109 (62%) |
| Minor ECG repolarization TFC | 10/109 (9%) |
| Major ECG depolarization TFC | 6/109 (6%) |
| Minor ECG depolarization TFC | 33/109 (30%) |
| Major arrhythmia TFC | 32/109 (29%) |
| Minor arrhythmia TFC | 41/109 (38%) |
| Major family history TFC | 57/109 (52%) |
| Minor family history TFC | 1/109 (1%) |
| RV-GLS, % | −17.6±6.1 |
| RV-FWS, % | −17.7±7.6 |
| RV-MD, ms | 68.9 (40.2–1078) |
| RV-FWMD | 50.8 (23.7—1 16.0) |
| RVOT-SAX, mm | 39 (34–44) |
| FAC, % | 29.0±12.1 |
| LVEF, % | 55.2±7.6 |
| LV-GLS, % | — 19.9±3.7 |
ARVC indicates arrhythmogenic right ventricular cardiomyopathy; FAC, fractional area change; IQR, interquartile range; LVEF, left ventricular ejection fraction; LV-GLS, left ventricular global longitudinal strain; RV-FWMD, right ventricular free wall mechanical dispersion; RV-FWS, right ventricular free wall strain; RV-GLS, right ventricular global longitudinal strain; RV-MD, right ventricular mechanical dispersion; RVOT-SAX, right ventricular outflow tract diameter at end-diastole in short axis view; and TFC, 2010 ARVC Revised Task Force Criteria.
Diagnostic Utility of RV Strain
Measurement of RV strain was technically feasible in 99 patients (91% of the study cohort), and the prevalence of definite or borderline ARVC by TFC was 83/99 (84%) in this group. An example RV strain analysis is shown in Figure 1. LV strain was feasible in 85 patients (78% of the study cohort). The diagnostic performance of RV strain is summarized in Figure 2 and Table 2. Table 2 also outlines optimal cutoffs for diagnosis based on the Youden index. RV-GLS had area under the receiver operating characteristic curve 0.76 (0.64–0.88; P<0.001), and RV-GLS worse (less negative) than −17.9% had 61% sensitivity and 81% specificity for the diagnosis of ARVC, while RV-FWS had area under the receiver operating characteristic curve 0.77 (0.65–0.90; P<0.001), and RV-FWS worse than −21.2% had 83% sensitivity and 52% specificity for ARVC. Neither RV mechanical dispersion and RV free wall mechanical dispersion nor LV-GLS was significantly diagnostic (P=0.19, P=0.32, and P=0.13, respectively). There was no significant difference in the diagnostic performance of RV-GLS and RV-FWS (P difference=0.65).
Figure 1. Example right ventricular strain analysis.
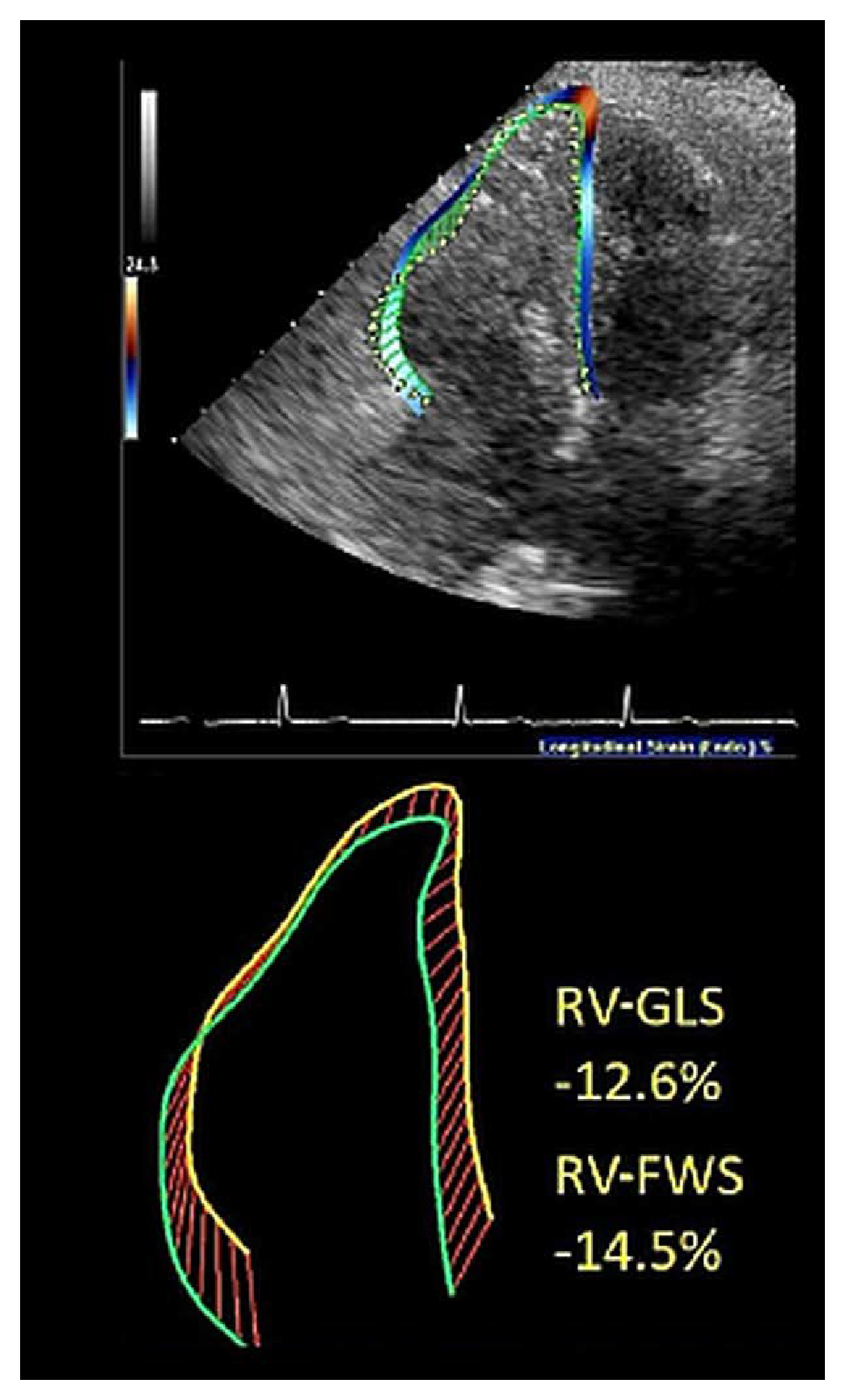
RV-FWS indicates right ventricular free wall strain; and RV-GLS, right ventricular global longitudinal strain.
Figure 2. Diagnostic performance of right ventricular strain in arrhythmogenic right ventricular cardiomyopathy.
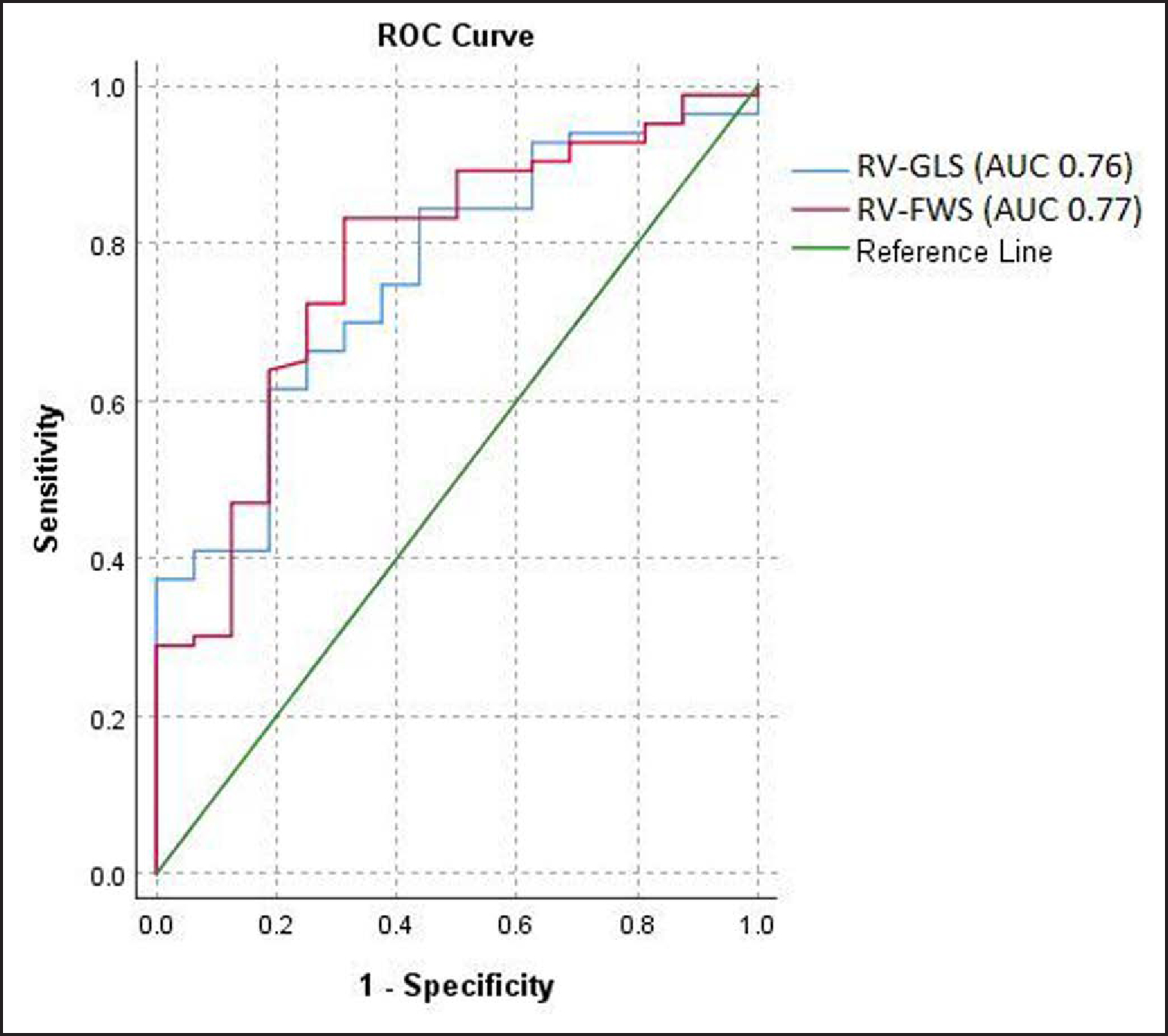
Full model details are given in Table 2. AUC indicates area under the receiver operating curve; ROC, receiver operating curve; RV-FWS, right ventricular free wall strain; and RV-GLS, right ventricular global longitudinal strain.
Table 2.
Diagnostic Performance of Right Ventricular Strain Metrics
| RV strain metric | AUC | P value | Optimal cutoff* | Sensitivity, % | Specificity, % | PPV, % | NPV, % | PLR | NLR | Accuracy, % |
|---|---|---|---|---|---|---|---|---|---|---|
| RV-GLS, % | 0.76 (0.64–0.88) | <0.001 | −179 | 61.4 | 81.3 | 94.4 | 28.9 | 3.3 | 0.5 | 64.6 |
| RV-FWS, % | 0.77 (0.65–0.90) | <0.001 | −21.2 | 83.1 | 51.9 | 93.2 | 44.0 | 2.7 | 0.2 | 80.8 |
AUC indicates area under the receiver operating curve; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; RV, right ventricular; RV-FWS, right ventricular free wall strain; and RV-GLS, right ventricular global longitudinal strain.
Determined by the Youden index.
Both RV-GLS and RV-FWS were significantly different between TFC affected (RV-GLS, −16.7±6.0% and RV-FWS, −16.5±7.2%) and unaffected subjects (RV-GLS, −22.2±4.8% and RV-FWS, −23.8±6.9%; P=0.001 for both RV-GLS and RV-FWS). We sought to evaluate the utility of strain in those missed by TFC. We analyzed RV-GLS in the subset of patients diagnosed with ARVC who did not fulfill the echocardiographic component of TFC with the use of standard echocardiographic 2-dimensional measurements alone (n=75 diagnosed with ARVC in the absence of 2-D echocardiographic component, with RV strain available in n=69). Table S2 describes the diagnostic criteria used to classify ARVC in this cohort, in the absence of echocardiographic TFC phenotype. In this subset, 41/69 (59%) patients had RV-GLS worse than our optimal cutoff of −17.9%, and 56/69 (81%) patients had RV-FWS worse than the optimal cutoff of −21.2%, meaning that the ARVC phenotype could be reliably detected using RV strain in a large majority of patients where traditional echocardiographic components of the TFC were not met. In net reclassification analyses, compared with the conventional echocardiographic approach, strain analyses resulted in greater case detection (NRI+, 46%,[95% CI, 36%–56%]) although at the cost of some unfavorable noncase reclassification (NRI−, −39% [95% CI, −61% to −15%]). Overall net reclassification index was favorable (0.073 [95% CI, −0.18 to 0.32]). Detailed reclassification results are shown in Table 3.
Table 3.
Reclassification Analysis Comparing Traditional Echocardiographie Criteria for ARVC Diagnosis to Derived RV Strain Cutoffs (RV-GLS or RV-FWS)
| Strain (RV-GLS or RV-FWS abnormal) | |||
|---|---|---|---|
| Traditional echo | No | Yes | |
| ARVC | No | 13 | 43† |
| Yes | 1* | 34 | |
| NOT ARVC | No | 10 | 7* |
| Yes | 1† | 34 | |
| NRI + | 46% (36% to 56%) | ||
| NRI− | −39% (−61% to −15%) | ||
| NRI | 0.073 (−0.18 to 0.32) | ||
ARVC indicates arrhythmogenic right ventricular cardiomyopathy; NRI, net reclassification index; NRI+, case reclassification; NRI—, noncase reclassification; RV, right ventricular; RV-FWS, right ventricular free wall strain; and RV-GLS, right ventricular global longitudinal strain.
Inappropriate reclassification.
Appropriate reclassification.
Prognostic Utility of LV and RV Strain
During a median follow-up time of 2.9 years (maximum follow-up, 4.3 years), 1 death, 5 heart transplants, and 13 ventricular arrhythmia events were observed. As there were overlapping events in some patients, the total composite event rate for death, heart transplantation, or ventricular arrhythmia was 17/109 (16%) in the study cohort and 15/99 (15%) in those with RV strain measurements. An RV-GLS worse than (less negative) −17.9% was associated with more composite events in follow-up (P=0.039; χ2), but an RV-FWS worse than (less negative) −21.2% was not associated with a significantly different event rate (NS P=0.9; χ2). However, while the event ratio by χ2 showed a favorable signal for RV-GLS, by Kaplan-Meier analysis, there was no significant difference in time-dependent event rate using these cut points for RV-GLS and RV-FWS (NS P=0.39 log-rank and NS P=0.46, respectively). By contrast, LV-GLS worse than the recommended normal cutoff of −18.0%5 was a significant predictor of the composite outcome in time-dependent event analysis (P=0.04 log-rank; Figure 2). Importantly, left ventricular ejection fraction ≤55% was not prognostic for outcome (NS P=0.36 log-rank).
There was no significant difference in age, height, weight, and systolic or diastolic blood pressure in LV-GLS groups ≤−18% and >18% (Table 4).
Table 4.
Comparison of Baseline Factors Between Left Ventricular Global Longitudinal Strain Groups
| Variable | LV-GLS ≤-18% | LV-GLS >-18% | Significance |
|---|---|---|---|
| Age, y | 44±15 | 49±15 | NS (0.70) |
| Height, cm | 172±9 | 173±9 | NS (0.99) |
| Weight, kg | 79 (64–95) | 86 (67–103) | NS (0.27) |
| SBP | 115±16 | 116±20 | NS (0.36) |
| DBP | 68 (60 –74) | 70 (65–81) | NS (0.32) |
DBP indicates diastolic blood pressure; LV-GLS, left ventricular global longitudinal strain; NS, nonsignificant; and SBP systolic blood pressure.
DISCUSSION
In a prospective, multicenter registry, we evaluated the utility of LV and RV strain in ARVC. Our study’s key findings are given as follows.
RV strain (Figure 1) had good diagnostic performance against a reference diagnostic standard of the revised TFC (which incorporates clinical information pertaining to imaging, tissue and electrocardiographic abnormalities, history of arrhythmia, and family history) and could identify ARVC phenotype in a large proportion of patients who did not meet current echocardiographic TFC.
While LV strain did not add diagnostic value, it identified high-risk patients and is, therefore, a good prognostic marker (Figure 3).
Figure 3. Prognostic value of left ventricular strain in arrhythmogenic right ventricular cardiomyopathy showing freedom from composite end point of death (all-cause mortality), heart transplantation, or ventricular arrhythmia.
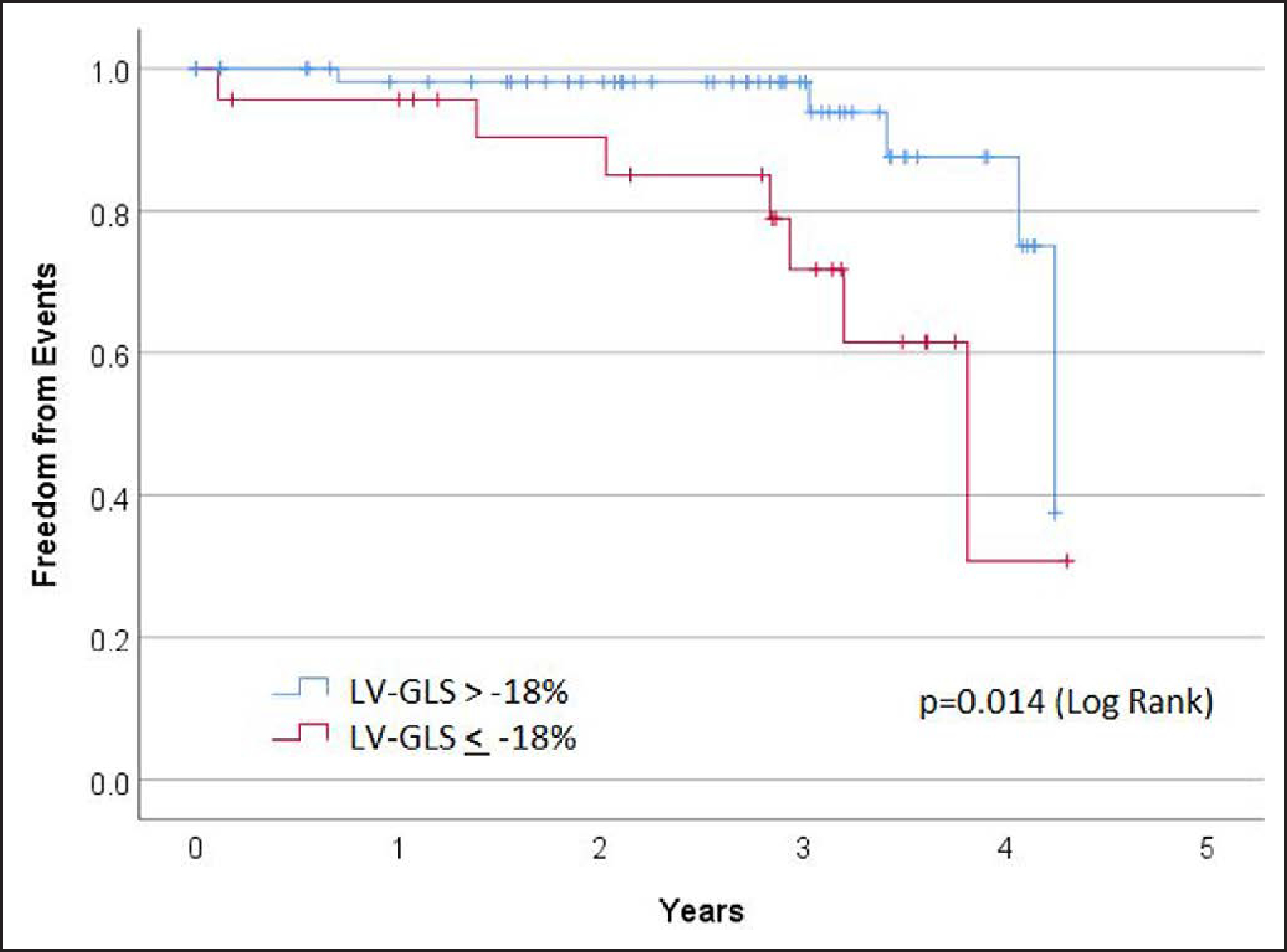
LV-GLS indicates left ventricular global longitudinal strain.
Our study highlights the value of adding strain analysis to the evaluation of ARVC patients. Diagnostic yield can be improved with RV strain, and prognosis can be better informed using LV strain. LV and RV strain are easy to perform from routinely acquired clinical ECGs and can easily be done using postprocessing software to evaluate previously obtained echocardiographic images. While LV strain is commonly used, RV strain is less commonly applied in routine practice, but we found that it is easily applied. RV strain was feasible in 91% of our prospectively recruited, unselected, multicenter cohort.
Additionally, the combined use of RV-GLS and RV-FWS can improve diagnosis due to their comparative strengths. Using the Youden index–based diagnostic cutoffs, RV-GLS was specific, while RV-FWS was sensitive, meaning that in combination, these metrics are complementary. With this said, this observation is influenced by using Youden index cutoffs that weigh sensitivity and specificity equally, but nonetheless, when taking this neutral approach, such a pattern emerges and can potentially be exploited in practice. Absolute strain cutoffs are different in RV-GLS than RV-FWS as expected due to the influence of the interventricular septum. While there has been increasing focus on magnetic resonance imaging in ARVC, echocardiography is more accessible than magnetic resonance imaging, more comfortable for patients, and lower cost, avoids concerns about magnetic fields and cardiac implantable electronic devices, does not require gadolinium administration, and is more convenient for serial assessment. The use of strain echocardiography in the first instance could better identify patients through greater sensitivity and could then triage those needing further assessments or closer surveillance. We believe that future diagnostic guidelines should incorporate strain analyses to supplement traditional echocardiographic approaches.
Others have also highlighted the clinical utility of RV strain and derived similar clinical cut points to those observed in our data. Muraru et al11 noted that abnormal RV-GLS could be denoted by a value worse than −20%, while Corrado et al12 note that a strain worse than −18% identifies regional dysfunction in ARVC. These values are in line with the optimal GLS cutoff that we found in our study (−17.9%). Additionally, our diagnostic cutoff for RV-FWS of −21.2% is supportive of (albeit slightly more lenient than) consensus recommendations suggesting a normal limit RV-FWS as −23%.5
Furthermore, a growing body of evidence has documented links between RV strain parameters and disease progression and clinical arrhythmia in ARVC. Teske et al13 and Sarvari et al14 have correlated RV strain to disease severity and the likelihood of malignant arrhythmia. More recently, Mast et al15 have demonstrated the role of abnormal mechanical dispersion in the basal RV in predicting disease progression in the preclinical stages of ARVC.16 Kirkels et al17 have shown the value of RV strain deformation pattern and mechanical dispersion in predicting ventricular arrhythmia. Malik et al18 also recently reported in a retrospective evaluation of 40 patients that RV-FWS worse than −20% was associated with worse structural progression (defined by RV dilatation) in ARVC.
Our finding that LV strain is a good prognostic marker is an important finding. Recent work has shown that LV pathology exists in a significant proportion of ARVC patients undergoing postmortem analysis.8 Our findings support the idea that patients with LV involvement are in a higher risk group than those without. Importantly, the prognostic importance of LV involvement is not detected by conventional measures (left ventricular ejection fraction), highlighting the value of LV strain analysis.19 Moreover, there were no differences in baseline characteristics between LV-GLS groups, suggesting that this is not a result confounded by LV-GLS being a marker of other factors such as advanced age or hypertension. Our data add to the body of evidence driving the push to rename ARVC as simply, arrhythmogenic cardiomyopathy, AC, removing the RV designation and acknowledging the increasingly recognized biventricular involvement. Indeed, the recent Padua criteria highlight the importance of LV involvement in ARVC and also highlight the use of strain to analyze ventricular dysfunction.20 Our data, and the preceding work of others, highlight the close attention that should be paid to patients with ARVC and impaired LV strain. While it would be potentially premature to suggest that these patients should necessarily be protected from events with implantable defibrillator devices based on our data alone, we advocate for urgent further research in this area. At the least, we would support closer surveillance and evaluation, perhaps with magnetic resonance imaging analyses for gadolinium uptake or an electrophysiology study, for arrhythmic risk stratification.
Some limitations must be considered when interpreting our data. Strain was not feasible in all patients, reflecting real-world practice. RV strain was feasible in 91% patients, and LV strain was feasible in 78% patients. Patients with inadequate RV or LV strain were not necessarily congruous. That is, RV strain could be feasible when LV strain was not and vice versa. For this reason, we did not constrain the cohort to only cases where RV or LV strain were feasible to maximize available strain data for analyses. The cohort was enriched for ARVC, with a prevalence of ≈80% by revised TFC. While this affects the positive and negative predictive values of the diagnostic performance of RV strain, sensitivity, specificity, and likelihood ratio data are less susceptible to the influence of high prevalence. Further studies evaluating the performance of RV strain with a less enriched cohort, or a reference population of healthy subjects, would be worth evaluating. Our analysis defined and tested optimal cutoffs for diagnostic performance within one cohort; therefore, the results presented represent a best-case scenario. While this practice is commonplace in the literature, particularly in rare diseases where large datasets are impractical, it is important to highlight this point. The fact that our optimal cutoffs are closely aligned with prior investigations is reassuring. Prognostic findings could be influenced by low patient cohort numbers and low event rates, albeit despite prospective follow-up over a median of approximately 3 years in a multicenter North American registry. Our cohort had a balanced sex mix but was majority (95%) White race, limiting external validity in other ethnicities. Future work must occur in patients from diverse ethnic backgrounds to validate the findings presented here. We acknowledge that multicenter studies can have confounding due to variations in patient demographics, phenotype, and practice patterns between centers. Because our study was evaluating genetic cardiomyopathy (with limited influence of known geographic variation in phenotype within North America) and was part of a national registry with standardized surveillance and management protocols, we believe that the potential confounding effects of a multicenter approach are limited. Moreover, given the rarity of ARVC, a multicenter approach was inherently necessary for our study. The addition of genotype data could have enriched this analysis, but unfortunately, such data were not available to us. Notwithstanding the fact that genotype-phenotype interaction in ARVC is incompletely understood, future analyses could benefit from including such data. While considering future studies that can build upon the findings presented, investigators might consider 3-dimensional approaches to RV strain analysis.21,22
CONCLUSIONS
In a prospective, multicenter registry of ARVC, RV strain demonstrated diagnostic utility and added to current echocardiographic criteria by identifying ARVC phenotype in a large proportion of patients missed by conventional echocardiographic criteria, while LV strain identified high-risk patients.
Supplementary Material
CLINICAL PERSPECTIVE.
In a multicenter registry of arrhythmogenic right ventricular (RV) cardiomyopathy, RV strain analysis demonstrated diagnostic utility, while left ventricular strain demonstrated prognostic utility. For diagnosis of arrhythmogenic RV cardiomyopathy, RV global longitudinal strain was more sensitive, while RV free wall strain was more specific. RV global longitudinal strain and RV free wall strain were abnormal (above optimal cutoff) in 59% and 81% of patients, respectively, who met conventional Task Force diagnostic criteria for arrhythmogenic RV cardiomyopathy using the non-imaging parameters but would have been missed by traditional echocardiographic criteria. Left ventricular strain analysis did not add diagnostic value but was more prognostic than RV strain. RV strain assessment adds to conventional approaches to echocardiographic diagnosis of arrhythmogenic RV cardiomyopathy while left ventricular strain identifies patients at increased risk of adverse outcomes.
Acknowledgments
Additional information:
Frank I Marcus, MD, died December, 2022; authorship listed with permission of his estate. His co-authors acknowledge Dr Marcus’ invaluable contributions over decades to the understanding of arrhythmogenic right ventricular cardiomyopathy.
Sources of Funding
Drs Namasivayam, Bernard, Bertrand, Churchill and Khurshid have received support from Clinical and Research Fellowships from Massachusetts General Hospital and Harvard Medical School. Dr Namasivayam has received support from the St. Vincent’s Clinic Foundation/Applied Medical Research Institute, the National Heart Foundation of Australia Postdoctoral Fellowship, the New South Wales Early-Mid Career Fellowship, Ramaciotti Foundation Health Investment Grant and Nvidia Corporation Academic Hardware Grant. Dr Churchill is supported by the National Institutes of Health (1K23HL159262–01A1). The registry study was supported by a NIH grant R01 HL 116906. Dr Mestroni is supported by NIH grants R01 HL69071, HL116906, 1R01HL147064 NCATS/CTSA UL1 TR001082, AHA17GRNT33670495 and in part by the Foundation Leducq TNE (14-CVD 03).
Nonstandard Abbreviations and Acronyms
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- LV
left ventricle
- LV-GLS
left ventricular global longitudinal strain
- RV
right ventricle/ventricular
- RV-FWS
right ventricular free wall strain
- RV-GLS
right ventricular global longitudinal strain
- TFC
2010 ARVC Revised Task Force Criteria
Footnotes
Disclosures
Dr Namasivayam received support from the Nvidia Corporation Academic Hardware Grant for work unrelated to this article. The other authors report no conflicts.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.123.015671.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Mayooran Namasivayam, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Cardiology, St Vincent’s Hospital, Faculty of Medicine and Health, University of New South Wales, Victor Chang Cardiac Research Institute, Sydney, Australia.
Philippe B. Bertrand, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Cardiology, Ziekenhuis Oost-Limburg, Genk, Belgium.
Samuel Bernard, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston; Division of Cardiology, NYU Langone Health, New York University.
Timothy W. Churchill, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston.
Shaan Khurshid, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston.
Frank I. Marcus, University of Arizona College of Medicine, Tucson.
Luisa Mestroni, Division of Cardiology and Cardiovascular Institute, University of Colorado Anschutz Medical Campus, Aurora.
Jeffrey E. Saffitz, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston.
Jeffrey A. Towbin, St. Jude Children’s Research Hospital, University of Tennessee Health Science Center, Memphis.
Wojciech Zareba, University of Rochester Medical Center, NY.
Michael H. Picard, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston.
Danita Yoerger Sanborn, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston.
REFERENCES
- 1.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. doi: 10.1056/NEJMra1509267 [DOI] [PubMed] [Google Scholar]
- 2.Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Am Coll Cardiol. 2018;72:784–804. doi: 10.1016/j.jacc.2018.05.065 [DOI] [PubMed] [Google Scholar]
- 3.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoerger DM, Marcus F, Sherrill D, Calkins H, Towbin JA, Zareba W, Picard MH; Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Echocardiographic findings in patients meeting task force criteria for arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2005;45:860–865. doi: 10.1016/j.jacc.2004.10.070 [DOI] [PubMed] [Google Scholar]
- 5.Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O, Galderisi M, Habib G, Knuuti J, Lancellotti P, et al. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy – an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J. 2017;18:237–253. doi: 10.1093/ehjci/jew229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoit BD. Right ventricular strain comes of age. Circ Cardiovasc Imaging. 2018;11:e008382. doi: 10.1161/CIRCIMAGING.118.008382 [DOI] [PubMed] [Google Scholar]
- 7.Prakasa KR, Wang J, Tandri H, Dalal D, Bomma C, Chojnowski R, James C, Tichnell C, Russell S, Judge D, et al. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:507–512. doi: 10.1016/j.amjcard.2007.03.053 [DOI] [PubMed] [Google Scholar]
- 8.Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B, Basu J, Parry-Williams G, Papatheodorou E, Paterson C, et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D’Hooge J, Donal E, Fraser AG, Marwick T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Img. 2018;19:591–600. doi: 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 10.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org [Google Scholar]
- 11.Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, Romeo G, Cavalli G, Iliceto S, Badano LP. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ Cardiovasc Imaging. 2016;9:e003866. doi: 10.1161/CIRCIMAGING.115.003866 [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teske AJ, Cox MG, De Boeck BW, Doevendans PA, Hauer RN, Cramer MJ. Echocardiographic tissue deformation imaging quantifies abnormal regional right ventricular function in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Soc Echocardiogr. 2009;22:920–927. doi: 10.1016/j.echo.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 14.Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E, Amlie JP, Edvardsen T. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J. 2011;32:1089–1096. doi: 10.1093/eurheartj/ehr069 [DOI] [PubMed] [Google Scholar]
- 15.Mast TP, Taha K, Cramer MJ, Lumens J, van der Heijden JF, Bouma BJ, van den Berg MP, Asselbergs FW, Doevendans PA, Teske AJ. The prognostic value of right ventricular deformation imaging in early arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol Img. 2019;12:446–455. doi: 10.1016/j.jcmg.2018.01.012 [DOI] [Google Scholar]
- 16.Haugaa KH, Lie OH. Reveal the concealed: the quest for early disease detection in family members at risk of developing arrhythmogenic cardiomyopathy. J Am Coll Cardiol Img. 2019;12:456–457. doi: 10.1016/j.jcmg.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Kirkels FP, Lie ØH, Cramer MJ, Chivulescu M, Rootwelt-Norberg C, Asselbergs FW, Teske AJ, Haugaa KH. Right ventricular functional abnormalities in arrhythmogenic cardiomyopathy: association with life-threatening ventricular arrhythmias. J Am Coll Cardiol Img. 2021;14:900–910. doi: 10.1016/j.jcmg.2020.12.028 [DOI] [PubMed] [Google Scholar]
- 18.Malik N, Win S, James CA, Kutty S, Mukherjee M, Gilotra NA, Tichnell C, Murray B, Agafonova J, Tandri H, et al. Right ventricular strain predicts structural disease progression in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc. 2020;9:e015016. doi: 10.1161/JAHA.119.015016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Voss WB, Lentz RW, Thomas JD, Akhter N. The diagnostic and prognostic value of echocardiographic strain. JAMA Cardiol. 2019;4:580–588. doi: 10.1001/jamacardio.2019.1152 [DOI] [PubMed] [Google Scholar]
- 20.Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, Migliore F, Pilichou K, Rampazzo A, Rigato I, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 21.Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations in integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther. 2018;8:101–117. doi: 10.21037/cdt.2017.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atsumi A, Seo Y, Ishizu T, Nakamura A, Enomoto Y, Harimura Y, Okazaki T, Abe Y, Aonuma K. Right ventricular deformation analyses using a three-dimensional speckle-tracking echocardiographic system specialized for the right ventricle. J Am Soc Echocardiogr. 2016;29:402–411.e2. doi: 10.1016/j.echo.2015.12.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


