Abstract
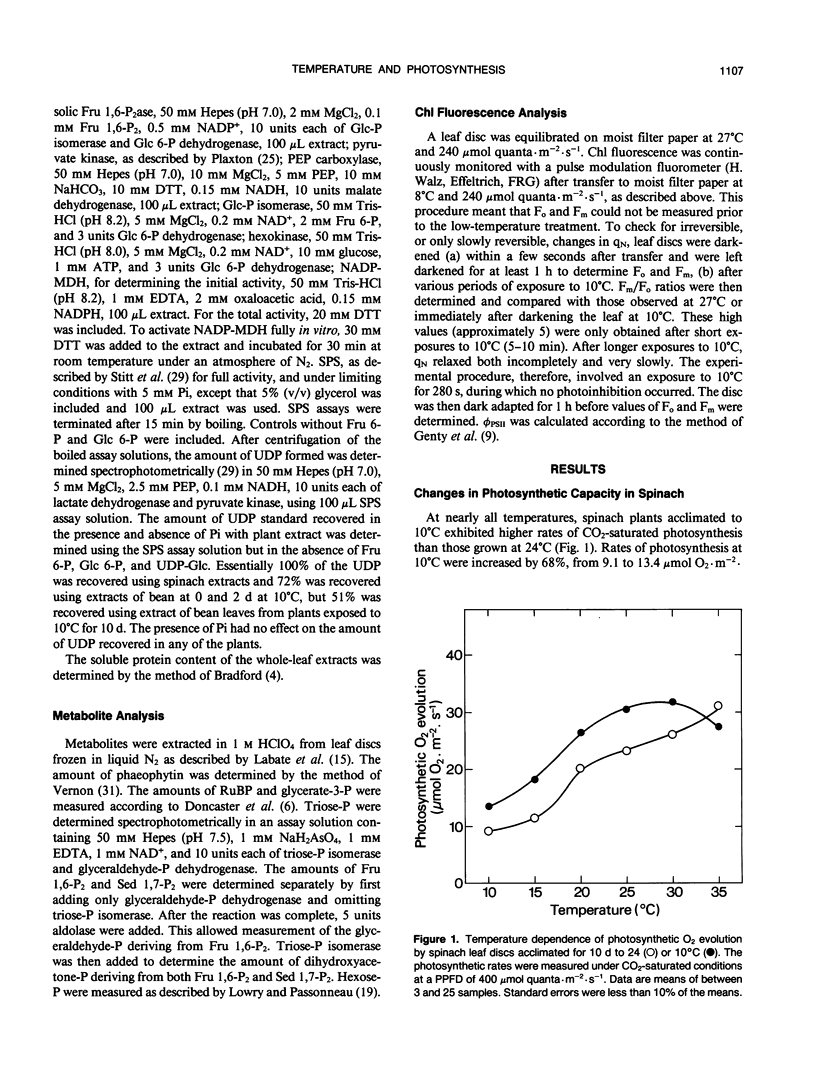
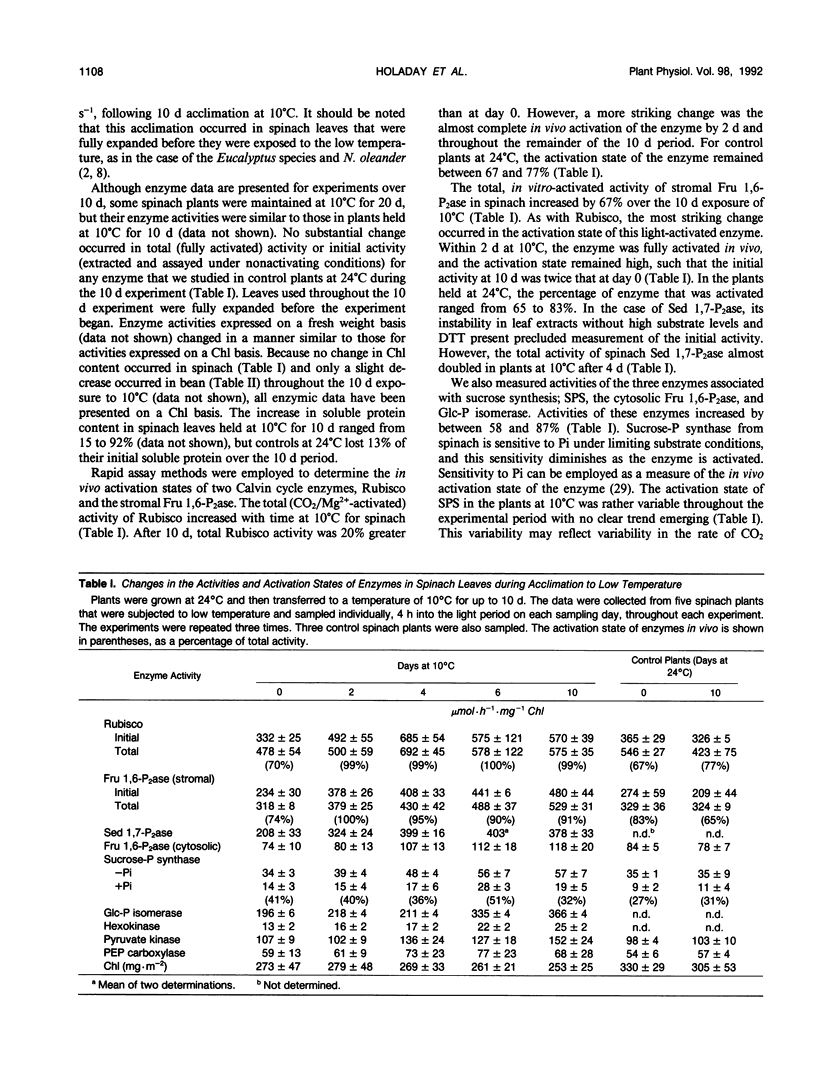
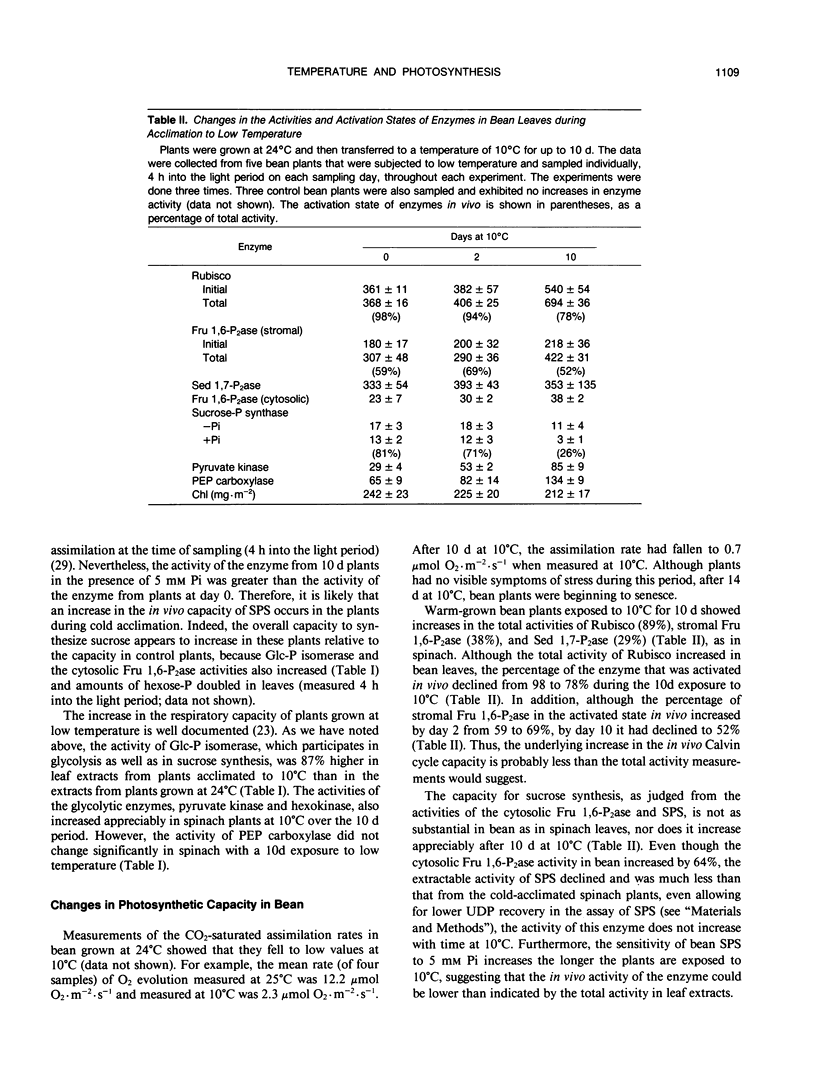
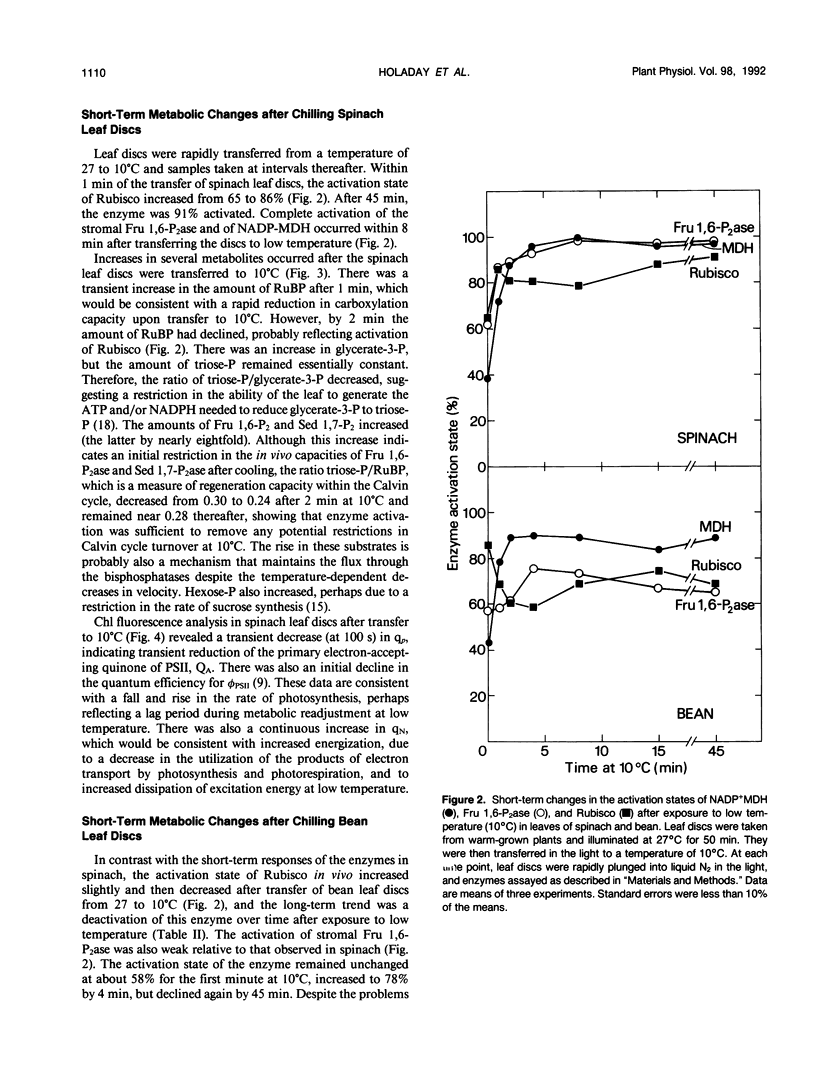
The aim of this study was to determine the response of photosynthetic carbon metabolism in spinach and bean to low temperature. (a) Exposure of warm-grown spinach and bean plants to 10°C for 10 days resulted in increases in the total activities of a number of enzymes, including ribulose 1,5-bisphosphate carboxylase (Rubisco), stromal fructose 1,6 bisphosphatase (Fru 1,6-P2ase), sedoheptulose 1,7-bisphosphatase (Sed 1,7-P2ase), and the cytosolic Fru 1,6-P2ase. In spinach, but not bean, there was an increase in the total activity of sucrose-phosphate synthase. (b) The CO2-saturated rates of photosynthesis for the cold-acclimated spinach plants were 68% greater at 10°C than those for warm-acclimated plants, whereas in bean, rates of photosynthesis at 10°C were very low after exposure to low temperature. (c) When spinach leaf discs were transferred from 27 to 10°C, the stromal Fru 1,6-P2ase and NADP-malate dehydrogenase were almost fully activated within 8 minutes, and Rubisco reached 90% of full activation within 15 minutes of transfer. An initial restriction of Calvin cycle fluxes was evident as an increase in the amounts of ribulose 1,5-bisphosphate, glycerate-3-phosphate, Fru 1,6-P2, and Sed 1,7-P2. In bean, activation of stromal Fru 1,6-P2ase was weak, whereas the activation state of Rubisco decreased during the first few minutes after transfer to low temperature. However, NADP-malate dehydrogenase became almost fully activated, showing that no loss of the capacity for reductive activation occurred. (d) Temperature compensation in spinach evidently involves increases in the capacities of a range of enzymes, achieved in the short term by an increase in activation state, whereas long-term acclimation is achieved by an increase in the maximum activities of enzymes. The inability of bean to activate fully certain Calvin cycle enzymes and sucrose-phosphate synthase, or to increase nonphotochemical quenching of chlorophyll fluorescence at 10°C, may be factors contributing to its poor performance at low temperature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hahn M., Walbot V. Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol. 1989 Nov;91(3):930–938. doi: 10.1104/pp.91.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel J. R., Prosser C. L. Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev. 1974 Jul;54(3):620–677. doi: 10.1152/physrev.1974.54.3.620. [DOI] [PubMed] [Google Scholar]
- Kobza J., Edwards G. E. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987 Jan;83(1):69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney H. A., Björkman O., Collatz G. J. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata: I. Carbon Dioxide Exchange Characteristics of Intact Leaves. Plant Physiol. 1978 Mar;61(3):406–410. doi: 10.1104/pp.61.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. D., Kobza J., Seemann J. R. Measurement of 2-carboxyarabinitol 1-phosphate in plant leaves by isotope dilution. Plant Physiol. 1991 May;96(1):208–213. doi: 10.1104/pp.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R. W. Acclimation of Photosynthetic and Respiratory Carbon Dioxide Exchange to Growth Temperature in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1977 May;59(5):795–799. doi: 10.1104/pp.59.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath G. F., Ort D. R., Portis A. R., Jr Impaired reductive activation of stromal bisphosphatases in tomato leaves following low-temperature exposure at high light. Arch Biochem Biophys. 1990 Nov 1;282(2):302–308. doi: 10.1016/0003-9861(90)90121-e. [DOI] [PubMed] [Google Scholar]


