Abstract
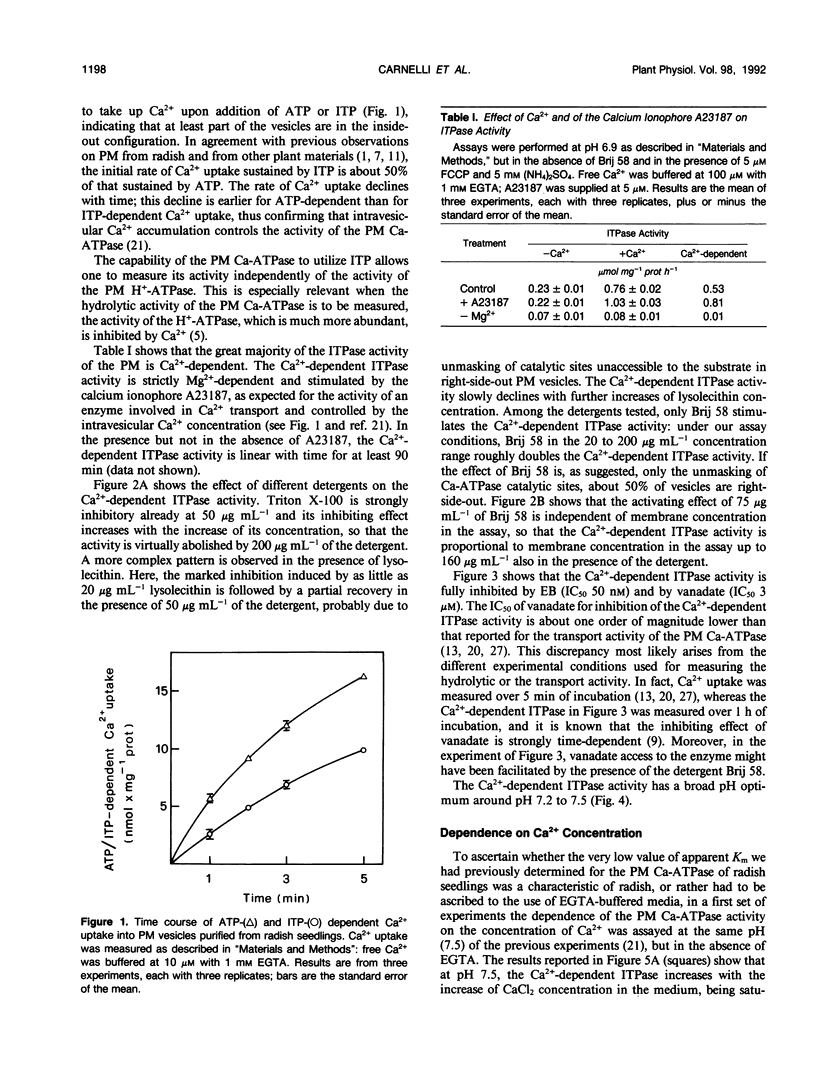
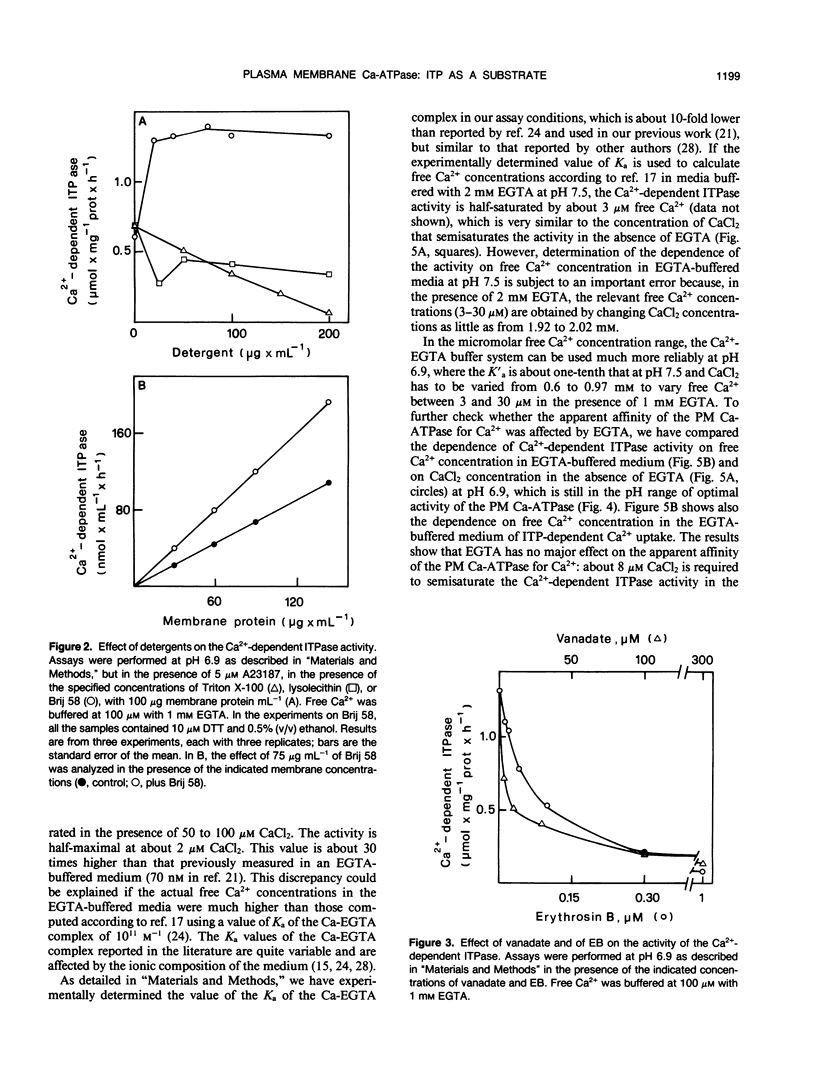
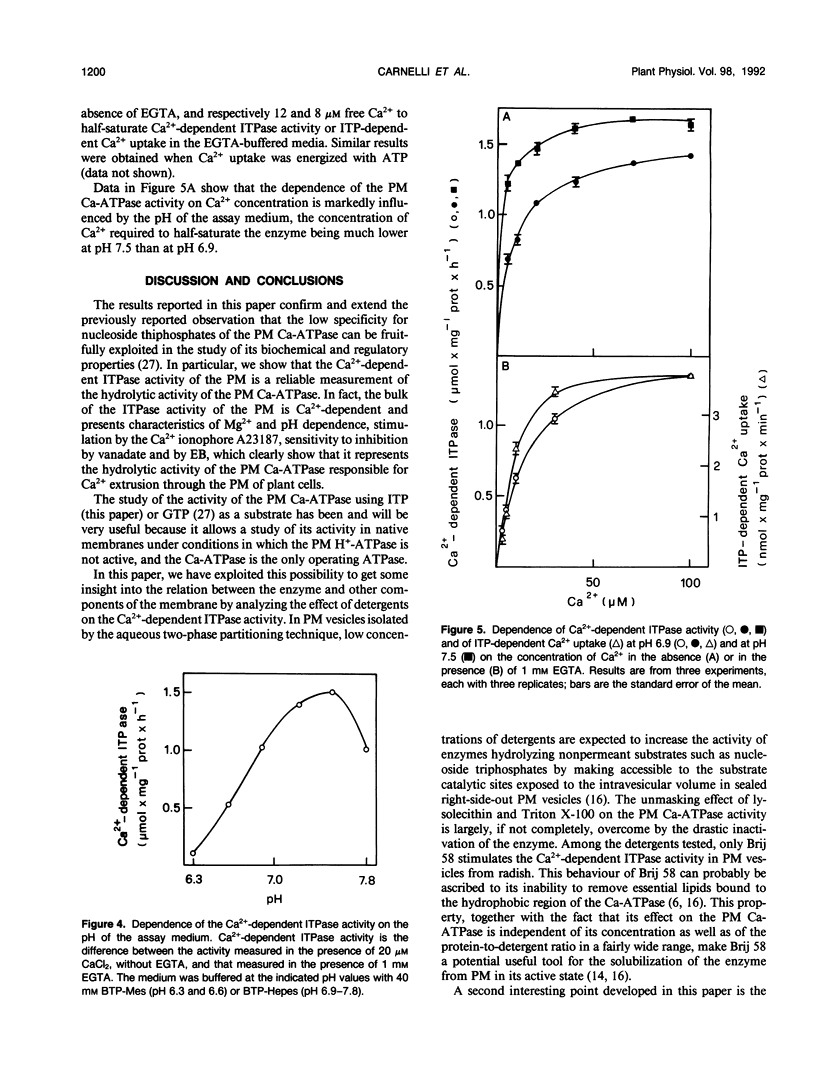
In this work, we exploited the capability of the plasma membrane Ca-ATPase to utilize ITP as a substrate to study its characteristics in plasma membrane vesicles purified from radish (Raphanus sativus L.) seedlings. The majority of the ITPase activity of plasma membrane was Ca2+-dependent. The Ca2+-dependent ITPase activity was Mg2+-dependent and was stimulated by the calcium ionophore A23187. It was inhibited by erythrosin B (concentration giving 50% inhibition, 50 nanomolar) and by vanadate (concentration giving 50% inhibition, 3 micromolar) and displayed a broad pH optimum around pH 7.2 to 7.5. Both the hydrolytic and the transport activity of the plasma membrane Ca-ATPase were half-saturated by Ca2+ in the micromolar concentration range. No major effect of EGTA on the saturation kinetics of the enzyme was observed. The affinity of the plasma membrane Ca-ATPase for Ca2+ was about fourfold higher at pH 7.5 than at pH 6.9. The Ca2+-dependent ITPase activity was stimulated about twofold by polyoxyethylene 20 cetyl ether, although it was inhibited by Triton X-100 and by lysolecithin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briskin D. P. Ca-translocating ATPase of the plant plasma membrane. Plant Physiol. 1990 Oct;94(2):397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobak B. K., Allan E. F., Comerford J. G., Roberts K., Dawson A. P. Presence of guanine nucleotide-binding proteins in a plant hypocotyl microsomal fraction. Biochem Biophys Res Commun. 1988 Feb 15;150(3):899–903. doi: 10.1016/0006-291x(88)90713-9. [DOI] [PubMed] [Google Scholar]
- Giannini J. L., Ruiz-Cristin J., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : II. Characterization of Ca Uptake into Plasma Membrane Vesicles. Plant Physiol. 1987 Dec;85(4):1137–1142. doi: 10.1104/pp.85.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf P., Weiler E. W. Functional Reconstitution of an ATP-Driven Ca-Transport System from the Plasma Membrane of Commelina communis L. Plant Physiol. 1990 Oct;94(2):634–640. doi: 10.1104/pp.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Ulvskov P., Larsson C. Effect of detergents on the H(+)-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta. 1990 Jan 29;1021(2):133–140. doi: 10.1016/0005-2736(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. A high affinity calcium-stimulated magnesium-dependent adenosine triphosphatase in rat adipocyte plasma membranes. J Biol Chem. 1980 May 10;255(9):4087–4093. [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Carnelli A., De Michelis M. I. Plasma Membrane Ca-ATPase of Radish Seedlings : II. Regulation by Calmodulin. Plant Physiol. 1992 Mar;98(3):1202–1206. doi: 10.1104/pp.98.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. The Ca-Transport ATPase of Plant Plasma Membrane Catalyzes a nH/Ca Exchange. Plant Physiol. 1987 Apr;83(4):994–1000. doi: 10.1104/pp.83.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., Olivari C., De Michelis M. I. Identification and Characterization of the Ca-ATPase which Drives Active Transport of Ca at the Plasma Membrane of Radish Seedlings. Plant Physiol. 1989 Aug;90(4):1429–1434. doi: 10.1104/pp.90.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobo A., Brown L., Roufogalis B. D. Kinetic properties of the purified Ca2+-translocating ATPase from human erythrocyte plasma membrane. Biochim Biophys Acta. 1986 Jan 16;854(1):9–20. doi: 10.1016/0005-2736(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Williams L. E., Schueler S. B., Briskin D. P. Further Characterization of the Red Beet Plasma Membrane Ca-ATPase Using GTP as an Alternative Substrate. Plant Physiol. 1990 Mar;92(3):747–754. doi: 10.1104/pp.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Foresta B., le Maire M., Orlowski S., Champeil P., Lund S., Møller J. V., Michelangeli F., Lee A. G. Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry. 1989 Mar 21;28(6):2558–2567. doi: 10.1021/bi00432a032. [DOI] [PubMed] [Google Scholar]


