Abstract
We assessed the applicability of an in vitro model of low-level infection of human monocytes to the characterization of the virulence of strains of the Mycobacterium tuberculosis family. Peripheral blood monocytes were infected at a 1:1 ratio with the virulent M. tuberculosis strain H37Rv, the avirulent M. tuberculosis strain H37Ra, and the attenuated M. bovis strain BCG. Both the percentages of cells infected by the three strains and the initial numbers of intracellular organisms were equivalent, as were levels of monocyte viability up to 7 days following infection. Intracellular growth reflected virulence, as H37Rv replicated in logarithmic fashion throughout the assay, BCG growth reached a plateau at 4 days, and H37Ra did not grow at all. The same patterns of growth were observed following infection of human alveolar macrophages with H37Rv and H37Ra. Monocyte production of tumor necrosis factor alpha was significantly higher following infection with virulent H37Rv than with either BCG or H37Ra. In contrast, there was no clear correlation of interleukin 10 production with virulence. Nonadherent cells of purified-protein-derivative-positive donors mediated equivalent degrees of reduction of the intracellular growth of H37Rv, BCG, and H37Ra. Low-level infection of human monocytes with H37Rv, BCG, and H37Ra thus provides an in vitro model for assessment of the virulence of these M. tuberculosis family strains. Furthermore, it is suggested that the virulence of these strains is expressed primarily by their differing abilities to adapt to the intracellular environment of the mononuclear phagocyte.
Tuberculosis remains the most frequent cause of death due to an infectious disease throughout the world (37). Despite this, little is known about the capacity of the human immune response to eliminate Mycobacterium tuberculosis or about the virulence mechanisms used by the organism to evade these defenses. These two aspects of the pathogenesis of infection with M. tuberculosis converge at the level of the mononuclear phagocyte. Following activation by lymphocytes, mononuclear phagocytes serve as the final effectors in the killing of intracellular M. tuberculosis. Nevertheless, the organisms can survive, and even thrive, in the intracellular environment of human blood monocytes and tissue macrophages.
Virulence may be defined as the capacity of a microorganism to overcome host defenses. The course of disease following infection with an organism is clearly the most meaningful measure of virulence, and accordingly, the assessment of the virulence of strains of M. tuberculosis has traditionally been based on the lethality of these strains for mice and guinea pigs and on the replication of bacilli at tissue sites such as the lungs. However, studies of tuberculosis in animals are time-consuming and expensive. Furthermore, although mouse and guinea pig models can effectively assess the virulence of strains of M. tuberculosis, animal studies may be less helpful in clarifying the mechanisms by which the bacilli interact with or subvert host immune responses to result in disease. Such mechanistic studies are difficult to perform with guinea pigs due to the relatively limited scope of available immunologic reagents. In mice, containment of M. tuberculosis by mononuclear phagocytes has been shown be mediated predominantly by gamma interferon-mediated production of nitric oxide (NO) (8, 26, 27). Production of NO in human cells has proven much more difficult to demonstrate, however, and may be more effectively induced by signals other than gamma interferon (1, 17, 21, 60), indicating that immunologic findings from the murine model may not be directly applicable to the pathogenesis of tuberculosis in humans (19, 46).
Advances in the molecular biology of mycobacteria have provided new tools which should facilitate more definitive studies of the virulence of M. tuberculosis. Molecular epidemiology based on the DNA fingerprinting of restriction fragment length polymorphisms has made it possible to identify specific isolates of M. tuberculosis as responsible for outbreaks of disease (2, 15, 54, 55). Assessment of the virulence of these strains may help clarify the degree to which case clustering can be attributed to bacterial as opposed to host factors. In addition, the development of techniques for genetic manipulation of mycobacteria has accelerated the search for virulence genes of M. tuberculosis (34), an effort which is likely to be facilitated by ongoing sequencing of the M. tuberculosis genome (59). The exploitation of these advances would be greatly aided by the availability of in vitro assays of virulence that are more rapid as well as more directly applicable for understanding the pathogenesis of tuberculosis in humans.
We recently described a model of infection of human monocytes with the virulent M. tuberculosis strain H37Rv in which a reproducible low-level infection was achieved (53). Using this model, we demonstrated the ability of lymphocytes to activate monocytes to limit the growth of intracellular M. tuberculosis. In the current study, we investigated the applicability of this model to the assessment of the virulence of strains of the M. tuberculosis family. Virulent M. tuberculosis H37Rv, attenuated M. bovis BCG, and avirulent M. tuberculosis H37Ra were chosen for study because their virulence in animal models is well-characterized (11, 44). H37Rv grows progressively (in the initial weeks of infection), whereas growth of BCG is more limited, and H37Ra does not grow. The ability to distinguish the degrees of virulence of these particular strains in an in vitro system is of particular interest in that comparisons of their DNA sequences and levels of gene expression have already been used in attempts to identify virulence factors of M. tuberculosis (36, 39). We determined that growth of H37Rv, BCG, and H37Ra within human mononuclear phagocytes following low-level infection correlates with virulence as determined in animals models. We then compared the abilities of the strains to induce cytokines and to modulate lymphocyte-dependent monocyte effector functions.
MATERIALS AND METHODS
Processing and quantification of mycobacterial stocks.
Broth cultures of mycobacteria were grown in sterile Middlebrook 7H9 medium with 10% Middlebrook ADC enrichment and 0.2% glycerol (subsequently referred to as 7H9 medium). Plated cultures were grown on Middlebrook 7H10 agar with 10% Middlebrook OADC enrichment (Difco, Detroit, Mich.).
M. tuberculosis H37Rv, M. bovis BCG (Pasteur), and M. tuberculosis H37Ra (catalog no. 25618, 35734, and 25177, respectively; American Type Culture Collection, Rockville, Md.) were initially grown as broth cultures in 1.7-liter expanded-surface rolling bottles (catalog no. 2528-1700; Corning, Corning, N.Y.) at 37°C. These initial cultures were divided into aliquots and immediately stored at −70°C.
The aliquots prepared above were utilized to directly inoculate all subsequent roller-bottle cultures for use in infection of monocytes and macrophages, so that all infections were performed with organisms which had undergone only one previous laboratory passage. To minimize clumping and allow for accurate quantification, infecting cultures of mycobacteria were processed in a manner based on that described by Schlesinger (48). Briefly, mid-log-phase roller-bottle cultures were aliquoted into 50-ml polypropylene tubes (Falcon, model no. 2098; Becton Dickinson Labware, Lincoln, N.J.) and centrifuged at 3,000 rpm (1200 × g) for 30 min. The resulting bacterial pellets were resuspended in 35 ml of fresh 7H9 medium. Five milliliters of washed and autoclaved 3-mm-diameter glass beads (catalog no. 11-312A; Fisher Scientific, Pittsburgh, Pa.) were then added to each conical tube, which was then vortexed for 5 min and centrifuged at 600 rpm (50 × g) for 10 min. Mycobacteria remaining suspended in the supernatant were aliquoted into sterile 1.6-ml cryotubes (Sarstedt, Newton, N.C.) and stored at −70°C.
When ready for use, bacterial aliquots thus processed were thawed at 37°C. Three to four sterile glass beads were placed in each cryotube. Tubes were vortexed for 5 min and centrifuged in a microcentrifuge at 2,000 rpm (325 × g) for 10 min. Bacteria still in suspension were used for infections. Quantification was performed by assessment of CFU of plated serial 10-fold dilutions of this supernatant. Aliquots of bacteria prepared from a single initial roller-bottle culture were found to have reproducible CFU after all processing was complete. CFU assessment of one aliquot of mycobacteria was therefore used to calculate the infecting inoculum for all cryotubes of a batch. Bacterial suspensions were diluted to appropriate concentrations in Iscove’s modified Dulbecco’s medium with NaHCO3, 25 mM HEPES, and l-glutamine (IMDM, catalog no. 12-722; Bio-Whittaker, Walkersville, Md.).
Human subjects.
Subjects for blood donations were volunteers aged 22 to 50 without signs or symptoms of lung disease. Studies of intracellular growth and cytokine induction were performed with cells from tuberculin-negative subjects. For studies of the ability of nonadherent cells (NAC) to limit intracellular growth within human monocytes, blood was obtained from subjects who had a history of a positive tuberculin (purified-protein-derivative [PPD]) skin test. The subjects for bronchoalveolar lavage (BAL) were two female volunteers, aged 34 and 47 years. Neither had a history of any lung disease, and both were PPD-negative nonsmokers. Protocols for both blood drawing and BAL were approved by the Institutional Review Board of the Case Western Reserve University and University Hospitals of Cleveland.
Isolation of cell populations.
Peripheral blood was obtained by venipuncture from healthy individuals, and mononuclear cells were isolated by density sedimentation with Ficoll-sodium diatrizoate (Ficoll-Paque; Pharmacia, Uppsala, Sweden) and washed three times in RPMI 1640 (Bio-Whittaker). Monocytes were then separated by adherence to tissue-culture-grade 100-mm-diameter polystyrene petri dishes (Falcon, model no. 3003; Becton Dickinson Labware) that had been precoated with 1 to 2 ml of pooled human serum. Plates were incubated for 1 h at 37°C, and NAC were removed by gently rinsing the plates with warmed RPMI 1640 plus 10% fetal bovine serum (catalog no. A-1111-L; HyClone, Logan, Utah). Cells that remained adherent to petri dishes were covered with cold phosphate-buffered saline (Bio-Whittaker) and cooled to 4°C for 15 to 30 min, after which they were dislodged with a sterile plastic scraper (Cell Lifter 3008; Costar, Cambridge, Mass.). This population was found to be 99% positive with a nonspecific esterase staining kit (catalog no. 181-B; Sigma, St. Louis, Mo.) and is henceforth referred to as human monocytes.
Human alveolar macrophages were obtained by BAL as previously described (32). Briefly, after we obtained informed consent from a subject, flexible fiberoptic bronchoscopy was performed via nasal intubation. The bronchoscope was wedged into a segment of the right middle lobe, and BAL was performed by instilling and then aspirating six 30-ml aliquots of sterile 0.9% NaCl. Cells were isolated by centrifugation of lavage fluid at 300 × g for 15 min. This cell population was 90 to 95% positive by nonspecific esterase staining and is henceforth referred to as alveolar macrophages.
Infection of human monocytes and alveolar macrophages with M. tuberculosis and assessment of phagocytosis, monocyte viability, and intracellular growth.
Isolated human monocytes and alveolar macrophages were resuspended at a density of 106/ml in IMDM and 5% fresh autologous serum without antibiotics. The contents of triplicate wells of 100-μl aliquots of this suspension (i.e., 105 monocytes or macrophages/well) were then aliquoted into round-bottomed 96-well plates (model no. 2585; Corning) for quantification of bacteria by CFU at each time point to be studied. Two additional wells were plated for assessment of cell viability at each time point. For assessment of phagocytosis, triplicate 50-μl aliquots of this suspension were plated onto 60-mm-diameter petri dishes (Falcon, model no. 1007; Becton Dickinson). Plates were incubated overnight at 37°C to allow for readherence of monocytes and adherence of alveolar macrophages.
The next day, supernatants were removed from all plates and replaced with a 1:1 infecting ratio of mycobacteria in IMDM with 30% fresh autologous serum. For 96-well plates, 100 μl was added to each well. Phagocytosis assays used 50-μl suspensions of bacteria applied directly to the “spots” of adherent cells plated the previous day. Plates were returned to a 37°C incubator for 1 h, after which supernatants were aspirated. For 96-well plates, each well was washed three times with RPMI 1640 and 10% fetal calf serum to remove the remaining noningested mycobacteria. Wells were then refilled with 100 μl of IMDM 10% autologous serum.
For phagocytosis assays, washing of petri dishes was followed by staining of infected monocytes with Kinyoun carbol fuschin stain (Difco). The flat surface of each plate was then cut away from the remainder and mounted onto glass microscope slides. Phagocytosis was then expressed as the percentage of monocytes infected with M. tuberculosis as determined by counting of 100 cells under light microscopy.
At time points 1 h, 4 days, and 7 days following infection, cell survival and bacterial growth were assessed. Viability studies were made of monocyte cultures from duplicate wells by the method of Nakagawara and Nathan (41). Briefly, supernatants were removed from these wells and replaced with 100 μl of naphthol blue-black (NBB; Aldrich Chemical Co., Milwaukee, Wis.). Following a 10-min incubation at room temperature, monocytes were mixed with a pipettor, 10-μl aliquots were transferred to a hemocytometer, and nuclei were counted. Percent survival was calculated as the number of viable cells per milliliter divided by the initial concentration of 106 cells/ml times 100.
For the assessment of intracellular mycobacterial growth, supernatants of the triplicate wells were first aspirated and saved. Monocytes were lysed by the addition of 50 μl of 0.067% sodium dodecyl sulfate in 7H9 medium to each well, followed by incubation at 37°C for 10 min. Lysis was halted by the addition of 50 μl of 20% bovine serum albumin in phosphate-buffered saline to each well. Four 10-fold serial dilutions of each supernatant and lysate were then prepared. Triplicate 10-μl volumes of each dilution were plated onto Middlebrook 7H10-OADC and incubated in a 5% CO2 atmosphere at 37°C until visible colonies were large enough to be counted (usually 2 to 3 weeks). Results were expressed as CFU per milliliter of lysate, which is equivalent to CFU per 106 initial monocytes.
Induction of TNF-α and IL-10.
Supernatants of 96-well cultures established in the manner described above were collected at 0, 6, 24, and 48 h following infection and passed through 0.22-μm-pore-size filters (Millex-GS; Millipore, Bedford, Mass.). Samples were frozen at −20°C until they were ready for use. Concentrations of tumor necrosis factor alpha (TNF-α) and interleukin 10 (IL-10) were determined by sandwich enzyme-linked immunosorbent assay as previously described (51).
For some studies, preparations of bacilli for infection were passed through 0.22-μm-pore-size filters to determine whether cytokine production was induced by soluble antigens within these preparations. In these experiments, monocytes from the same individual were incubated for 1 h with either bacilli or bacillus-free filtrate. Mycobacteria or filtrates were rinsed in the manner described above, and supernatants were again collected at 0, 6, 24, and 48 h following incubation, filtered, and frozen until they were ready for cytokine determination by enzyme-linked immunosorbent assay.
Growth inhibition by nonadherent cells.
Studies of the ability of lymphocytes to inhibit intracellular growth of the three strains within monocytes were performed using cells obtained from PPD-positive subjects. NAC which were rinsed from petri dishes following adherence of monocytes as described above were washed, resuspended in IMDM with 5% autologous serum, and incubated overnight at 37°C. The next day, these cells were again washed and resuspended at a density 10 × 106/ml. Following washing of nonphagocytosed mycobacteria from infected monocytes, 100 μl of NAC (106 NAC) was added to each well containing infected monocytes. The resulting 10:1 NAC-to-monocyte ratio was selected to approximate that of peripheral blood mononuclear cells. CFU were then assessed at days 4 and 7 as described above. Growth of intracellular mycobacteria from cocultured monocytes and NAC was expressed as a percentage of the growth exhibited in cultures of monocytes only from the same subject.
RESULTS
Initial phagocytosis and monocyte viability.
We previously demonstrated that incubation of human monocytes with virulent M. tuberculosis H37Rv at a 1:1 ratio for 1 h resulted in a reproducible infection which did not alter monocyte viability (53). We used the same methods to infect human monocytes with the attenuated M. bovis strain BCG and with avirulent M. tuberculosis H37Ra and virulent H37Rv.
Phagocytosis was assessed with monocytes from five subjects for each of the strains. H37Rv infected 20.2% ± 5.6% of cells, BCG infected 22.8 % ± 3.6% of cells, and H37Ra infected 17.8% ± 5.5% of cells. The percentages of cells infected with a bacterium-to-cell infecting ratio of 1:1 thus did not vary significantly with the virulence of the infecting strain (by Student’s t test).
Monocyte viability was determined by NBB staining immediately after infection and at 4 and 7 days. Again, cells from five donors were assessed for each of the three strains. Rinsing of cells and removal of supernatants prior to addition of NBB was presumed to have eliminated cells which had detached from the plates. In Table 1, monocyte viability is reported as the percentage of intact cells remaining adherent to wells at each time point compared to the initial 105 cells plated per well. As indicated in the table, viability was not significantly altered by infection with low doses of H37Rv or H37Ra when viabilities were compared to each other or to that of uninfected cells (assessed by Student’s t test). Viability of monocytes infected with BCG was somewhat higher than that of uninfected cells as well as those infected with the other two strains. This difference was statistically significant relative to the viability at day 7 of cells infected with H37Rv (P = 0.045).
TABLE 1.
Viability of human monocytes in culture following infection with M. tuberculosis strains of various degrees of virulence
| Time (day) | % of viable monocytes (± 1 SD)
following infection witha:
|
|||
|---|---|---|---|---|
| No. bacilli | H37Rv | H37Ra | BCG | |
| 0 | 68.5 ± 8.7 | 66.0 ± 10.5 | 71.2 ± 16.9 | 66.0 ± 8.0 |
| 4 | 53.8 ± 9.8 | 58.3 ± 9.1 | 59.7 ± 19.7 | 67.0 ± 13.0 |
| 7 | 55.0 ± 10.6 | 54.3 ± 6.4 | 56.6 ± 15.1 | 67.0 ± 12.0b |
All results are expressed as percentages of monocytes aliquoted into microtiter wells the night prior to infection (at a 1:1 bacterium-to-cell ratio). Each value is a ±1 standard deviation of results of experiments with five subjects for each strain.
The greater viability of BCG-infected monocytes at day 7 is significant only in comparison to cells infected with H37Rv (P = 0.045 by Student’s t test).
Virulence-related patterns of intracellular growth in blood monocytes and in alveolar macrophages.
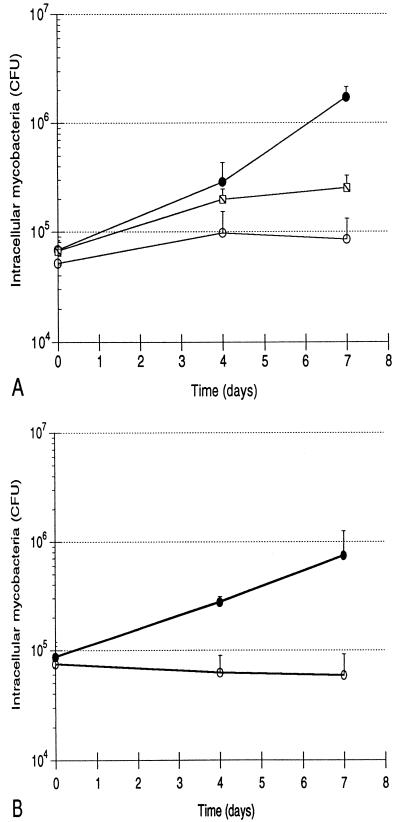
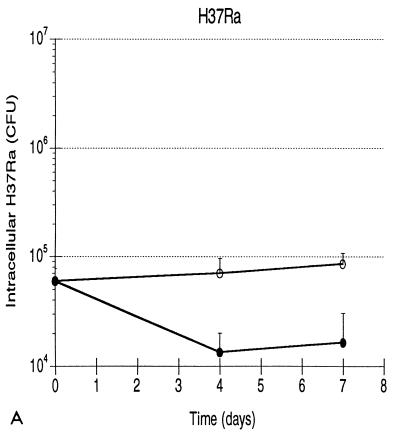
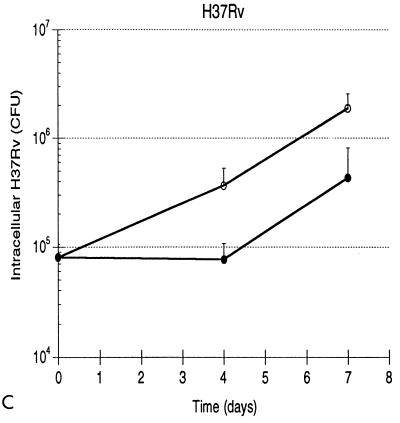
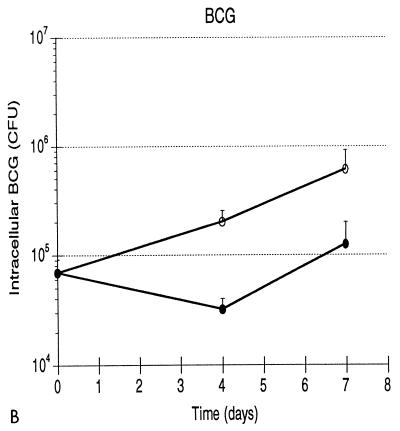
Growth patterns of the three strains of mycobacteria are illustrated in Fig. 1. Mean levels of growth of H37Rv, BCG, and H37Ra within human monocytes from five subjects are shown in Fig. 1A. Each error bar indicates 1 standard deviation. As indicated, both the initial intracellular bacterial burden and the growth pattern of each strain were highly reproducible. The effective intracellular inocula of the three strains were substantially lower than those which were anticipated from the phagocytosis assay, most likely reflecting the different culture formats used in the two assays (i.e., round-bottomed wells as opposed to flat spots of monocytes). Nevertheless, the initial burdens of intracellular H37Rv, BCG, and H37Ra did not display statistically significant differences (by Student’s t test), indicating that the culture method used in CFU assays also provided the same starting point for assessing the results of infection of monocytes with the three strains. Intracellular H37Rv replicated throughout the assay, resulting in 25-fold growth by day 7, or a bacterial doubling time of 36.2 h. Growth of BCG paralleled that of H37Rv through the first 4 days, but the attenuated strain subsequently did not replicate further. Overall, BCG thus replicated only 3.7-fold, exhibiting an 88.9-h doubling time. The difference in levels of intracellular growth of H37Rv and BCG was not significant at day 4 but was significant at day 7 (P < 0.001). The 1.6-fold greater load of intracellular H37Ra at day 7 was not statistically different from the bacterial load of the avirulent strain at time zero (P = 0.187) and reflected an intracellular doubling time of 247.0 h. The growth pattern of H37Ra was significantly different from those of H37Rv and BCG at both day 4 (P = 0.027 and 0.016, respectively) and at day 7 (P = 0.001 and 0.003, respectively). Because growth of H37Ra within human monocytes has been previously reported (25, 35), infections with H37Ra were repeated following the preparation of a fresh stock of the avirulent strain. These studies confirmed the lack of significant growth of H37Ra following low-level infection (data not shown). In addition, H37Ra stocks were assessed with AccuProbe for M. tuberculosis (Gene-Probe, San Diego, Calif.), which confirmed that the strain was of the M. tuberculosis family rather than a nonpathogenic laboratory contaminant. The patterns of growth of H37Rv, BCG, and H37Ra within human monocytes following low-level infection thus correlated well with those observed in in vivo animal studies.
FIG. 1.
Growth of H37Rv, BCG, and H37Ra within human monocytes correlates with virulence of the infecting strain, as does growth of H37Rv and H37Ra within human alveolar macrophages. (A) Intracellular H37Rv ( ), BCG (-⧅-), and H37Ra (-○-) within human blood monocytes following incubation at a 1:1 bacterium-to-cell ratio. Growth curves plot mean CFU at each time point from results of five experiments for each strain. Error bars indicate 1 standard deviation. (B) Growth of H37Rv ( ) and H37Ra (-○-) within alveolar macrophages. Curves plot means and 1 standard deviation of results of studies using cells from two subjects.
Growth of H37Rv and H37Ra was also studied with fresh human alveolar macrophages from two subjects. As illustrated in Fig. 1B, initial intracellular loads of the two strains following incubation at a 1:1 infection ratio were identical. Both strains grew less well in macrophages than in monocytes, as H37Rv grew 8.6-fold and intracellular H37Ra decreased to 0.8 of the initial infecting load. Although statistical significance was not demonstrated with this small number of subjects, the relative patterns of intracellular growth of H37Rv and H37Ra in human alveolar macrophages remained consistent with those observed in blood monocytes (and in experimental animals), suggesting that the growth patterns of these strains are independent of the level of in vivo differentiation of the infected mononuclear phagocytes.
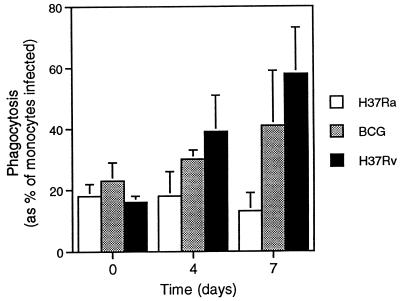
With the culture format of the phagocytosis assay, evaluation of the progress of infection in monocyte cultures at days 0, 4, and 7 was performed by light microscopy and indicated that the differences in numbers of intracellular bacilli detected over the course of the assay reflected both growth within individual monocytes and release and subsequent reingestion of bacilli. As illustrated in Fig. 2, the percentage of monocytes containing H37Ra did not change over the culture period, whereas a progressive increase in the percentage of cells infected was seen with BCG and, to an even greater extent, H37Rv. For all three strains, more than 95% of infected cells contained five or few organisms at time zero. More than 95% of H37Ra-infected monocytes contained five or fewer bacilli at day 7, whereas 30% of BCG-infected monocytes and 70% of H37Rv-infected monocytes contained six or more bacilli at day 7; however, most of the H37Rv-infected cells contained substantially more than six bacilli. An example of the successful achievement of low-level initial infection and subsequent strain-specific progression of intracellular growth in cells from one subject is illustrated in Fig. 3.
FIG. 2.
Virulence-related growth of mycobacteria is reflected in percentages of monocytes infected over a 7-day assay. Following a 1-h incubation with mycobacteria added at a 1:1 bacterium-to-cell ratio, initial levels of phagocytosis of H37Ra, BCG, and H37Rv were equivalent. As illustrated, progressively higher percentages of monocytes were observed to be infected with BCG over the 7-day period. This finding was made to an even greater extent with H37Rv. The graph represents means and standard deviations of results of infections of monocytes from five subjects for each of the strains studies.
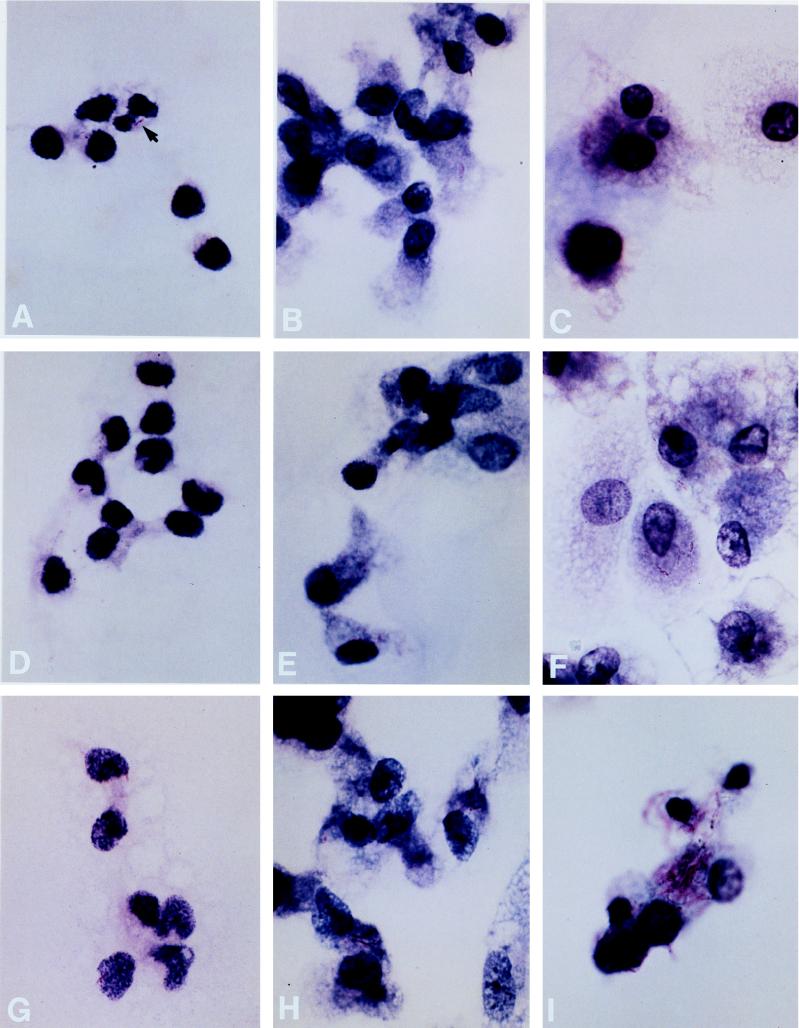
FIG. 3.
Virulence-related growth of mycobacteria within human monocytes. The courses of infection at days 0, 4, and 7 are illustrated for H37Ra (A to C), BCG (D to F), and H37Rv (G to I). An arrow in panel A indicates the appearance of bacilli at this magnification (×960 for all photos). Equivalently low levels of infection of human monocytes were achieved at time zero following incubation with H37Ra (A), BCG (D), and H37Rv (G). Subsequently, intracellular H37Ra was not observed to have grown at either day 4 (B) or day 7 (C). In contrast, by day 4, growth of BCG was observed (E), although there was relatively little further change by day 7 (F). Growth of H37Rv was apparent at day 4 (H) and was much more striking by day 7 (I). All infected monocytes pictured are from a single experiment with cells from one donor.
Differences in levels of release of bacilli into the culture supernatant were also assessed. The efficiency of the initial rinsing procedure at removing nonphagocytosed bacteria was indicated by the finding that mean CFU of bacilli within the culture supernatants at time zero were at least 10-fold lower than those of intracellular bacilli at time zero for all three strains. Subsequently, mean CFU of H37Ra and BCG within culture supernatants remained at least 10-fold lower than CFU within cells at days 4 and 7. This finding was also true at day 4 for cultures with H37Rv, although by day 7, CFU of H37Rv within culture supernatants was only fivefold lower than CFU of H37Rv within cells.
Induction of TNF-α and IL-10.
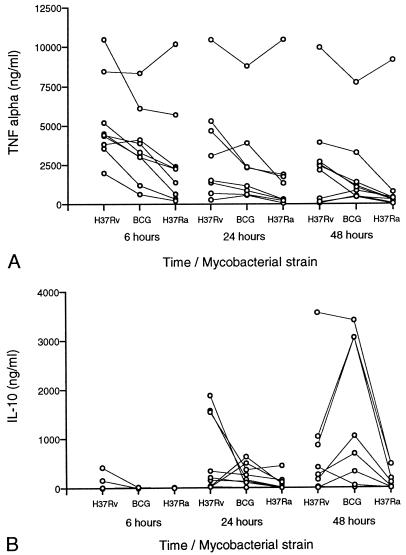
To determine whether infection with M. tuberculosis strains of various degrees of virulence altered the balance of activating and immunosuppressive cytokines produced by monocytes, we measured concentrations of TNF-α and IL-10 in culture supernatants at 6, 24, and 48 h following equivalent levels of infection with H37Rv, BCG, and H37Ra. Infections with the three strains were established simultaneously for each subject, and results were analyzed by Student’s paired t test. The results obtained for nine subjects are illustrated in Fig. 4.
FIG. 4.
Induction of monocyte TNF-α is greater following infection with H37Rv than with BCG or H37Ra, but induction of IL-10 does not correlate with virulence. (A) TNF-α concentrations within supernatants of human blood monocytes collected at 6, 24, and 48 h following infection with H37Rv, BCG, and H37Ra. Connected dots indicate TNF-α induction by cells from a single subject at one time point. Although absolute levels of the TNF-α responses of the nine subjects varied considerably, the trend to greater induction by H37Rv was statistically significant in comparison to induction by BCG at 6 and 48 h and in comparison to induction by H37Ra at all three time points (by paired t test). (B) Concentrations of IL-10 following infection of monocytes from the same nine subjects. The only finding of statistical significance was greater induction of IL-10 at 48 h by BCG than by H37Ra.
Figure 4A illustrates monocyte TNF-α production following infection with H37Rv, BCG, and H37Ra. Although there was substantial donor-to-donor variation in levels of TNF-α production, peak levels of TNF-α were generally observed 6 h following infection for all three strains. The TNF-α level correlated with the virulence of the infecting strain, being highest following infection with H37Rv, intermediately high with BCG, and lowest with H37Ra at all time points. Induction of TNF-α by H37Rv was significantly greater than that of H37Ra at 6, 24, and 48 h (P = 0.005, 0.006, and 0.008, respectively, by paired t test). TNF-α induction by H37Rv was also significantly greater than that by BCG at 6, and 48 h by paired t test (P = 0.008 and 0.045, respectively) but not at 24 h (P = 0.061). Differences between levels of TNF-α production in response to BCG and H37Ra were nonsignificant at all time points.
Figure 4B illustrates IL-10 concentrations within the same supernatants. Unlike that of TNF-α, production of IL-10 showed no clear correlation with virulence. Again, substantial donor-to-donor variation was observed. IL-10 induction by BCG was significantly greater than that by H37Ra at 48 h (P = 0.031 by paired t test), whereas the differences in levels of induction of IL-10 by H37Rv and BCG as well as by H37Rv and H37Ra were not statistically significant at any of the time points assessed.
Although all bacilli had been rinsed of culture medium in preparation for the infections, we sought to confirm that the differences in levels of production of TNF-α observed reflected monocyte responses to phagocytosis of the bacilli rather than to stimulation of the cultures by residual soluble mycobacterial products. Levels of induction of TNF-α by H37Ra, BCG, and H37Rv samples prepared as described above were therefore compared with those induced by filtrates of these samples (from which bacilli had been removed by passage though 0.22-μm-pore-size filters). Monocytes from four subjects were incubated in parallel with either filtrates or unfiltered bacterial preparations. For all three strains, only a small proportion of TNF-α induction could be attributed the effects of filterable bacterial products. At 6 h, filtrates of H37Ra, BCG, and H37Rv elicited TNF-α levels equivalent to 10% (±15%), 9% (±10%), and 22% (±17%), respectively, of that elicited by the unfiltered bacteria. At 24 h, TNF-α induction by filtrates represented 13% (±24%) of that elicited by H37Ra, 5% (±10%) of that elicited by BCG, and 8% (±9%) of that elicited by H37Rv. TNF-α levels in cultures exposed to filtrates declined more rapidly than the levels in cultures to which unfiltered bacilli were added, so that at 48 h, induction by filtrates of H37Ra, BCG, and H37Rv accounted for only 5% (±10%), 3% (±6%), and 4% (±8%), respectively, of induction by the unfiltered bacteria. The differences in the relative abilities of the filtrates of the three strains to induce TNF-α were nonsignificant at all time points studied (by paired t test). Thus, the observed strain-specific differences in levels of induction of TNF-α by H37Ra, BCG, and H37Rv were attributable to monocyte responses following phagocytosis of the organisms rather than differences in the abilities of the soluble products of these mycobacterial strains to elicit cytokine production.
Ability of NAC to limit intracellular growth of mycobacterial strains.
The addition of NAC from PPD-positive subjects to cultures of infected monocytes was performed in order to determine whether H37Rv, BCG, and H37Ra differed in their abilities to evade lymphocyte-dependent monocyte effector mechanisms. The growth curves of the three strains within monocytes alone and following addition of NAC are illustrated in Fig. 5. As illustrated, both the time courses and the degrees of limitation of growth (or, with H37Ra, reduction in the number of intracellular bacilli) were similar for the three strains.
FIG. 5.
Addition of lymphocytes from PPD-positive subjects to monocytes infected with H37Ra (A), BCG (B), and H37Rv (C) results in comparable reductions in intracellular mycobacteria at both 4 and 7 days. NAC were added in a 10:1 ratio relative to the number of infected monocytes. For H37Ra and BCG, results indicate means and 1 standard deviation of results of studies of three subjects. H37Rv results are means and 1 standard deviation for four subjects studied.
In order to allow for direct comparison of the magnitudes of NAC-mediated reduction of intracellular H37Rv, BCG, and H37Ra, the intracellular burden of each strain of mycobacteria at day 7 within monocytes cocultured with NAC was reexpressed as the percentage of that within monocytes alone. This calculation confirms that the three strains do not differ in their susceptibilities to the effects of NAC. At day 4 following addition of NAC, intracellular CFU were reduced to 15.9% ± 14.7% of that present within monocytes alone for H37Ra, compared to reductions of 16.0% ± 5.7% for BCG and 19.9% ± 13.2% for H37Rv. CFU of intracellular bacilli at day 7 following addition of NAC were 19.3% ± 11.3% of those observed within monocytes alone for H37Ra, 20.4% ± 9.4% of those for BCG, and 20.3% ± 19.9% of those for H37Rv. The efficacies of NAC-mediated reductions in the intracellular burdens of the three strains did not differ significantly either by day 4 or by day 7 (as assessed by t test).
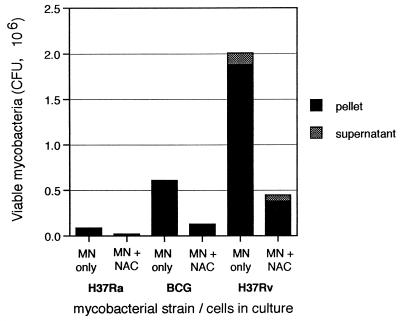
To confirm that the reduction in the numbers of intracellular bacilli did not simply represent killing of infected monocytes and/or release of the bacilli from the adherent layer, CFU of the three strains within culture supernatants were also determined. Figure 6 illustrates the absolute numbers of bacilli in the cultures of monocytes alone and monocytes plus nonadherent cells. Although the percentages of bacilli in culture supernatants relative to those within cells increased following addition of NAC, the absolute numbers of bacilli in the supernatants could not account for the observed decreases in numbers of bacilli within cells. Thus, the reduction in numbers of intracellular bacilli reflected predominantly killing, rather than release, of the infecting mycobacteria.
FIG. 6.
Lymphocyte-mediated reduction in numbers of intracellular H37Ra, BCG, and H37Rv bacilli reflects killing rather than release of the organisms. For each of the three strains, bars represent mean CFU of both the intracellular pellet and the culture supernatant within cultures of monocytes (MN) alone and monocytes to which lymphocytes have been added. As illustrated, CFU of both H37Ra and BCG within supernatants under both conditions are so minimal as to be inapparent at this scale. CFU of H37Rv within culture supernatants following addition of NAC are lower than those seen within cultures of monocytes alone and are not of a great enough magnitude to account for the observed reduction in the number of intracellular H37Rv bacilli.
DISCUSSION
In this study we investigated the applicability of a recently described method of reproducible low-level infection of human monocytes with virulent M. tuberculosis (53) to the assessment of the virulence of strains of the M. tuberculosis family. We found that intracellular growth of three well-characterized laboratory strains, M. tuberculosis H37Rv, M. bovis BCG, and M. tuberculosis H37Ra, correlated with the virulence of these strains as measured in murine and guinea pig models of infection. Intracellular growth of H37Rv and H37Ra within human alveolar macrophages also demonstrated virulence-related patterns of growth. Monocyte TNF-α was induced to a significantly greater degree by H37Rv than by BCG or H37Ra, whereas production of the immunosuppressive cytokine IL-10 showed no clear correlation with virulence. Addition of nonadherent cells from tuberculin-positive donors to infected monocytes led to significant inhibition of the growth of all three strains, and the magnitudes of the protective effects of addition of lymphocytes on the growth of H37Ra, BCG, and H37Ra were not significantly different.
Although various investigators have utilized in vitro models to study the effector function of human mononuclear phagocytes infected with M. tuberculosis (12, 18, 32, 46), few studies have made use of these models to assess intracellular growth as an indicator of virulence. Crowle and May previously reported correlation of growth of the virulent M. tuberculosis Erdman strain and various preparations of BCG within monocytes with the virulence of those strains in animal models (13). Since this particular study was aimed at assessing the potential immunogenicities of vaccine strains, however, intracellular growth of H37Ra was not evaluated. More recently, Paul et al. compared levels of intracellular growth of H37Rv and H37Ra within monocyte-derived macrophages infected at day 6 of culture with the specific goal of addressing the potential use of this human model in the assessment of virulence (43). The investigators reported no significant differences in the levels of intracellular growth of the two strains. However, except for the findings of preliminary studies which used different methodologies, Paul et al.’s findings differed from ours primarily by demonstrating much less growth of intracellular H37Rv. In the preliminary studies, the significant growth of H37Ra which was observed most likely reflected methods which resulted in high initial burdens of intracellular bacteria, whereas the limited growth of H37Rv may reflect conditions of culture of both the bacteria and the infected cells.
Growth of H37Ra within human monocytes following infection with high bacterium-to-cell ratios has been reported previously (32), and the extent of mycobacterial growth within infected animals is in part a function of the initial infecting inoculum (38, 49). In Paul et al.’s initial experiments assessing the effects of in vitro differentiation of monocytes on subsequent intracellular growth of H37Ra, rinsing of nonphagocytosed bacteria was not performed. Although infection at 2 days of culture, the time point most similar to one used in our study, was followed by roughly 16-fold growth of H37Ra over 7 days, the initial infectious burden of bacteria in these studies was approximately 1.25 × 107 H37Ra bacilli per 106 monocytes, or 250-fold higher than the mean of 5 × 104 bacilli per 106 monocytes achieved in our study. In subsequent experiments in which levels of growth of H37Rv and H37Ra were compared, nonphagocytosed M. tuberculosis were rinsed from cultures. The resulting initial intracellular bacterial burden at time zero of approximately 9 × 104 bacilli per 106 monocytes was followed by replication of H37Ra with a doubling time of 104 h. This corresponds to 3.1-fold growth over 7 days, compared to the 1.6-fold growth we observed. The effect of initial inoculum in Paul et al.’s study is further emphasized by the finding that H37Ra grew less than twofold within cells of the one subject in whom the initial infectious burden was most comparable to those of our subjects.
Intracellular growth of H37Rv was relatively slow in Paul et al.’s study, as the reported doubling time of 96 h corresponds to 3.4-fold growth by day 7, compared to the 25-fold growth of H37Rv we observed. Although in vitro differentiation of monocytes as used in the study by Paul et al. increases the ability of the phagocytes to contain M. tuberculosis (20), our study of alveolar macrophages indicates that virulence-related patterns of intracellular growth are maintained even in differentiated mononuclear phagocytes. The slow growth of H37Rv may instead primarily reflect the cultivation of the bacilli in medium containing the detergent Tween 80. Tween 80 was not used in our study because it and other detergents are known to reduce the virulence of M. tuberculosis (11, 58). An additional consideration is that the 96-well, round-bottomed plate format used in our culture system is likely to have facilitated rephagocytosis of released organisms to a greater extent than the 24-well, flat-bottomed plate format used by Paul et al. Greater reuptake of released bacilli may tend to amplify differences in the growth capacities of the strains and therefore increase the ability of the assay to detect such differences.
Our subsequent studies were aimed at using the model of low-level infection of monocytes to further investigate mechanisms of virulence of the strains. Induction of TNF-α was assessed because both in vivo and in vitro studies have suggested a role for TNF-α in containment of intracellular M. tuberculosis (18, 28, 32). Failure to induce TNF-α, or active suppression of its production, is a mechanism of virulence of several pathogens, including M. avium (6, 29, 33, 50). Literature relating to the correlation of virulence and induction of TNF-α by M. tuberculosis has been confused by studies which showed that the mycobacterial cell wall component lipoarabinomannan (LAM) isolated from an avirulent mycobacterial strain induced significantly more TNF-α than LAM isolated from H37Rv (3, 9, 14). However, the avirulent source of LAM was not H37Ra, as initially reported, but a nonpathogenic mycobacterial species, and LAMs of the virulent Erdman strain, H37Rv, BCG, and H37Ra are all structurally identical (10, 35, 45). Our finding that TNF-α induction was greater with increasing strain virulence clearly indicates that the virulence-related growth patterns of these M. tuberculosis strains do not reflect the immunologic “silence” of more virulent strains that fail to induce this protective response. This observation is consistent with previous findings by Rook et al., who observed greater induction of TNF-α by human monocytes in response to H37Rv than to BCG in a study which did not include comparison with H37Ra (47). In contrast, TNF-α production by murine macrophages was reported to be lower following infection with H37Rv than with H37Ra, but this result may be an artifact of the reduced viability of cells infected with H37Rv as opposed to H37Ra in this system (52 versus 80%) (23).
Although in its activating effects, TNF-α may play a protective role following infection with M. tuberculosis, this cytokine may also play a role in the wasting and tissue necrosis which characterize active tuberculosis (7). A relationship between the destructive effects of TNF-α and the virulence of M. tuberculosis strains has been suggested by the finding that cells infected with virulent M. tuberculosis are more susceptible to TNF-α-mediated cytotoxicity than are cells infected with attenuated or avirulent strains (24, 25). Our observation of a correlation between the levels of virulence of H37Rv, BCG, and H37Ra and their abilities to induce TNF-α further supports the hypothesis that the ability of M. tuberculosis to stimulate production of TNF-α may serve primarily to promote pathogenesis rather than protection.
Induction of the downregulatory cytokine IL-10 by H37Rv, BCG, and H37Ra and the ability of lymphocytes to inhibit intracellular growth of the three strains were studied in order to determine whether the degrees of virulence of the strains were reflected in their immunosuppressive capacities. Active tuberculosis is characterized by immunosuppression, which is reflected by impairment of blastogenesis and cytokine production by T cells from patients in response to mycobacterial antigens (22, 56). Neutralizing antibody to IL-10 restores these in vitro responses (31, 32). Other immunosuppressive effects take place at the monocyte level. For example, infection with M. tuberculosis impairs antigen presentation by human monocytes (30), and some mycobacterial components can diminish monocyte responsiveness to lymphocyte-mediated activation (52). Our finding that the levels of induction of monocyte IL-10 by H37Rv, BCG, and H37Ra did not clearly correlate with the degrees of virulence of the infecting strains suggests that differential induction of this immunosuppressive pathway is not a mechanism by which virulent strains evade host defenses. The ability of nonadherent cells from PPD-positive subjects to mediate equivalent levels of reduction in intracellular growth of the three strains further implies that H37Rv, BCG, and H37Ra do not differ in their ability to suppress antigen-specific T-cell responses or lymphocyte-dependent monocyte activation. Our results suggest that a major aspect of the virulence of M. tuberculosis H37Rv, M. bovis BCG, and M. tuberculosis H37Ra is the relative abilities of the bacteria to adapt to the intracellular environment of the monocyte, as opposed to their abilities to induce immunosuppressive cytokines or otherwise modulate protective lymphocyte-mediated responses. This conclusion has a correlate in whole-animal studies of infection of SCID mice with M. tuberculosis strains of various degrees of virulence. H37Rv, BCG, and H37Ra all replicate more efficiently in these T-cell-deficient mice than in immunocompetent animals. Nevertheless, virulence-related differences in growth rates of the strains persist, implying that the strains differ in their abilities to survive nonspecific defenses such as those of mononuclear phagocytes (42).
Our studies demonstrate that low-level infection of human monocytes with H37Rv, BCG, and H37Ra can serve as a model of the virulence of M. tuberculosis whose results provide correlation with the findings of traditional animal models. If this correlation is found with clinical isolates as well, the human monocyte model may provide a rapid and useful tool for clarification of the role of bacterial virulence in the epidemiology of outbreaks of tuberculosis. The model may also be particularly well-suited to the investigation of virulence genes, in that virulence of many bacterial species is regulated by genes which respond to changes in environmental conditions (40). Such virulence mechanisms include the actions of regulatory genes which mediate adaptations essential to bacterial survival and growth within mononuclear phagocytes (4, 5). As M. tuberculosis genes with homologies to environmentally responsive regulatory genes of other species have recently been identified (16, 57), it is possible that similar mechanisms of virulence may be operative within M. tuberculosis as well. The human monocyte model of infection may therefore prove useful in assessing the results of manipulations of proposed virulence genes of M. tuberculosis.
ACKNOWLEDGMENTS
We thank Elizabeth Rich, Hiroe Shiratsuchi, and Zahra Toossi of Case Western Reserve University for helpful discussions of the development of the monocyte model and W. Henry Boom and Laurie R. Hall of Case Western Reserve University for critical reviews of the manuscript.
This research was supported by NIH grant AI35207. Q.L. received additional support from NIH grant AI45244. R.F.S. was funded in part by a Case Western Reserve University Research Initiation Grant sponsored by the Ohio Board of Regents and by a Parker B. Francis Fellowship in Pulmonary Research sponsored by the Francis Families Foundation.
REFERENCES
- 1.Albina J E. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukocyte Biol. 1995;58:643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- 2.Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 4.Baumler A J, Kusters J G, Stojilkovic I, Heffron F. Salmonella typhimuriumloci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belden W J, Miller S I. Further regulation of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect Immun. 1994;62:5095–5101. doi: 10.1128/iai.62.11.5095-5101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuscher H U, Rödel F, Forsberg Å, Röllinghoff M. Bacterial evasion of host immune defense: Yersinia enterocoliticaencodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1978;316:379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- 8.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosisby reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee D, Roberts A D, Lowell K, Brennan P J, Orme I M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992;60:1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee D, Lowell K, Rivoire B, McNeil M R, Brennan P J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannose residues in some strains. J Biol Chem. 1992;267:6228–6233. [PubMed] [Google Scholar]
- 11.Collins F M, Smith M M. A comparative study of the virulence of Mycobacterium tuberculosismeasured in mice and guinea pigs. Am Rev Respir Dis. 1969;100:631–639. doi: 10.1164/arrd.1969.100.5.631. [DOI] [PubMed] [Google Scholar]
- 12.Crowle A J, May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981;31:453–463. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowle A J, May M H. Replication of lyophilized and cultured BCG in human macrophages. Am Rev Respir Dis. 1983;128:673–679. doi: 10.1164/arrd.1983.128.4.673. [DOI] [PubMed] [Google Scholar]
- 14.Dahl K E, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R, Jr, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with human immunodeficiency virus. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 16.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMaria R, Cifone M G, Trotta R, Rippo M R, Festuccia C, Santoni A, Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis M. Killing of Mycobacterium tuberculosiswithin human monocytes: activation by cytokines and calcitriol. Clin Exp Immunol. 1991;84:200–206. doi: 10.1111/j.1365-2249.1991.tb08149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douvas G S, Looker D L, Vatter A E, Crowle A J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douvas G S, Berger E M, Repine J E, Crowle A J. Natural mycobacteristatic activity in human monocyte-derived adherent cells. Am Rev Respir Dis. 1986;143:44–48. doi: 10.1164/arrd.1986.134.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Dugas B, Mossalayi M D, Damais C, Kolb J-P. Nitric oxide production by human monocytes: evidence for a role of CD23. Immunol Today. 1995;16:574–580. doi: 10.1016/0167-5699(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 22.Ellner J J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978;121:2573–2578. [PubMed] [Google Scholar]
- 23.Falcone V, Bassey E B, Toniolo A, Conaldi P G, Collins F M. Differential release of tumor necrosis factor-α from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol Med Microbiol. 1994;8:225–232. doi: 10.1111/j.1574-695X.1994.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 24.Filley E A, Bull H A, Dowd P M, Rook G A W. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumor necrosis factor. Immunology. 1992;77:505–509. [PMC free article] [PubMed] [Google Scholar]
- 25.Filley E A, Rook G A W. Effect of mycobacteria on sensitivity to the cytotoxic effects of tumor necrosis factor. Infect Immun. 1991;59:2567–2572. doi: 10.1128/iai.59.8.2567-2572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flesch I E, Kaufmann S H E. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosisinfection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosisin mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 29.Furney S K, Skinner P S, Roberts A D, Appelberg R, Orme I M. Capacity of Mycobacterium aviumisolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992;60:4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gercken J, Pryjma J, Ernst M, Flad H-D. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong J-H, Zhang M, Modlin R L, Linsley P S, Iyer D, Lin Y, Barnes P F. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor-mediated uptake and tumor necrosis factor-α-mediated growth inhibition of Mycobacterium tuberculosisby human alveolar macrophages. J Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- 33.Huffnagle G B, Chen G-H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T-cell mediated pulmonary inflammation and immunity by a high melanin producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 34.Jacobs W R, Jr, Bloom B R. Molecular genetic strategies for identifying virulence determinants of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 253–270. [Google Scholar]
- 35.Khoo K-H, Dell A, Morris H R, Brennan P J, Chatterjee D. Onsitol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem. 1995;270:12380–12389. doi: 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- 36.Kinger A K, Tyagi J S. Identification and cloning of genes differentially expressed in the virulent strain of Mycobacterium tuberculosis. Gene. 1993;131:113–117. doi: 10.1016/0378-1119(93)90678-v. [DOI] [PubMed] [Google Scholar]
- 37.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 38.Lefford M J. The effect of inoculum size on the immune response to BCG in mice. Immunology. 1971;21:369–381. [PMC free article] [PubMed] [Google Scholar]
- 39.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mekalanos J J. Environmental signals controlling expression of virulence gene determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawara A, Nathan C F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages, and multinucleated giant cells. J Immunol Methods. 1983;56:261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- 42.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivodoubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul S, Laochumroonvorapong P, Kaplan G. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J Infect Dis. 1996;174:105–112. doi: 10.1093/infdis/174.1.105. [DOI] [PubMed] [Google Scholar]
- 44.Pierce C H, Dubos R J, Schaefer W B. Multiplication and survival of tubercle bacilli in the organs of mice. J Exp Med. 1953;97:189–205. doi: 10.1084/jem.97.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prinzis S, Chatterjee D, Brennan P J. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovisBCG. J Gen Microbiol. 1993;139:2649–2658. doi: 10.1099/00221287-139-11-2649. [DOI] [PubMed] [Google Scholar]
- 46.Rook G A W, Steele J, Ainsworth M, Champion B R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333–338. [PMC free article] [PubMed] [Google Scholar]
- 47.Rook G A W, Taverne J, Leveton C, Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumor necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987;62:229–234. [PMC free article] [PubMed] [Google Scholar]
- 48.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosisis mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 49.Shier D R, Long M W. The relationship between infecting dosage and median survival in tuberculous guinea pigs. Am Rev Respir Dis. 1971;104:206–214. doi: 10.1164/arrd.1971.104.2.206. [DOI] [PubMed] [Google Scholar]
- 50.Shiratsuchi H, Toossi Z, Mettler M A, Ellner J J. Colonial morphotype as a determinant of cytokine expression by human monocytes infected with Mycobacterium avium. J Immunol. 1993;150:2945–2954. [PubMed] [Google Scholar]
- 51.Shiratsuchi H, Hamilton B, Toossi Z, Ellner J J. Evidence against a role for interleukin-10 in the regulation of growth of Mycobacterium aviumin human monocytes. J Infect Dis. 1996;173:410–417. doi: 10.1093/infdis/173.2.410. [DOI] [PubMed] [Google Scholar]
- 52.Sibley L D, Adams L B, Krahenbuhl J L. Inhibition of interferon gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin Exp Immunol. 1990;80:141–148. doi: 10.1111/j.1365-2249.1990.tb06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silver, R. F., Q. Li, W. H. Boom, and J. J. Ellner. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J. Immunol., in press. [PubMed]
- 54.Small P M, Shafer R W, Hopewell P C, Singh S P, Murphy M J, Desmond E, Sierra M F, Schoolnik G K. Exogenous reinfection with multi-drug resistant Mycobacterium tuberculosisin patients with advanced HIV infection. N Engl J Med. 1993;328:1137–1144. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 55.Small P M, Hopewell P C, Singh S P, Paz A, Parsonnet J, Ruston D C, Schecter G F, Daley C L, Schoolnik G. The epidemiology of tuberculosis in San Francisco. N Engl J Med. 1994;330:1136–1144. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 56.Toossi Z, Kleinhenz M E, Ellner J J. Defective interleukin-2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Via L E, Curcic R, Mudd M H, Dhandayuthapani S, Ulmer R J, Deretic V. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J Bacteriol. 1996;178:3314–3321. doi: 10.1128/jb.178.11.3314-3321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wayne L G. Cultivation of Mycobacterium tuberculosis for research purposes. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 73–84. [Google Scholar]
- 59.Williams N. Europeans move on from yeast to TB. Science. 1996;272:27. doi: 10.1126/science.272.5258.27b. [DOI] [PubMed] [Google Scholar]
- 60.Zembala M, Siedlar M, Marcinkiewicz J, Pryjma J. Human monocytes are stimulated for nitric oxide release in vitro by some tumor cells but not by cytokines and lipopolysaccharide. Eur J Immunol. 1994;24:435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]