Abstract
Background
Precision medicine has the potential to improve cardiovascular disease (CVD) risk prediction in individuals with Type 2 diabetes (T2D).
Methods
We conducted a systematic review and meta-analysis of longitudinal studies to identify potentially novel prognostic factors that may improve CVD risk prediction in T2D. Out of 9380 studies identified, 416 studies met inclusion criteria. Outcomes were reported for 321 biomarker studies, 48 genetic marker studies, and 47 risk score/model studies.
Results
Out of all evaluated biomarkers, only 13 showed improvement in prediction performance. Results of pooled meta-analyses, non-pooled analyses, and assessments of improvement in prediction performance and risk of bias, yielded the highest predictive utility for N-terminal pro b-type natriuretic peptide (NT-proBNP) (high-evidence), troponin-T (TnT) (moderate-evidence), triglyceride-glucose (TyG) index (moderate-evidence), Genetic Risk Score for Coronary Heart Disease (GRS-CHD) (moderate-evidence); moderate predictive utility for coronary computed tomography angiography (low-evidence), single-photon emission computed tomography (low-evidence), pulse wave velocity (moderate-evidence); and low predictive utility for C-reactive protein (moderate-evidence), coronary artery calcium score (low-evidence), galectin-3 (low-evidence), troponin-I (low-evidence), carotid plaque (low-evidence), and growth differentiation factor-15 (low-evidence). Risk scores showed modest discrimination, with lower performance in populations different from the original development cohort.
Conclusions
Despite high interest in this topic, very few studies conducted rigorous analyses to demonstrate incremental predictive utility beyond established CVD risk factors for T2D. The most promising markers identified were NT-proBNP, TnT, TyG and GRS-CHD, with the highest strength of evidence for NT-proBNP. Further research is needed to determine their clinical utility in risk stratification and management of CVD in T2D.
Subject terms: Prognostic markers, Diabetes complications
Plain language summary
People living with type 2 diabetes (T2D) are more likely to develop problems with their heart or blood circulation, known as cardiovascular disease (CVD), than people who do not have T2D. However, it can be difficult to predict which people with T2D are most likely to develop CVD. This is because current approaches, such as blood tests, do not identify all people with T2D who are at an increased risk of CVD. In this study we reviewed published papers that investigated the differences between people with T2D who experienced CVD compared to those who did not. We found some indicators that could potentially be used to determine which people with T2D are most likely to develop CVD. More studies are needed to determine how useful these are. However, they could potentially be used to enable clinicians to provide targeted advice and treatment to those people with T2D at most risk of developing CVD.
Ahmad, Lim, Morieri, Tam et al perform a systematic review and meta-analysis of biomarkers, genetic markers, and risk scores for prediction of cardiovascular outcomes in Type 2 diabetes. A few prognostic markers are identified that provide incremental predictive utility beyond established cardiovascular risk factors.
Introduction
Individuals with type 2 diabetes (T2D) have a 1.5 to 2-fold higher risk of developing cardiovascular disease (CVD) compared to those without T2D1,2. This is particularly concerning given the high global prevalence of diabetes and the aging population. More than 500 million individuals worldwide are affected by this chronic disease, resulting in substantial human and economic costs3,4. However, predicting CVD risk in T2D remains a challenge, and existing risk algorithms, such as the UK Prospective Diabetes Study (UKPDS) Risk Engine and Framingham Risk Score (FRS), have shown only modest predictive value in external validation studies5–7. Thus, it is essential to identify or develop readily available and cost-effective measures that can accurately identify individuals with a higher absolute risk of developing CVD beyond the risk estimated from established risk factors.
Precision medicine provides a promising approach to optimize risk prediction by integrating multidimensional data (i.e., genetic, clinical, sociodemographic), accounting for individual differences8. Recognizing the potential value of precision medicine in improving diabetes prevention and care, the Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD) and is led by global leaders in precision diabetes medicine9. This systematic review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine10. As part of this broader initiative, we conducted a systematic review and meta-analyses addressing precision prognosis for CVD outcomes.
While previous systematic reviews of biomarkers for prediction of CVD have been conducted in the general population11–25, this review focused on patients with T2D. We sought to answer two questions: (1) Which novel markers predict CVD in people with T2D? (2) Is there any evidence that these markers enhance risk prediction beyond current practice? Addressing these questions may inform the development of more effective strategies for detecting and predicting CVD in individuals with T2D, ultimately leading to improved management and prevention of this complication.
Therefore, to identify those biomarkers with most promising clinical utility for CV risk assessment, we followed a rigorous stepwise approach, including evaluation of the incremental value of each biomarker beyond traditional risk factors (i.e. with evaluation of improvement in different metrics such as c-statistic and net reclassification improvement – NRI), as recommended by the statement from the American Heart Association for identification of novel markers for CV disease26.
In summary, employing a stringent study selection process, this systematic review and meta-analysis identified four prognostic factors with high predictive utility, supported by moderate to high-strength evidence. Furthermore, three prognostic factors demonstrated moderate predictive utility, backed by low to moderate-strength evidence, and six prognostic factors showed low predictive utility, with evidence levels ranging from low to moderate. Risk scores demonstrated modest discrimination on internal validation, with diminished performance in external validation, particularly in cohorts diverging from the original population.
Methods
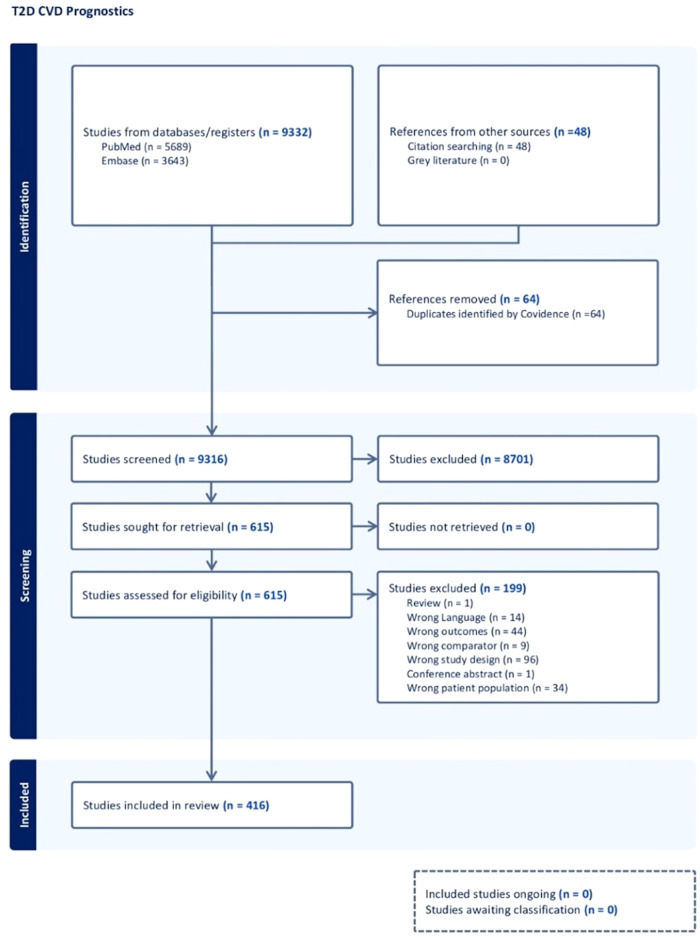
As a reporting guidance, we followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement27. Figure 1 presents the PRISMA flow diagram, illustrating the process that led to the final selection of studies for review. Prior to data collection, the proposed systematic review and meta-analysis was registered on PROSPERO (Registration number: CRD42021262843).
Fig. 1. PRISMA flow diagram detailing the process that led to final study inclusion for review.
Flowchart illustrating the screening of studies through title and abstract review, screening, and inclusion. n number of studies.
Inclusion and exclusion criteria
This review included longitudinal studies (prospective or retrospective cohorts, including secondary analyses of cohorts from randomized controlled trials) of participants with T2D (youth-onset and adult-onset). Inclusion criteria included observational studies published from 1990–2021 that reported on the association between a prognostic factor or risk score and one or more CVD outcomes among participants with T2D. During the period of our review, the diagnostic criteria for T2D underwent some modifications (e.g. change in fasting glucose threshold, addition of hemoglobin A1C). We accepted studies that reported the inclusion of participants with T2D as defined in each individual study. Exclusion criteria included cross-sectional studies, studies utilizing surrogate endpoints for cardiovascular (CVD) outcomes such as carotid intima-media thickness, endothelial dysfunction, and arterial stiffness, and studies including only participants with pre-diabetes or only participants with type 1 diabetes. Studies with mixed populations of diabetes were included only if results were reported separately for participants with T2D. Supplemental Table 1 summarizes the Participant Intervention Comparison Outcomes and Studies (PICOS) framework.
Outcomes
Only studies reporting outcomes on fatal or non-fatal coronary heart disease (CHD) or cardiovascular mortality (either alone or as individual component of composite outcomes) were included. A broad definition of CHD, including any outcomes defined by terms such as myocardial infarction, ischemic heart events, cardiac events, coronary artery disease, and major cardiovascular events was used.
Search strategy
We conducted a comprehensive search on Medline and Embase of studies published from January 1990 to March 2021 using keywords and MeSH (Medical Subject Headings) terms relevant to T2D and CVD (see Supplemental Note 1). In addition, we searched the reference lists of eligible studies and systematic reviews to identify any further relevant studies. The search strategy was designed by a multi-professional team of researchers with expertise in precision medicine, clinical diabetes, cardiovascular disease, biomarker development and evaluation, genetic markers, and predictive analytics, supported by two librarians with expertise in conducting systematic reviews and meta-analyses. References identified were exported to EndNote (Clarivate Analytics) and imported to Covidence, where studies were assessed for eligibility. After the removal of duplicates, 14 authors participated in screening each title/abstract, and full-text articles were obtained if abstracts were considered eligible by at least one author. Each full-text article was assessed for inclusion independently by two authors (among 12 total authors), and disagreements were resolved by consensus.
Data extraction
All data were extracted and coded by one author and reviewed by a second author to ensure data accuracy. After undergoing training to ensure consistency in the process, thirteen authors participated in the data extraction process (A.A., C.T., L.L., M.F.G., M.L.M., N.M., R.C.W.M., S.K., C.H., G.Y., Y.Z., M.D.P., S.C.T.). To minimize inter-reviewer variability and ensure consistency in data extraction, all authors underwent training sessions via video conferences and participated in mock assessments.
During data extraction, studies were classified into three categories based on the primary type of prognostic factors reported, namely biomarkers, genetic markers, and risk scores. Biomarkers were broadly defined as non-genetic laboratory tests, clinical conditions, socio-demographics, vital signs, diagnostic procedures, and imaging tests. Genetic markers included specific DNA sequences or variations, such as single nucleotide polymorphisms (SNPs), restriction fragment length polymorphisms (RFLP), or short tandem repeats (STR). Risk scores were defined as predictive models, algorithms, or risk calculation tools that estimated the overall likelihood or category of cardiovascular disease (CVD) based on a set of risk factors. When multiple genetic variants were combined to predict risk (using SNPs), the study was classified as a genetic marker (i.e., genetic risk score) rather than a risk score. Additional details about the included studies can be found in Supplemental Note 2.
The following data were extracted from each article using a standardized data form in Covidence and Excel data tables: study characteristics (country or countries of the study population, study start and end year, study design, inclusion/exclusion criteria, study setting, data sources), participant characteristics (years of follow-up, follow-up duration, total number of participants, race/ethnicity/ancestry, and baseline characteristics), prognostic factor(s) characteristics (name, prognostic factor type, units of measurement, units and cut-offs in regression analyses, transformation methods, effect measures [hazard ratio, odds ratio, c-statistic, net reclassification improvement (NRI), integrated discrimination index (IDI), etc.] and 95% confidence intervals, adjusted covariates), and outcomes (CVD outcome definition, number of events and non-events), and validation methods. For genetic markers, we collected risk variants, risk alleles, and closest gene (locus).
For continuous variables, we collected mean and standard deviation or median and interquartile range as reported in the study. We collected fully adjusted effect measures (HR, RR, OR, c-statistic) and their corresponding 95% CIs reported in the original articles. When studies reported multiple multivariate-adjusted effect measures, we collected the estimate from the most fully adjusted model. We did not contact primary authors to obtain data that were not reported. Furthermore, data were collected to evaluate the risk of biases in each study as summarized in Supplemental Table 2 and described in the quality assessment paragraph.
Quality assessment
We used a modified Newcastle-Ottawa Scale (NOS) to assess quality and risk of biases. The scale assesses studies based on six common domains, including representativeness of the exposed and non-exposed cohorts, ascertainment of exposure and outcome, and adequacy of study follow-up for primary and secondary CVD events, as well as the adequacy of cohort follow-up28. For biomarker studies, we added two additional domains to the NOS to address bias due to confounding by evaluating the number of covariates and established CVD risk factors included in the adjusted models. Each study was given a score for each domain and an overall quality evaluation was determined by adding up these scores. The possible range of scores for non-genetic biomarkers, based on 8 domains, was 2 to 28, while for genetic biomarkers and risk scores, scores ranged from 2 to 18 based on 6 domains. Two authors assessed study quality independently, and a third author resolved any disagreements.
We reported the overall risk of bias based on the distribution of scores in each prognostic factor category, with higher scores representing lower risk of bias. Studies in the top, second, and lowest tertiles (according to the distribution specific for each type of study, i.e. non-genetic biomarkers, genetic biomarkers and risk scores) were considered to have low, medium, and high risk of bias, respectively. The score of each domain was also classified as low, medium, or high risk of bias for graphical purposes, as clarified in Supplemental Table 2.
Statistical analysis
A random-effects model was used to pool the overall effect estimates in all meta-analyses, only if the heterogeneity test was statistically significant. For studies reporting the same effect measure (e.g. HR), we calculated the pooled effect estimate with 95% CIs for each biomarker or genetic marker and assessed heterogeneity between studies using the Cochran’s Q statistic (p < 0.1), the I2 index >75%, and τ2. Due to the limited number of studies per prognostic factor, subgroup analyses by population characteristics or outcomes were not performed. We performed sensitivity analyses by excluding studies with high risk of bias. As the number of studies per prognostic factor was always less than 10, we were unable to assess publication bias using funnel plots. We used R, version 4.2.3 (R Project for Statistical Computing), with the “meta”, “metafor”, and “forestplot” packages for all analyses29. Two-sided statistical tests were used with a significance threshold of <0.05.
Strength of the evidence
We considered aspects of the GRADE approach30 and the JBI critical appraisal tools31 in grading the strength of evidence for individual biomarkers and genetic markers/risk scores. We applied relevant GRADE criteria, including indirectness, inconsistency, and imprecision, throughout the study. Since we only included studies that involved patients with T2D and a “hard” clinical CVD outcome, the evidence is considered direct by definition. We analyzed the results from T2D patients with and without baseline CVD and specified all relevant CVD outcomes to assess the applicability of individual biomarkers in specific populations and outcomes. To ensure robustness and validity of our findings, we established strict eligibility criteria, excluding studies that did not adjust for established CVD risk factors (listed in Supplemental Table 3). Furthermore, we scored studies based on the adequacy of adjustment for covariates, including the total number of covariates and established CVD risk factors, in accordance with the JBI criterion for statistical adjustment of confounders.
We used the American Heart Association scientific consensus report for stepwise evaluation of novel markers for CVD risk26 to identify promising biomarkers and genetic markers based on their strength of evidence progressing from measures of association, discrimination, improvement in discrimination, net reclassification index (NRI) or integrated discrimination index (IDI). This approach is summarized in Supplemental Table 4. For biomarkers and genetic markers, we progressed from those with significant adjusted association in at least one study to those with net positive number of studies showing significant association in a consistent direction. The net positive number of studies was calculated by summing up all studies with positive association and subtracting studies with no association (e.g., three studies showing positive association and two studies with no association yielded a net positive number of one). We identified biomarkers that improved prediction performance when added to established models, based on improvement in at least one of c-statistic, NRI (the probability that a person is appropriately classified into either high- or low-risk), or IDI (quantification of predicted probabilities of events and non-events based on inclusion of the biomarker in the model), and further narrowed down the list to those with improvement in all three indicators.
Accordingly, for each of the prognostic factors that passed our evidence-based screening criteria, predictive utility was classified as high (3 points), moderate (2 points), or low (<2 points) based on three criteria: number of studies with all three performance indicators satisfied (1 point if >0 studies, 0 points if 0 studies), number of pooled meta-analyses showing significant association (1 point if >0, 0 points if 0 studies), non-pooled analysis showing 75% of studies had a significant association (1 point if yes, 0 points if no). Strength of Evidence was classified as high (4 points), moderate (2 or 3 points), or low (<2 points) based on four criteria: at least one meta-analysis was conducted regardless of outcome (1 point if yes, 0 points if no), exclusion of high risk of bias studies did not alter inferences from meta-analyses (1 point if unaltered, 0 points if altered), exclusion of high risk of bias studies did not alter inferences from non-pooled analyses (1 point if unaltered, 0 points if altered), and consistencies in the definition of the prognostic marker used in analyses (1 point if yes, 0 points if no).
For the risk scores, we provide a complete assessment of risk of bias and pooled c-statistics; however, we decided not to conduct a corresponding stepwise approach to evidence grading as explained above for biomarkers/genetic markers due to the complexity in verifying specifications of each model over time and across comparisons. Inferences from the risk score results are here meant to guide future work that would permit analyses to handle this complexity.
Inclusion and ethics statement
This research is a part of a broader initiative, Precision Medicine in Diabetes Initiative (PMDI), that was established by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD) and is led by global leaders in precision diabetes medicine. Therefore, researchers from multiple countries and continents have contributed to this study. The roles and responsibilities of co-authors were collaboratively agreed upon before the start of the review process. This study is exempt from ethical review due to the use of publicly available data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Study selection and characteristics
Out of 9380 studies identified from databases/registries (N = 9332) and other sources (N = 48), there were 9316 unique studies after removing 64 duplicates. Of these, 615 articles were selected for full-text review, and finally, 416 articles were considered appropriate for inclusion in the analysis5,32–446. Outcomes were reported for 321 biomarker studies, 48 genetic marker studies, and 47 risk score/model studies, as shown in Supplemental Data 1, 2, and 3. Figures 1 and 2 provide an overview of the screening and selection process.
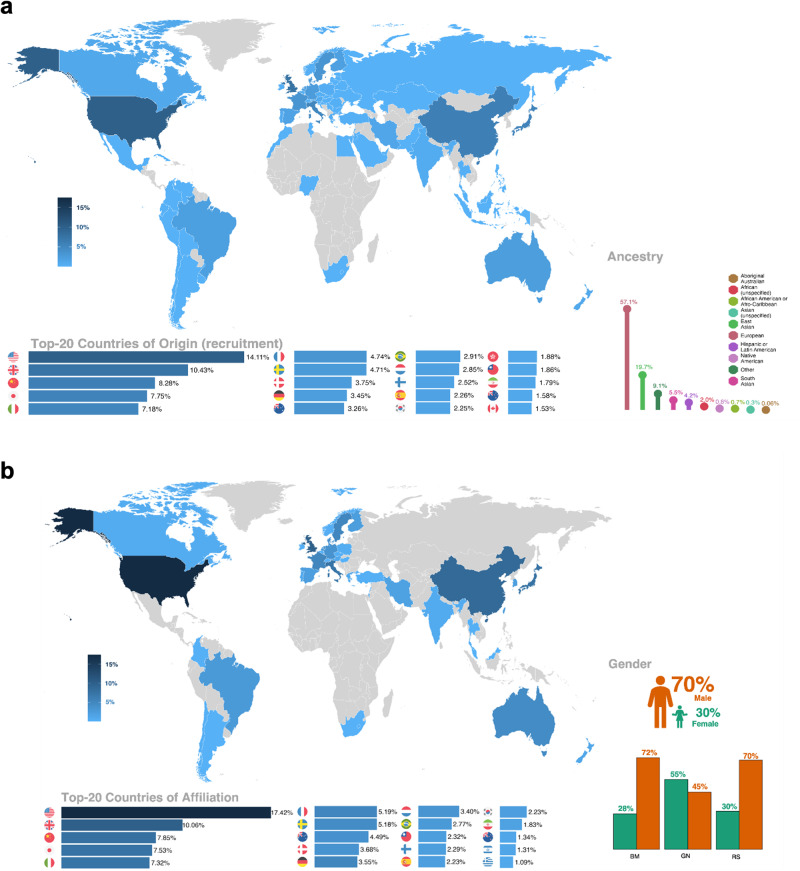
Fig. 2. Selection of studies to be included for evaluating the associations of biomarkers, genetic markers and non-genetic risk scores with cardiovascular outcomes.
This figure shows the selection criteria used to identify included biomarkers, genetic biomarkers, and non-genetic risk scores. No. number. *See Supplemental Table 3.
Predominant ancestry in the studied populations were European (57.1%), East Asian (19.7%), South Asian (5.5%) and Hispanic or Latin American (4.2%). Geographically, the United States, United Kingdom, China, Japan, and Italy were the top five represented countries with regards to origin of study participants and author affiliation in the included studies. Figure 3 and online interactive figures (https://hugofitipaldi.shinyapps.io/T2D_prognostic/) offer a detailed breakdown of ethnic and geographic distributions447.
Fig. 3. Global distribution of origin and ancestry of the study populations and countries of affiliation and gender distribution of authors of the included studies.
Panel A shows the top 20 countries of origin and ancestry of the study populations evaluated in the included studies. Panel B shows the top 20 countries of affiliation and gender distribution of authors of the included studies. The data used for this visualization was obtained from PubMed and PubMed Central through manual curation and by applying text mining functions developed using R software version 4.1.2. The final proportions of ancestries were calculated for each unique study and then aggregated as described in detail here448.
CVD outcomes
There was heterogeneity in the CVD outcomes evaluated across the analyzed studies (see Supplemental Fig. 1). The median duration of follow-up reported across studies was 5 years (IQR 3.1 to 7.8 years). The most frequently reported outcomes were coronary heart disease, cardiovascular mortality, and stroke, either individually or combined. The vast majority (87%) of studies had a clearly defined outcome based on ICD-10 codes, clinical documentation, or adjudication, with 9% relying on registry or record linkage, and 4% using either patient self-report or having an unclear definition. We classified primary prevention as the prediction of CVD in individuals without a history of the disease, secondary prevention as the prediction of recurrent CVD events or CVD progression in those already diagnosed with the disease, and mixed populations as a combination of both primary and secondary prevention.
Biomarkers
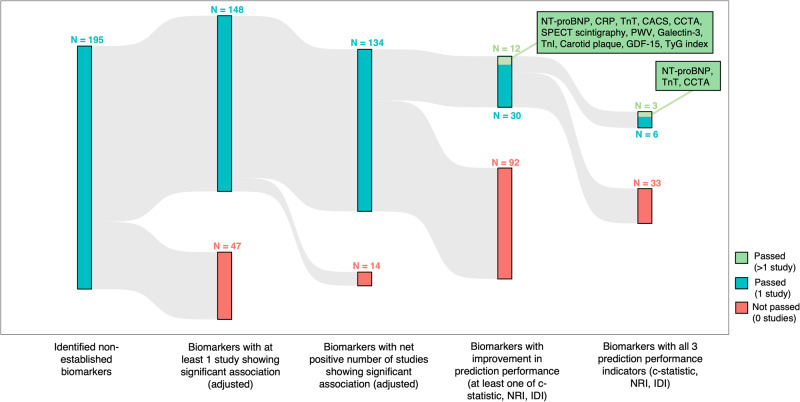
Among 416 included studies, 321 (77.2%), 48 (11.5%), and 47 (11.2%) were studies of non-genetic biomarkers, genetic biomarkers, and non-genetic risk scores, respectively. Among the 321 studies of non-genetic biomarkers, 70 (21.8%) evaluated established CVD risk factors and were excluded, while 30 studies (9.3%) were included because they used a novel approach (e.g., variability, setting) for an established risk factor (Fig. 2). Further, three studies did not adjust for any CVD risk factors and were excluded, leaving 218 studies consisting of 195 unique biomarkers in the analysis.
Among these 195 biomarkers analyzed, 134 (69%) had a significant adjusted association for predicting CVD, based on a net positive number of studies (Fig. 4 and Supplemental Data 4). Out of these, 12 (9%) showed improvement in c-statistic, NRI, or IDI in more than one study: N-terminal pro b-type natriuretic peptide (NT-proBNP), C-reactive protein (CRP), troponin T (TnT), coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA), single-photon emission computed tomography (SPECT) scintigraphy, pulse wave velocity (PWV), galectin-3 (Gal-3), troponin I (TnI), carotid plaque, growth differentiation factor-15 (GDF-15), and triglyceride-glucose (TyG) index. The following biomarkers showed prediction performance but in only one study: SPECT, TnI, TyG, 25-hydroxyvitamin D, poly (ADP-ribose) polymerase (PARP), and interleukin-6 (IL-6).
Fig. 4. Sankey diagram showing the funneling of identified non-genetic biomarkers through sequential filtering steps.
The number of biomarkers passing or not passing each step (based on the criteria specified at the bottom of the diagram) is depicted at the top of the colored bars, with biomarkers passing all steps having the strongest predictive performance value.
Biomarkers with all three prediction performance indicators satisfied in more than one study were NT-proBNP, TnT, and CCTA, with results summarized in Table 1. For NT-proBNP, 5 studies reported improvement in c-statistics ranging from 0.01 to 0.07, significant increase in NRI ranging from 0.04 to 0.50, and significant rIDI ranging from 0.012 to 0.48 (in four studies). For TnT, 3 studies reported improvement in c-statistics ranging from 0.02 to 0.10, significant NRI ranging from 0.150 to 0.44, and rIDI ranging from 0.03 to 0.05. For CCTA, 3 studies reported improvement in c-statistics ranging from 0.08 to 0.35, with one study reporting statistically significant improvements in NRI of 0.55 and rIDI of 0.046. Of these three biomarkers, NT-proBNP showed the strongest incremental predictive value based on the magnitude of these indicators. Supplemental Data 5 shows the degree of variation in measurement methods used for each of these biomarkers.
Table 1.
Performance of the prediction of 3 biomarkers with the most evidence.
| Biomarker | Study | Clinical factors / biomarkers to be compared | Improvement in C-statistics | NRI | IDI | |||
|---|---|---|---|---|---|---|---|---|
| Yes /No | Estimate (95% CI) and P-value | NRI (95% CI) | P-value | IDI (95% CI) | P-value | |||
| NT-proBNP | Sharma 2020 | Age, sex, SBP, history of HF, duration of diabetes, prior MI, hypertension, hyperlipidemia, smoking, eGFR. | Yes | 0.05 (NR) |
0.39 (0.30–0.47) |
NR |
0.09 (0.08–0.10) |
<0.05 |
| Wolsk 2017 | Prior MI, BMI, NSTEMI (index event), heart rate, HbA1c, percutaneous coronary intervention at the index event, cerebrovascular disease, AF, prior HF, sodium concentration, macroalbuminuria, PAD, age, and LDL concentration. | Yes |
0.01 (CI NR), P < 0.05 |
0.11 (5.7–16.6) |
<0.05 |
0.08 (0.03–1.6) |
<0.05 | |
| Wong 2019 | Age ≥ 65 years, Male, T2D, Hypertension | Yes |
0.03 (CI NR), P = 0.001 |
0.35 (0.24–0.45) |
<0.001 |
0.01 (0.01–0.02) |
<0.001 | |
|
Scirica 2016 |
Treatment arms, age, SBP, sex, history of HF-, duration of diabetes, prior MI, history of hypertension, history of hyperlipidemia, smoking, and eGFR | Yes |
0.07 (CI NR), P < 0.001 |
0.040 (0.03–0.04) |
<0.05 |
0.48 (0.41–0.55) |
<0.05 | |
| Van der Leeuw 2016 | Female sex, age at diabetes diagnosis, duration of diabetes, HbA1c, square of HbA1c, SBP, square of SBP, TC/HDL ratio, urinary ACR, current smoking status, history of major macrovascular disease | Yes | 0.02 (0.00–0.04), P-value NR |
0.2 (0.10–0.44) |
<0.05 | NR | NR | |
| Van der Leeuw 2016 | Female sex, age at diabetes diagnosis, duration of diabetes, HbA1c, systolic blood pressure, TC/HDL ratio, eGFR, current smoking status, history of major macrovascular disease | Yes | 0.02 (0.00–0.05), P-value NR |
0.50 (0.26–0.73) |
<0.05 | NR | NR | |
| TnT |
Lepojarvi 2016 |
Age, sex, history of acute MI, BMI, Canadian Cardiovascular Society grading of angina pectoris, left ventricular ejection fraction and mass index, HDL cholesterol, ACR, HbA1c, and type of glucose metabolism disorder | Yes |
0.10 (CI and P-value NR), |
0.231 (0.067–0.394) |
<0.01 |
0.05 (0.03–0.08) |
<0.001 |
|
Scirica 2016 |
Treatment arms, age, SBP, sex, history of HF, duration of diabetes, prior MI, history of hypertension, history of hyperlipidemia, smoking, and eGFR | Yes |
0.07 (CI NR), P < 0.001 |
0.440 (0.380–0.510) |
<0.05 |
0.03 (0.02–0.03) |
<0.05 | |
|
Rørth 2019 |
Natural logarithm of NT-proBNP, age, sex, treatment effect, ejection fraction, NYHA class, BMI, heart rate, SBP, creatinine, LDL, prior angina pectoris, AF and pacemaker implantation. | Yes |
0.02 (CI NR), P = 0.02 |
0.150 (0.051–0.261) |
0.007 |
0.03 (0.01–0.06) |
<0.001 | |
| CCTA |
Lee 2017 |
Age, male sex, HTN, smokers, hyperlipidemia, eGFR, and HbA1c | Yes |
0.07 (CI NR), P = 0.03 |
0.550 (0.343–0.757) |
<0.0001 |
0.05 (0.02–0.07) |
0.0006 |
|
Halon 2016 |
UKPDS and log CAC Score | Yes |
0.35 (CI NR), P = 0.021 |
0.63 (CI NR) |
NR |
0.65 (CI NR) |
NR | |
Data on improvement in C-statistics was collected from the study, either as reported or derived by comparing the C-statistic from the reference model with the C-statistic obtained from the combination of the reference model and the novel biomarker. NR Not Reported, SBP systolic blood pressure, HF heart failure, MI myocardial infarction, BMI body mass index, AF atrial fibrillation, PAD peripheral artery disease, T2D Type 2 diabetes, eGFR estimated glomerular filtration rate, ACR albumin-creatinine ratio. P-values were extracted from studies as reported, therefore some p-values are given as <0.05 or <0.001 because exact p-values were unavailable.
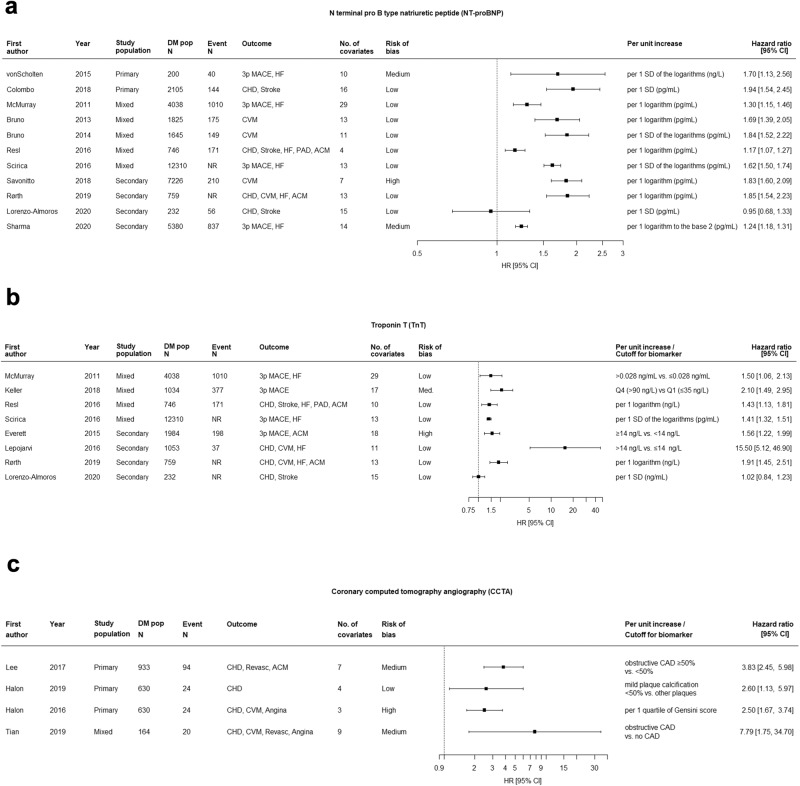
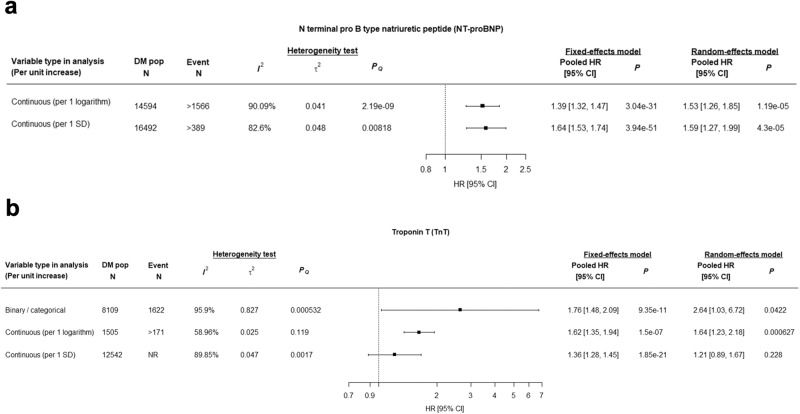
Forest plots in Fig. 5a show the HRs for 11 studies evaluating NT-proBNP, conducted in heterogeneous populations (2 primary, 5 mixed, and 4 secondary), outcomes, units in regression analyses (i.e., SD, SD of log), and laboratory units (ng/L, pg/mL). Nonetheless, all studies except one showed a significant association with a CVD outcome. Eight out of 11 (73%) studies were assessed to be at low risk of bias. Figure 6a and Supplemental Figs. 2a, b show the meta-analysis of NT-proBNP as a continuous variable per logarithmic and per 1 SD unit increase, confirming the highly significant association with CVD (pooled HR 1.53, 95% CI 1.26-1.85 per log increase; pooled HR 1.59, 95% CI 1.27–1.99 per SD increase) after accounting for heterogeneity with the random effects models (I2 90% and I2 83%, respectively). Interestingly, although our review excluded studies focusing exclusively on heart failure patients, among three studies that incorporated EF as a covariate in their models, NT-proBNP was shown to have predictive value for cardiovascular outcomes independent of EF241,326,397 (Supplemental Data 5).
Fig. 5. Forest plots for three biomarkers (NT-proBNP, TnT, and CCTA) with the most evidence for prediction of CVD outcomes.
Panel a (NT-proBNP); Panel b (TnT); Panel c (CCTA). HR hazard ratio, CI confidence interval, DM pop N, sample size for diabetes population; Event N, number of individuals developed CVD outcomes; 3p MACE, 3-point major adverse cardiovascular events; HF heart failure, CHD coronary heart disease, CVM cardiovascular mortality, PAD peripheral artery disease, ACM all-cause mortality.
Fig. 6. Meta-analysis of NT-proBNP and TnT for predicting cardiovascular outcomes.
Panel a (NT-proBNP); Panel b: TnT; PQ is the p-value obtained from the Cochran’s Q test. HR, hazard ratio; CI, confidence interval; DM pop N, sample size for diabetes population; Event N, number of individuals developed CVD outcomes.
Forest plots in Fig. 5b show the HRs for 8 studies evaluating TnT, conducted primarily for mixed or secondary populations with variable CVD outcomes. Studies differed with respect to cut-offs and categories for TnT, units of measurement (ng/ml, ng/L) and analysis (per log, per 1 SD log). Among these studies, all but one showed a positive association. Notably, the study by Lepojarvi 2016 was an outlier in its magnitude of effect and confidence intervals. Overall, for TnT, study quality was good with 6 out of 8 (75%) assessed to be at low risk of bias227. A significant association for TnT was observed in studies where the biomarker was evaluated as a continuous variable per 1 log increase with pooled HR 1.64 (95% CI 1.23, 2.18) and I2 59% (Fig. 6b and Supplemental Fig. 3a); similarly, when treated as a binary or categorical variable, the pooled HR was 2.64 (95% CI 1.03, 6.72) with I2 = 95.9% (Fig. 6b and Supplemental Fig. 3b). However, when treated as a continuous variable per 1 SD, there was no longer a significant association in a random effects model (Fig. 6b and Supplemental Fig. 3c).
Forest plots in Fig. 5c show the HRs for 5 studies evaluating CCTA conducted primarily for primary CVD prevention with variable CVD outcomes. Studies differed significantly with respect to CCTA definition of subclinical or clinical CHD. All 5 studies showed a significant association; however, 2 of the 5 studies (40%) were assessed to be at a high risk of bias.
Apart from these three biomarkers, SPECT, TnI, TyG, 25-hydroxyvitamin D, poly(ADP-ribose) polymerase (PARP), and interleukin-6 (IL-6) showed prediction performance in all three performance indicators but in only one study. Forest plots for the remaining 9 biomarkers that showed improvement in at least one performance indicator in more than one study (CACS, carotid plaque, CRP, gal-3, GDF-15, PWV, SPECT scintigraphy, TnI, and TyG) are shown in Supplemental Figs. 4–6. Again, there was substantial heterogeneity with respect to study populations, outcomes, and units of analysis for these biomarkers. Biomarkers showing positive association in at least 75% of studies included CACS, carotid plaque, gal-3, PWV, SPECT scintigraphy, TnI, and TyG. While CRP did not meet the threshold of 75% of studies showing an association, when meta-analyzed as a binary or categorical variable, it showed a significant pooled association; PWV and TyG also demonstrated significant association in pooled analysis (Supplemental Fig. 7).
Genetic markers
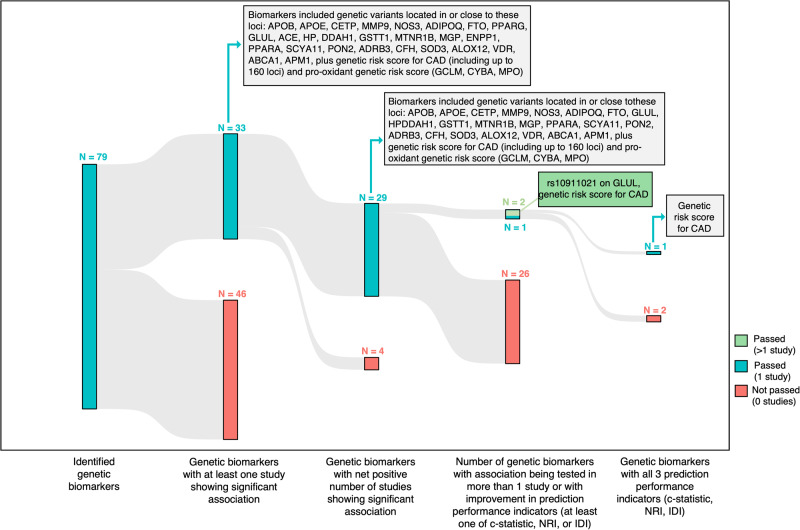
Among the 48 genetic studies analyzed (Supplemental Data 2), 79 genetic biomarkers were examined for their association with incident CVD events (Supplemental Data 6), mainly in populations of European (65%) or Asian (26%) ancestries, with sparse representation of populations of other ancestries (e.g., African 12% or Hispanic 3%), with 12% of associations being tested in mixed populations. Most of the studies (70 out of 79) used single variants as distinct genetic biomarkers (exposure), while 9 studies used a combination of different SNPs into genetic risk scores (GRS) as the exposure. Remarkably, most of these exposures were tested only in one study, and external validation was performed in only 4 out of 48 studies, with only one study using a longitudinal cohort as a validation set, i.e., GRS for CHD. Overall, among the 79 genetic biomarkers, 33 (41.8%) had at least one study showing significant association, out of which 29 had a net positive number of studies showing significant association. Out of these 29 genetic biomarkers, two were tested in more than one study (rs10911021 on GLUL, GRS for CHD [GRS-CHD]), one had improvement in any performance indicator in a single study (isoform e4 in APOE), and one had improvement in all three performance indicators in a single study (GRS-CHD) (Fig. 7).
Fig. 7. Sankey diagram showing the funneling of identified genetic biomarkers through sequential filtering steps.
The number of biomarkers passing or not passing each step (based on the criteria specified at the bottom of the diagram) is depicted at the top of the colored bars, with biomarkers passing all steps having the strongest predictive performance value.
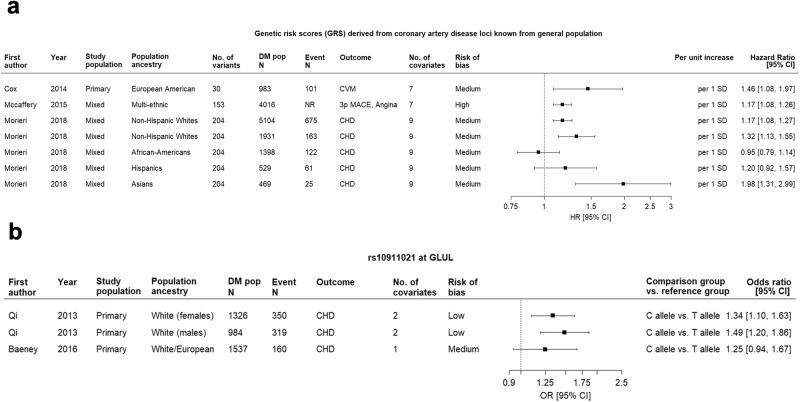
Notably, the rs10911021 variant in GLUL was the only single variant that showed an association with CVD in several studies. This variant was initially identified in T2D patients using a genome-wide approach and subsequently confirmed for its association with CVD in selected populations from two additional studies. For GRS-CHD, four separate studies investigated the combination of up to 204 CHD variants from 160 distinct loci derived from the general population. These studies had distinct but overlapping and increasing numbers of loci and variants tested in more recent investigations. The most recently performed GRSs were externally validated and demonstrated significant improvements in CVD risk reclassification (cNRI) as well as notable enhancements of 8% in relative IDI (rIDI). However, these findings were identified in subjects of European ancestry and ancestry-specific analyses showed consistency in Asian subjects but not in other ancestral backgrounds. Forest plots for variants located on the GRS-CHD and GLUL are shown in Fig. 8, while their meta-analyses can be found in Supplemental Fig. 8.
Fig. 8. Forest plots of genetic risk scores and GLUL variant rs10911021 for predicting cardiovascular outcomes.
Panel a: Genetic risk scores; Panel b: GLUL variant rs10911021; HR, hazard ratio; CI, confidence interval; DM pop N, sample size for diabetes population; Event N, number of individuals developed CVD outcomes; 3p MACE, 3-point major adverse cardiovascular events.
Risk scores/models
Forty-seven studies reported results of 27 unique CVD risk scores (Supplemental Data 3 and 7). Supplemental Figs. 9 and 10 provides the c-statistics from internal and external validation analyses, respectively. On both internal and external validation, discrimination was modest. Most risk scores were developed in the United States, Europe, and East Asia and 61.1% of the internal validation studies were assessed to be at a high risk of bias. Model performance tended to decline when validated in countries that differed from the development cohort (Supplemental Fig. 11). For example, the FDS study achieved high c-statistics (>0.80) when validated in an Australian cohort, but lower ones (0.58-0.69) when tested in European countries. In line with previous studies5–7, discrimination for the UKPDS and FRS was generally poor on external validation. Most prediction models focused on baseline characteristics and did not account for time-varying factors that may modify CVD risk (e.g., statin, SGLT-2i, GLP-1 RA). An exception was the BRAVO risk engine, published in 2020 and validated in trials of SGLT-2i patients, showing that this risk engine effectively predicted CV health benefits through improvements in common clinical measures (e.g., A1C, SBP, and BMI)343.
Supplemental Figs. 12, 13 provide the pooled c-statistics from external validation studies on those risk scores for which the analysis was possible: ADVANCE, CHS, CVD-EDIC, NDR, NZ DCS and UKPDS risk scores. All risk scores exhibited modest discrimination (pooled c-statistics ranging from 0.63 to 0.68), with no individual risk score substantially outperforming the others.
Supplemental Fig. 14a, b provide a histogram of the total number of adjusted covariates and number of adjusted traditional CVD risk factors in each of the studies, respectively. Supplemental Fig. 15 is a network figure representing the connections of the adjusted covariates in the 416 included studies.
Sensitivity analyses
The results of sensitivity analyses excluding studies with high risk of bias from meta-analyses of biomarkers, genetic risk score, and for risk scores where pooled analyses were possible, respectively, are shown in Supplemental Figs. 16–18.
Synthesis
Table 2 provides a summary of findings of studies assessing the most promising biomarkers and genetic markers/scores for precision prognosis of CVD in T2D, along with our conclusions regarding their predictive utility and strength of evidence. In our synthesis of the evidence, we took into account the results from the sensitivity analyses described in the previous paragraph. The highest predictive utility was observed for NT-proBNP (high-evidence), TnT (moderate-evidence), TyG (high-evidence), and GRS-CHD (moderate-evidence). Prognostic factors with moderate predictive utility were CCTA (low-evidence), SPECT scintigraphy (low-evidence), and PWV (moderate-evidence). Prognostic factors with low predictive utility included CRP (moderate-evidence), CACS (low-evidence), Gal-3 (low-evidence), TnI (low-evidence), carotid plaque (low-evidence), and GDF-15 (low-evidence). Supplemental Figs. 19–22, 23, 24 provide the quality assessment for the included biomarker, genetic marker, and risk score studies, respectively.
Table 2.
Conclusion and strength of the evidence.
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| Prognostic Biomarker | No. of studies with all 3 performance indicators satisfied | No. of pooled meta-analyses showing significant association | No. of pooled meta-analyses showing significant association (excluding high risk of bias) | Non-pooled analyses showed that >=75% of studies had significant association | Persistent association on sensitivity analysis for non-pooled analyses (excluding high risk of bias) | Consistency in definition of prognostic biomarker used in analysis | Conclusion (predictive utility) | Strength of Evidence |
| NT-proBNP | 3 | 2/2 | 2/2 | Yes | Yes | Yes | High | High |
| CRP | 0 | 1/2 | 1/2 | No | No | No | Low | Moderate |
| TnT | 4 | 2/3 | 1/3 | Yes | Yes | No | High | Moderate |
| CACS | 0 | 0 | 0 | Yes | No | No | Low | Low |
| CCTA | 2 | 0 | 0 | Yes | No | No | Moderate | Low |
| SPECT | 1 | 0 | 0 | Yes | No | No | Moderate | Low |
| PWV | 0 | 1/1 | 1/1 | Yes | Yes | No | Moderate | Moderate |
| Gal-3 | 0 | 0 | 0 | Yes | Yes | No | Low | Low |
| TnI | 1 | 0 | 0 | No | No | No | Low | Low |
| Carotid plaque | 0 | 0 | 0 | Yes | Yes | No | Low | Low |
| GDF-15 | 0 | 0 | 0 | No | No | No | Low | Low |
| TyG | 1 | 1/1 | NA | Yes | No | Yes | High | Moderate |
| GRS-CHD | 1 | 1/1 | 1/1 | Yes | No | No | High | Moderate |
Table includes genetic and non-genetic biomarkers showing improvement in prediction performance (i.e. at least one of c-statistic, NRI, IDI) and in more than 1 study (corresponding to all markers in green boxes in Fig. 4 and Fig. 5). Notes: Predictive utility was classified as high (3 point) moderate (2 points) or low (<2 points) based on the criteria defined in columns A, B and D (Column A: >0 = 1 point; Column B: >0 = 1 point, Column D: “Yes” = 1 point). Strength of Evidence was classified as High (4 points), moderate (2 or 3 points) and low (<2 points) based on criteria defined in columns B, C, E and F (Column B: at least one meta-analysis conducted [regardless of outcome] = 1 point; Column C: exclusion of high risk of bias studies did not alter inferences from meta-analyses [same number as in column B] = 1 point; Column E: exclusion of high risk of bias studies did not alter inferences from non-pooled analyses [YES] = 1 point; Column F: “yes” = 1 point).
Discussion
Our systematic review of prognostic markers for CVD in individuals with T2D has revealed several notable findings. First, among the numerous studies that investigated the prognostic significance of CVD risk markers, only a few have been consistently found to be significantly associated with cardiovascular risk. Namely, NT-proBNP, TnT, TyG, and GRS-CHD demonstrated the highest predictive utility, with NT-proBNP having the strongest evidence. However, most of the remaining markers have not been adequately tested or compared against established CVD risk factors. Finally, even though some markers have demonstrated the capability of predicting cardiovascular events beyond what current risk factor-based models can offer, their application in clinical practice remains limited, as there is inadequate evidence of their contemporary clinical utility.
During the search process, a considerable number of studies were found ineligible for inclusion in our systematic review. Available studies were primarily cross-sectional in design, and only a limited number of them focused specifically on individuals with T2D and examined the early utility of risk factors and biomarkers in predicting future cardiovascular events. A major limitation in many studies was inadequate adjustment for established CVD risk factors; and even if studies considered adjustments, only a small fraction evaluated clinical utility beyond the use of established risk factors. These findings emphasize the need for better-designed studies to improve our understanding of the prognostic value of markers for CVD in T2D.
Most studies included in the final analysis were conducted in people of European, East or South Asian ancestry, with the top-5 countries of recruitment being the United States, UK, China, Japan and Italy. African ancestry and countries were underrepresented. A skewed geographical distribution was also evident regarding countries of author affiliation, with the same top-5 countries dominating the volume of publications. Although the geographical and ancestral imbalance reported here for biomarker studies is less pronounced than what was recently reported for GWAS studies448, it highlights the pressing need to enhance data collection, biomarker discovery and validation, as well as the development of population-specific cardiovascular risk prediction models in underrepresented populations and ancestries to hopefully help reduce healthcare disparities449.
In our analyses, the novel biomarker emerging as the best predictor was NT-proBNP; indeed, it fulfilled all criteria of predictive and clinical utility with multiple studies showing improvement in all prediction performance indicators, with consistency of results across studies and meta-analyses. Notably, this biomarker had also been found to be useful as a prognostic marker for incident CVD in the general population450. Our findings suggest that NT-proBNP, beyond its established role in the diagnosis and management of patients with heart failure, might also be used as a marker to predict CVD. Another biomarker found in the general population to improve primary CVD risk prediction among asymptomatic middle-aged adults is high-sensitivity CRP (hs-CRP). In our review, CRP was found to have low predictive utility with moderate strength of evidence, which may be due to variability in cut-offs used for this marker, the relatively small numbers of studies, differential effects in diabetes, or less sensitive to detect low-grade vascular inflammation (compared with hs-CRP).
Despite numerous genetic studies probing the link between polymorphisms and cardiovascular outcomes in diabetes, few genetic markers have been consistently examined in longitudinal studies or reliably found to be associated with these outcomes. Only one study from the systematic review utilized a genome-wide association study (GWAS) approach, identifying the rs10911021 variant near GLUL to be associated with CV outcome in diabetes, at genome-wide significance. The variant at GLUL was subsequently confirmed in two independent studies172. A more recent GWAS conducted among Chinese patients with T2D identified a variant at PDE1A for CHD in T2D, which was not included in our systematic review as it fell beyond our study inclusion period451. Polygenic risk scores also appear to emerge as promising tools, and GRS constructed from variants associated with CHD in the general population seem helpful for cardiovascular risk stratification in diabetes257.
Based on these limited findings, it becomes clear that we need a greater number of adequately powered GWAS to identify genetic markers associated with CVD in T2D. Nevertheless, we found several examples of studies that evaluated the utility of applying polygenic risk scores, or genome-wide polygenic risk scores, derived from the general population, for CVD risk stratification in T2D. In general, these have fair performance and a similar ability to stratify as in patients without diabetes. Considering the substantially larger sample sizes in currently published meta-analyses of GWAS for CHD in the general population, this approach will probably be more fruitful for the integration of genetic markers into risk stratification of cardiovascular complications. In the limited studies that have evaluated the added benefit of polygenic risk scores above clinical markers, there is, in general, a modest but significant improvement in prediction. Whether polygenic risk scores will become viable options for future risk stratification would partly depend on the availability of these tools, and the cost-effectiveness of adding these measures into clinical practice.
Beyond individual prognostic markers, our review identified several studies that evaluated CVD risk prediction models. While the UKPDS risk engine (developed among subjects with newly diagnosed T2D the UK) and the Framingham risk equation (developed from the general population in the US) were the most widely studied, they do not perform well in contemporary studies of people with T2D. This suggests difficulties in applying certain risk models to current healthcare settings. Nevertheless, our literature review shows that clinical risk models are perhaps the “readiest” for implementation in clinical practice to improve risk stratification in diabetes. On external validation, newer risk scores generally achieved higher discrimination compared to UKPDS and FRS, with Fremantle Diabetes Study 2 (FDS-2) having the highest c-statistic of 0.81 (developed and validated in different populations in Australia). We found that risk models performed better when validated in cohorts similar to the derivation cohort, with c-statistics of 0.699 0.015 and 0.668 0.006 (95% CI) (P = 0.018) for concordant and discordant studies, respectively.
In an era when electronic medical record (EMR)-based prediction models are being increasingly used, our results suggest that researchers should focus on the development of population-specific risk models that are intended to be deployed in the same population from which they were developed since the goal should be to achieve the highest predictive accuracy rather than to find a generic model that performs modestly well in all settings. Despite their potential utility and low implementation costs, we found a paucity of evidence showing integration of risk engine calculators into clinical practice. We are aware of several notable exceptions. For example, the Joint Asia Diabetes Evaluation (JADE) program has incorporated several risk prediction algorithms derived from Asian patients with diabetes into a web-based e-health portal, together with a graphical interface and decision support452, and has been evaluated in different clinical settings, including in randomized clinical trials453–456. Many EMR systems offer quick calculations of CVD risk using the American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Equations based on inputs available in the patient’s record, and we recommend that future risk scores found to have high predictive accuracy be made easily accessible to clinicians within their EMR workflow.
Given the limitations and gaps that emerged from this review, we recommend that future studies follow several guidelines to improve the quality and impact of studies on precision prognostics in diabetes. First, studies attempting to identify a risk marker should be conducted in prospective or longitudinal cohorts or trials, to provide more robust and reliable data. Second, studies should have sufficient sample size and duration of follow-up (at least 3 years for primary CVD events and at least 1 year for secondary CVD events) to ensure adequate statistical power. Third, studies must adjust for a minimal set of established clinical cardiovascular risk factors, to ensure that known risk factors do not confound any observed associations. Finally, studies must attempt to explore the added utility of biomarkers by comparing against prediction using established risk factors or models, or available risk engines for cardiovascular events. This would include evaluation of the change in c-statistics after adding risk markers/biomarkers of interest but also consider including additional metrics such as NRI and IDI. We believe that if journals make these requirements mandatory when evaluating such studies, it will help ensure that research funders are made aware and future studies are best suited for informing advances in this area especially in resource-limited countries. As in any other research field, harmonization of protocols, methods, and analysis pipelines should be encouraged to allow comparisons across studies and for clinical translation.
There are several unique strengths of this work. To our knowledge, this represents one of the most comprehensive overviews of the current status of knowledge about risk stratification of cardiovascular outcomes in T2D. We included studies from 1990 onwards, to capture some of the older studies, as well as more contemporary studies. Our inclusion of “biomarkers” in the broadest term allowed us to provide an objective overview of the different approaches currently being explored for better risk stratification. Limiting the analyses to studies using longitudinal cohorts allowed us to focus on studies that would inform prognostication. Limiting analyses to “hard” cardiovascular endpoints, rather than also including surrogate endpoints such as carotid intima-medial thickness, allowed us to focus on endpoints that would be of greatest clinical relevance. However, while this approach allows us to maximize the translational approach of our analyses, future studies focused on identification of biomarkers associated with early disease-informative endpoints (i.e. subclinical markers of atherosclerosis or minor cardiovascular disease) might identify different novel biomarkers for early-stage cardiovascular complications.
Our study does have limitations. We had to omit a considerable number of cross-sectional studies due to the extensive scope of the systematic review and the explained focus on longitudinal studies. We included only English language publications. Our search terms, potentially more sensitive towards detecting studies on clinical risk factors and biomarkers than genetic factors, may have led to fewer genetic studies being identified. However, we managed to supplement this by reintegrating some missing articles using the identified literature and the investigators’ expertise.
In conclusion, our systematic review on prognostic markers for cardiovascular endpoints in T2D identified several findings, which to the best of our knowledge, have not been previously reported, and has revealed some important knowledge gaps. We found that NT-proBNP, TnT, TyG, and GRS-CHD had high predictive utility beyond traditional CVD risk factors, with the highest strength of evidence for NT-proBNP. Among genetic markers, there was only sufficient evidence for the polygenic risk score for CHD, and among risk scores, predictive utility was modest on external validation. Given the relatively low number of studies analyzing these novel prognostic factors using a rigorous approach, these findings support the need for future studies testing these markers with convincing demonstration of incremental predictive utility. NT-proBNP appears to be the only biomarker ready to be tested prospectively to evaluate its utility in modifying clinical practice for prediction of CVD risk.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
A.A., M.D-P., H.F., M.F.G. acknowledge support from the Swedish Heart-Lung Foundation (20190470), Swedish Research Council (EXODIAB, 2009-1039; 2018-02837), Swedish Foundation for Strategic Research (LUDC-IRC, 15-0067), EU H2020-JTI-lMl2-2015-05 (Grant agreement number 115974 – BEAt-DKD) to M.F.G. L-L.L. acknowledge UK Medical Research Council Population and Systems Medicine Board (IF048-2022). M.L.M. is supported by Italian Ministry of Health Grant “Ricerca Finalizzata 2019” – GR-2019-12369702. C.H.T.T., C.H, and R.C.W.M. acknowledge support from the Research Grants Council of the Hong Kong Special Administrative Region (CU R4012-18), the Croucher Foundation Senior Medical Research Fellowship, University Grants Committee Research Grants Matching Scheme and Research Committee Postdoctoral Fellowship Scheme of the Chinese University of Hong Kong. F.F.C. acknowledge the Second Affiliated Hospital of Chongqing Medical University (No. 2022cffkyqdj). T.C. is an international training fellow supported by the Wellcome Trust grant (214205/Z/18/Z). R.W.K. was funded by a Novo Nordisk Foundation (NNF18OC0031650) postdoctoral fellowship. G.Y. and R.C.W.M. acknowledges support from the Provost’s Scheme for PhD scholarship from the Chinese University of Hong Kong. Y.Z. acknowledges a Postgraduate Studentship and Vice-Chancellor’s PhD scholarship from the Chinese University of Hong Kong. The authors wish to acknowledge the support of librarians from Lund University, Maria Björklund and Krister Aronsson for their expert support with the literature search. N.M. is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK125780, R01DK134955).
Author contributions
Drs. M.F.G., R.C.W.M., and N.M. had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: R.C.W.M., N.M., M.F.G., A.A.M., A.A., L-L.L., R.W.K., S.C.T., C.H.T., M.D-P., F.C., S.S. Acquisition, analysis, or interpretation of data: R.C.W.M., N.M., A.A., M.F.G., M.D-P., C.H.T., R.W.K., F.C., S.C.T., L-L.L., S.S., A.A.M., T.C., M.P., M.L.M., H.F., S.K., G.Y., Y.Z., C.H. Drafting of the manuscript: R.C.W.M., N.M., M.F.G., A.A., M.L.M., L-L.L., C.H.T., H.F., S.K. Critical revision of the manuscript for important intellectual content: R.C.W.M., N.M., M.F.G., A.A., L-L.L., M.L.M., C.H.T., F.C., T.C., M.D-P., H.F., C.H., S.K., S.S., R.W.K., A.A.M., S.C.T., G.Y., Y.Z., M.P., D.S., R.J.dS., D.K.T. Statistical analysis: C.H.T., M.L.M., N.M. A.A., L-L.L., M.L.M., C.H.T. contributed equally to this work and are considered co-first authors. M.F.G., R.C.W.M., and N.M. supervised all aspects of this work and are considered co-senior authors.
Peer review
Peer review information
Communications Medicine thanks Xiao Liu, Jairo Arturo Pinzon Cortes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The protocol for this systematic review and meta-analysis is publicly available through the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD42021262843. Comprehensive search strategies that can be reproduced are outlined in Supplemental Note 1. Any further details required are available from the corresponding author upon reasonable request. Complete lists of the publications where data were extracted for this study are provided as Excel files in Supplemental Data 1 (list of studies on non-genetic biomarkers), Supplemental Data 2 (list of studies on genetic biomarkers), and Supplemental Data 3 (list of studies on non-genetic risk scores). Supplemental Data 4, 6, and 7 provide source data used to generate forest plots and meta-analyses. The data presented in Fig. 3 is also available as online interactive figures (https://hugofitipaldi.shinyapps.io/T2D_prognostic/) and in a data repository (https://zenodo.org/records/10277173)447.
Competing interests
The authors declare the following competing interests: L-L.L. has served as an advisory committee member for Bayer, Boehringer Ingelheim, Novo Nordisk, Procter & Gamble Health, and Viatris; and as a speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Roche, Sanofi, Servier, and Zuellig Pharma Therapeutics. She has also received research grants from Abbott, AstraZeneca, and Boehringer Ingelheim. M.L.M. received lecture fees, consultancy, or advisory board fees from Amarin, Amgen, Eli Lilly, Merck Sharp & Dohme, Mylan, Novo Nordisk, Servier, and SlaPharma, all not directly related to this manuscript. R.W.K. has received consulting fees from Novo Nordisk. M.F.G. has received financial and non-financial (in kind) support from Boehringer Ingelheim Pharma GmbH, JDRF International, Eli Lilly, AbbVie, Sanofi-Aventis, Astellas, Novo Nordisk A/S, Bayer AG within EU grant H2020-JTI-lMl2-2015-05 (Grant agreement number 115974 – BEAt-DKD). She has also received financial and in-kind support from Novo Nordisk, Pfizer, Follicum, Coegin Pharma, Abcentra, Probi, Johnson & Johnson within a project funded by the Swedish Foundation for Strategic Research on precision medicine in diabetes (LUDC-IRC #15-0067). Dr. Gomez has received personal consultancy fees from Lilly and Tribune Therapeutics AB. R.C.W.M. has received research grants from AstraZeneca, Bayer, Novo Nordisk, Pfizer, Roche Diagnostics (HK) Ltd, Tricida Inc, and consultancy/speaker honorarium from AstraZeneca, Boehringer Ingelheim, Bayer, Merck. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. R.C.W.M. is a co-founder of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU). R.J.dS. has served as an external resource person to the World Health Organization’s Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012-2017 to present and discuss this work. He has presented updates of this work to the WHO in 2022. He has also done contract research for the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has received speaker’s fees from the University of Toronto, and McMaster Children’s Hospital. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada), co-chair of the Method working group of the ADA/EASD Precision Medicine in Diabetes group and is a co-opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England). He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator, and is a co-investigator on several funded team grants from the Canadian Institutes of Health Research. All other authors have no competing interests to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Abrar Ahmad, Lee-Ling Lim, Mario Luca Morieri, Claudia Ha-ting Tam.
These authors jointly supervised this work: Maria F. Gomez, Ronald C.W. Ma, Nestoras Mathioudakis.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Maria F. Gomez, Email: maria.gomez@med.lu.se
Ronald C. W. Ma, Email: rcwma@cuhk.edu.hk
Nestoras Mathioudakis, Email: nmathio1@jh.edu.
ADA/EASD PMDI:
Deirdre K. Tobias, Jordi Merino, Catherine Aiken, Jamie L. Benham, Dhanasekaran Bodhini, Amy L. Clark, Kevin Colclough, Rosa Corcoy, Sara J. Cromer, Daisy Duan, Jamie L. Felton, Ellen C. Francis, Pieter Gillard, Véronique Gingras, Romy Gaillard, Eram Haider, Alice Hughes, Jennifer M. Ikle, Laura M. Jacobsen, Anna R. Kahkoska, Jarno L. T. Kettunen, Raymond J. Kreienkamp, Lee-Ling Lim, Jonna M. E. Männistö, Robert Massey, Niamh-Maire Mclennan, Rachel G. Miller, Jasper Most, Rochelle N. Naylor, Bige Ozkan, Kashyap Amratlal Patel, Scott J. Pilla, Katsiaryna Prystupa, Sridharan Raghavan, Mary R. Rooney, Martin Schön, Zhila Semnani-Azad, Magdalena Sevilla-Gonzalez, Pernille Svalastoga, Wubet Worku Takele, Claudia Ha-ting Tam, Anne Cathrine B. Thuesen, Mustafa Tosur, Amelia S. Wallace, Caroline C. Wang, Jessie J. Wong, Jennifer M. Yamamoto, Katherine Young, Chloé Amouyal, Mette K. Andersen, Maxine P. Bonham, Mingling Chen, Tinashe Chikowore, Sian C. Chivers, Christoffer Clemmensen, Dana Dabelea, Adem Y. Dawed, Aaron J. Deutsch, Laura T. Dickens, Linda A. DiMeglio, Carmella Evans-Molina, María Mercè Fernández-Balsells, Stephanie L. Fitzpatrick, Stephen E. Gitelman, Mark O. Goodarzi, Jessica A. Grieger, Marta Guasch-Ferré, Nahal Habibi, Torben Hansen, Chuiguo Huang, Arianna Harris-Kawano, Heba M. Ismail, Benjamin Hoag, Randi K. Johnson, Angus G. Jones, Robert W. Koivula, Aaron Leong, Gloria K. W. Leung, Ingrid M. Libman, Kai Liu, S. Alice Long, William L. Lowe, Jr., Robert W. Morton, Suna Onengut-Gumuscu, James S. Pankow, Maleesa Pathirana, Sofia Pazmino, Dianna Perez, John R. Petrie, Camille E. Powe, Alejandra Quinteros, Rashmi Jain, Debashree Ray, Mathias Ried-Larsen, Zeb Saeed, Vanessa Santhakumar, Sarah Kanbour, Sudipa Sarkar, Gabriela S. F. Monaco, Denise M. Scholtens, Elizabeth Selvin, Wayne Huey-Herng Sheu, Cate Speake, Maggie A. Stanislawski, Nele Steenackers, Andrea K. Steck, Norbert Stefan, Julie Støy, Rachael Taylor, Gebresilasea Gendisha Ukke, Marzhan Urazbayeva, Bart Van der Schueren, Camille Vatier, John M. Wentworth, Wesley Hannah, Sara L. White, Gechang Yu, Yingchai Zhang, Shao J. Zhou, Jacques Beltrand, Michel Polak, Ingvild Aukrust, Elisa de Franco, Sarah E. Flanagan, Kristin A. Maloney, Andrew McGovern, Janne Molnes, Mariam Nakabuye, Pål Rasmus Njølstad, Hugo Pomares-Millan, Cécile Saint-Martin, Cuilin Zhang, Yeyi Zhu, Sungyoung Auh, Russell de Souza, Andrea J. Fawcett, Chandra Gruber, Eskedar Getie Mekonnen, Emily Mixter, Diana Sherifali, Robert H. Eckel, John J. Nolan, Louis H. Philipson, Rebecca J. Brown, Liana K. Billings, Kristen Boyle, Tina Costacou, John M. Dennis, Jose C. Florez, Anna L. Gloyn, Peter A. Gottlieb, Siri Atma W. Greeley, Kurt Griffin, Andrew T. Hattersley, Irl B. Hirsch, Marie-France Hivert, Korey K. Hood, Jami L. Josefson, Soo Heon Kwak, Lori M. Laffel, Siew S. Lim, Ruth J. F. Loos, Ronald C. W. Ma, Chantal Mathieu, Nestoras Mathioudakis, James B. Meigs, Shivani Misra, Viswanathan Mohan, Rinki Murphy, Richard Oram, Katharine R. Owen, Susan E. Ozanne, Ewan R. Pearson, Wei Perng, Toni I. Pollin, Rodica Pop-Busui, Richard E. Pratley, Leanne M. Redman, Maria J. Redondo, Rebecca M. Reynolds, Robert K. Semple, Jennifer L. Sherr, Emily K. Sims, Arianne Sweeting, Tiinamaija Tuomi, Miriam S. Udler, Kimberly K. Vesco, Tina Vilsbøll, Robert Wagner, Stephen S. Rich, and Paul W. Franks
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-023-00429-z.
References
- 1.Rawshani A, et al. Mortality and cardiovascular disease in Type 1 and Type 2 diabetes. Nw Eng. J. Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 2.Pearson-Stuttard J, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrin. 2021;9:165–173. doi: 10.1016/S2213-8587(20)30431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, et al. Glycemia Reduction in Type 2 Diabetes - Glycemic Outcomes. N Engl J Med. 2022;387:1063–1074. doi: 10.1056/NEJMoa2200433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.I. D. F. Diabetes Atlas. Diabetes around the world in 2021, https://diabetesatlas.org/ (2021).
- 5.van Dieren S, et al. External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with Type 2 diabetes. Diabetologia. 2011;54:264–270. doi: 10.1007/s00125-010-1960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szymonifka J, et al. Cardiovascular disease risk prediction for people with type 2 diabetes in a population-based cohort and in electronic health record data. JAMIA Open. 2020;3:583–592. doi: 10.1093/jamiaopen/ooaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho JC, et al. Evaluation of available risk scores to predict multiple cardiovascular complications for patients with type 2 diabetes mellitus using electronic health records. Comp. Methods Prog. Biomed. Update. 2023;3:100087. doi: 10.1016/j.cmpbup.2022.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung WK, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD) Diabetes Care. 2020;43:1617–1635. doi: 10.2337/dci20-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan JJ, et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care. 2022;45:261–266. doi: 10.2337/dc21-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias DK, et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 2023;29:2438–2457. doi: 10.1038/s41591-023-02502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damen JA, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhiting G, et al. Cardiovascular disease risk prediction models in the Chinese population- a systematic review and meta-analysis. BMC Public Health. 2022;22:1608. doi: 10.1186/s12889-022-13995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damen JA, et al. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. BMC Med. 2019;17:109. doi: 10.1186/s12916-019-1340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Cabrera JL, Ankeny J, Fernández-Montero A, Kales SN, Smith DL. A systematic review and meta-analysis of advanced biomarkers for predicting incident cardiovascular disease among asymptomatic middle-aged adults. Int. J. Mol. Sci. 2022;23:13540. doi: 10.3390/ijms232113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Holten TC, et al. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8:e62080. doi: 10.1371/journal.pone.0062080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guasti L, et al. TMAO as a biomarker of cardiovascular events: a systematic review and meta-analysis. Intern. Emerg. Med. 2021;16:201–207. doi: 10.1007/s11739-020-02470-5. [DOI] [PubMed] [Google Scholar]
- 17.Rienks J, Barbaresko J, Nöthlings U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: a systematic review and meta-analysis of observational studies. Nutrients. 2017;9:415. doi: 10.3390/nu9040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohel V, Jones JA, Wehler CJ. Salivary biomarkers and cardiovascular disease: a systematic review. Clin. Chem. Lab Med. 2018;56:1432–1442. doi: 10.1515/cclm-2017-1018. [DOI] [PubMed] [Google Scholar]
- 19.Kanbay M, et al. Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis. Int. Urol. Nephrol. 2016;48:2029–2042. doi: 10.1007/s11255-016-1387-8. [DOI] [PubMed] [Google Scholar]
- 20.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017;6:e004947. doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace TC, Slavin M, Frankenfeld CL. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. 2016;8:32. doi: 10.3390/nu8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emadian A, Andrews RC, England CY, Wallace V, Thompson JL. The effect of macronutrients on glycaemic control: a systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groups. Br. J. Nutr. 2015;114:1656–1666. doi: 10.1017/S0007114515003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun H, Noh NI, Lee EY. Genetic risk scores used in cardiovascular disease prediction models: a systematic review. Rev. Cardiovasc. Med. 2022;23:8. doi: 10.31083/j.rcm2301008. [DOI] [PubMed] [Google Scholar]
- 24.Jeong EG, et al. Depth and combined infection is important predictor of lower extremity amputations in hospitalized diabetic foot ulcer patients. Korean J. Intern Med. 2018;33:952–960. doi: 10.3904/kjim.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin JS, et al. Nontraditional risk factors in cardiovascular disease risk assessment: updated evidence report and systematic review for the US preventive services task force. Jama. 2018;320:281–297. doi: 10.1001/jama.2018.4242. [DOI] [PubMed] [Google Scholar]
- 26.Hlatky MA, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells GA, S. B., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 29.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J. Stat. Soft. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 30.Owens DK, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions–agency for healthcare research and quality and the effective health-care program. J. Clin. Epidemiol. 2010;63:513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Aromataris E. et al. JBI Manual for Evidence Synthesis. JBI, 10.46658/JBIMES-20-01 (2020).
- 32.Aboyans V, et al. The prognosis of diabetic patients with high ankle-brachial index depends on the coexistence of occlusive peripheral artery disease. J. Vasc. Surg. 2011;53:984–991. doi: 10.1016/j.jvs.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Lebdeh HS, Hodge DO, Nguyen TT. Predictors of macrovascular disease in patients with Type 2 diabetes mellitus. Mayo Clin. Proc. 2001;76:707–712. doi: 10.4065/76.7.707. [DOI] [PubMed] [Google Scholar]
- 34.Afarideh M, et al. Complex association of serum alanine aminotransferase with the risk of future cardiovascular disease in Type 2 diabetes. Atherosclerosis. 2016;254:42–51. doi: 10.1016/j.atherosclerosis.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Afsharian S, et al. Risk factors for cardiovascular disease and mortality events in adults with Type 2 diabetes- a 10-year follow-up: Tehran lipid and glucose study. Diabetes Metab. Res. Rev. 2016;32:596–606. doi: 10.1002/dmrr.2776. [DOI] [PubMed] [Google Scholar]
- 36.Alele JD, Luttrell LM, Hollis BW, Luttrell DK, Hunt KJ. Relationship between vitamin D status and incidence of vascular events in the Veterans Affairs Diabetes Trial. Atherosclerosis. 2013;228:502–507. doi: 10.1016/j.atherosclerosis.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Alkhalaf A, et al. Sex specific association between carnosinase gene CNDP1 and cardiovascular mortality in patients with Type 2 diabetes (ZODIAC-22) J. Nephrol. 2015;28:201–207. doi: 10.1007/s40620-014-0096-6. [DOI] [PubMed] [Google Scholar]
- 38.Anand DV, Lahiri A, Lim E, Hopkins D, Corder R. The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated Type 2 diabetic subjects. J. Am. Coll. Cardiol. 2006;47:1850–1857. doi: 10.1016/j.jacc.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 39.Anand DV, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur. Heart J. 2006;27:713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 40.Anavekar, N. S. et al. Predictors of cardiovascular events in patients with Type 2 diabetic nephropathy and hypertension: a case for albuminuria. Kidney Int Suppl, 66 S50-55 (2004). [DOI] [PubMed]
- 41.Angiolillo DJ, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541–1547. doi: 10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 42.Anyanwagu U, Donnelly R, Idris I. Albuminuria regression and all-cause mortality among insulin-treated patients with Type 2 Diabetes: analysis of a large UK primary care Cohort. Am. J. Nephrol. 2019;49:146–155. doi: 10.1159/000496276. [DOI] [PubMed] [Google Scholar]
- 43.Apperloo EM, Pena MJ, de Zeeuw D, Denig P, Heerspink HJL. Individual variability in response to renin angiotensin aldosterone system inhibition predicts cardiovascular outcome in patients with Type 2 diabetes: a primary care cohort study. Diabetes Obes. Metab. 2018;20:1377–1383. doi: 10.1111/dom.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araki S, et al. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36:1248–1253. doi: 10.2337/dc12-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avogaro A, et al. Incidence of coronary heart disease in Type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes Care. 2007;30:1241–1247. doi: 10.2337/dc06-2558. [DOI] [PubMed] [Google Scholar]
- 46.Azab B, Chainani V, Shah N, McGinn JT. Neutrophil-lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4-year follow-up study. Angiology. 2013;64:456–465. doi: 10.1177/0003319712455216. [DOI] [PubMed] [Google Scholar]
- 47.Azevedo MJ, et al. Value of diagnostic tools for myocardial ischemia used in routine clinical practice to predict cardiac events in patients with type 2 diabetes mellitus: a prospective study. Arq. Bras. Endocrinol. Metab. 2006;50:46–52. doi: 10.1590/S0004-27302006000100007. [DOI] [PubMed] [Google Scholar]
- 48.Bacci S, et al. The ENPP1 Q121 variant predicts major cardiovascular events in high-risk individuals: evidence for interaction with obesity in diabetic patients. Diabetes. 2011;60:1000–1007. doi: 10.2337/db10-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Backhaus SJ, et al. Cardiac magnetic resonance myocardial feature tracking for optimized risk assessment after acute myocardial infarction in patients with Type 2 Diabetes. Diabetes. 2020;69:1540–1548. doi: 10.2337/db20-0001. [DOI] [PubMed] [Google Scholar]
- 50.Basu S, et al. Validation of risk Equations for complications of Type 2 diabetes (RECODe) using individual participant data from diverse longitudinal Cohorts in the U.S. Diabetes Care. 2018;41:586–595. doi: 10.2337/dc17-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of risk equations for complications Of Type 2 diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5:788–798. doi: 10.1016/S2213-8587(17)30221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bates RE, et al. Impact of stress testing for coronary artery disease screening in asymptomatic patients with diabetes mellitus: a community-based study in olmsted county, Minnesota. Mayo Clin. Proc. 2016;91:1535–1544. doi: 10.1016/j.mayocp.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaney KE, et al. Variant rs10911021 that associates with coronary heart disease in type 2 diabetes, is associated with lower concentrations of circulating HDL cholesterol and large HDL particles but not with amino acids. Cardiovasc. Diabetol. 2016;15:115. doi: 10.1186/s12933-016-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beilin J, Stanton KG, McCann VJ, Knuiman MW, Divitini ML. Microalbuminuria in Type 2 diabetes: an independent predictor of cardiovascular mortality. Aust. N Z J. Med. 1996;26:519–525. doi: 10.1111/j.1445-5994.1996.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 55.Bell KJL, et al. Prognostic impact of systolic blood pressure variability in people with diabetes. PLoS One. 2018;13:e0194084. doi: 10.1371/journal.pone.0194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berkelmans GFN, et al. Prediction of individual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500,000 patients with Type 2 diabetes mellitus. Eur Heart J. 2019;40:2899–2906. doi: 10.1093/eurheartj/ehy839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard S, et al. Relation between XbA1 apolipoprotein B gene polymorphism and cardiovascular risk in a Type 2 diabetic cohort. Atherosclerosis. 2004;175:177–181. doi: 10.1016/j.atherosclerosis.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Bernard S, et al. Incremental predictive value of carotid ultrasonography in the assessment of coronary risk in a cohort of asymptomatic Type 2 diabetic subjects. Diabetes Care. 2005;28:1158–1162. doi: 10.2337/diacare.28.5.1158. [DOI] [PubMed] [Google Scholar]
- 59.Bianco HT, et al. Relevance of target-organ lesions as predictors of mortality in patients with diabetes mellitus. Arq Bras Cardiol. 2014;103:272–281. doi: 10.5935/abc.20140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biscetti F, et al. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascularization in diabetic patients: a prospective cohort study. Cardiovasc. Diabetol. 2020;19:170. doi: 10.1186/s12933-020-01151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonito B, Silva AP, Rato F, Santos N, Neves PL. Resistin as a predictor of cardiovascular hospital admissions and renal deterioration in diabetic patients with chronic kidney disease. J. Diabetes Complications. 2019;33:107422. doi: 10.1016/j.jdiacomp.2019.107422. [DOI] [PubMed] [Google Scholar]
- 62.Bosevski M, Borozanov V, Tosev S, Georgievska-Ismail L. Is assessment of peripheral endothelial dysfunction useful tool for risk stratification of Type 2 diabetic patients with manifested coronary artery disease? Int. J. Cardiol. 2009;131:290–292. doi: 10.1016/j.ijcard.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Bouchi R, et al. Fluctuations in HbA1c are associated with a higher incidence of cardiovascular disease in Japanese patients with Type 2 diabetes. J. Diabetes Invest. 2012;3:148–155. doi: 10.1111/j.2040-1124.2011.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brownrigg JR, et al. Microvascular disease and risk of cardiovascular events among individuals with Type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 2016;4:588–597. doi: 10.1016/S2213-8587(16)30057-2. [DOI] [PubMed] [Google Scholar]
- 65.Bruce DG, Davis WA, Starkstein SE, Davis TM. A prospective study of depression and mortality in patients with Type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2005;48:2532–2539. doi: 10.1007/s00125-005-0024-3. [DOI] [PubMed] [Google Scholar]
- 66.Bruno G, Barutta F, Landi A, Cavallo Perin P, Gruden G. NT-proBNP linking low-moderately impaired renal function and cardiovascular mortality in diabetic patients: the population-based Casale Monferrato Study. PLoS One. 2014;9:e114855. doi: 10.1371/journal.pone.0114855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno G, et al. N-terminal probrain natriuretic peptide is a stronger predictor of cardiovascular mortality than C-reactive protein and albumin excretion rate in elderly patients with type 2 diabetes: the Casale Monferrato population-based study. Diabetes Care. 2013;36:2677–2682. doi: 10.2337/dc13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruno G, et al. Fibrinogen and AER are major independent predictors of 11-year cardiovascular mortality in Type 2 diabetes: the Casale Monferrato Study. Diabetologia. 2005;48:427–434. doi: 10.1007/s00125-004-1667-1. [DOI] [PubMed] [Google Scholar]
- 69.Bruno G, et al. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in Type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 70.Burgess DC, et al. Incidence and predictors of silent myocardial infarction in Type 2 diabetes and the effect of fenofibrate: an analysis from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Eur. Heart J. 2010;31:92–99. doi: 10.1093/eurheartj/ehp377. [DOI] [PubMed] [Google Scholar]
- 71.Busch M, et al. The advanced glycation end product N(epsilon)-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. Am. J. Kidney Dis. 2006;48:571–579. doi: 10.1053/j.ajkd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Böger CA, et al. RANTES gene polymorphisms predict all-cause and cardiac mortality in Type 2 diabetes mellitus hemodialysis patients. Atherosclerosis. 2005;183:121–129. doi: 10.1016/j.atherosclerosis.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Cardona A, et al. Trimethylamine N-oxide and incident atherosclerotic events in high-risk individuals with diabetes: an ACCORD trial post hoc analysis. BMJ Open Diabetes Res Care. 2019;7:e000718. doi: 10.1136/bmjdrc-2019-000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardoso CR, Ferreira MT, Leite NC, Salles GF. Prognostic impact of aortic stiffness in high-risk Type 2 diabetic patients: the Rio deJaneiro Type 2 Diabetes Cohort Study. Diabetes Care. 2013;36:3772–3778. doi: 10.2337/dc13-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardoso CR, Leite NC, Salles GF. Prognostic importance of C-Reactive protein in high cardiovascular risk patients with Type 2 diabetes mellitus: the Rio de Janeiro Type 2 diabetes Cohort Study. J. Am. Heart Assoc. 2016;5:e004554. doi: 10.1161/JAHA.116.004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cardoso CR, Salles GF, Deccache W. Prognostic value of QT interval parameters in Type 2 diabetes mellitus: results of a long-term follow-up prospective study. J. Diabetes Complications. 2003;17:169–178. doi: 10.1016/S1056-8727(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 77.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with Type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc. Diabetol. 2018;17:33. doi: 10.1186/s12933-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cardoso CRL, Leite NC, Salles GF. Prognostic importance of visit-to-visit blood pressure variability for micro- and macrovascular outcomes in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc. Diabetol. 2020;19:50. doi: 10.1186/s12933-020-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cardoso CRL, Salles GC, Leite NC, Salles GF. Prognostic impact of carotid intima-media thickness and carotid plaques on the development of micro- and macrovascular complications in individuals with type 2 diabetes: the Rio de Janeiro Type 2 diabetes cohort study. Cardiovasc. Diabetol. 2019;18:2. doi: 10.1186/s12933-019-0809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlsson AC, et al. Growth differentiation factor 15 (GDF-15) is a potential biomarker of both diabetic kidney disease and future cardiovascular events in cohorts of individuals with type 2 diabetes: a proteomics approach. Ups J. Med. Sci. 2020;125:37–43. doi: 10.1080/03009734.2019.1696430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carlsson AC, et al. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in Type 2 diabetes. Cardiovasc. Diabetol. 2016;15:40. doi: 10.1186/s12933-016-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carnethon MR, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casiglia E, et al. Cardiovascular mortality in non-insulin-dependent diabetes mellitus. A controlled study among 683 diabetics and 683 age- and sex-matched normal subjects. Eur. J. Epidemiol. 2000;16:677–684. doi: 10.1023/A:1007673123716. [DOI] [PubMed] [Google Scholar]
- 84.Cavalot F, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in Type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–2243. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cavalot F, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in Type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 2006;91:813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 86.Cea Soriano L, Johansson S, Stefansson B, Rodríguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with Type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc. Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cederholm J, et al. Risk prediction of cardiovascular disease in Type 2 diabetes: a risk equation from the Swedish National Diabetes Register. Diabetes Care. 2008;31:2038–2043. doi: 10.2337/dc08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Celis-Morales CA, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: evidence from UK Biobank, a prospective population-based Cohort study. Diabetes Care. 2017;40:1710–1718. doi: 10.2337/dc17-0921. [DOI] [PubMed] [Google Scholar]
- 89.Ceriello A, et al. Empagliflozin reduced long-term HbA1c variability and cardiovascular death: insights from the EMPA-REG OUTCOME trial. Cardiovasc. Diabetol. 2020;19:176. doi: 10.1186/s12933-020-01147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cha SA, et al. Time- and frequency-domain measures of heart rate variability predict cardiovascular outcome in patients with Type 2 diabetes. Diabetes Res. Clin. Pract. 2018;143:159–169. doi: 10.1016/j.diabres.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cha SA, et al. Diabetic cardiovascular autonomic neuropathy predicts recurrent cardiovascular diseases in patients with Type 2 Diabetes. PLoS One. 2016;11:e0164807. doi: 10.1371/journal.pone.0164807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chacko KM, et al. Heart rate recovery predicts mortality and cardiovascular events in patients with type 2 diabetes. Med. Sci. Sports Exerc. 2008;40:288–295. doi: 10.1249/mss.0b013e31815c4844. [DOI] [PubMed] [Google Scholar]
- 93.Chan JC, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am. J. Med. 2014;127:616–624. doi: 10.1016/j.amjmed.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 94.Chang CW, et al. The first harmonic of radial pulse as an early predictor of silent coronary artery disease and adverse cardiac events in Type 2 diabetic patients. Cardiol. Res. Pract. 2018;2018:5128626. doi: 10.1155/2018/5128626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang LH, et al. The ankle brachial index exhibits better association of cardiovascular prognosis than non-high-density lipoprotein cholesterol in Type 2 diabetes. Am. J. Med. Sci. 2016;351:492–498. doi: 10.1016/j.amjms.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 96.Chang LH, et al. Upstroke time per cardiac cycle is associated with cardiovascular prognosis in Type 2 diabetes. Endocr. Pract. 2019;25:1109–1116. doi: 10.4158/EP-2019-0078. [DOI] [PubMed] [Google Scholar]
- 97.Charlton-Menys V, et al. Apolipoproteins, cardiovascular risk and statin response in Type 2 diabetes: the collaborative atorvastatin diabetes study (CARDS) Diabetologia. 2009;52:218–225. doi: 10.1007/s00125-008-1176-8. [DOI] [PubMed] [Google Scholar]
- 98.Chen HS, et al. Subclinical hypothyroidism is a risk factor for nephropathy and cardiovascular diseases in Type 2 diabetic patients. Diabet. Med. 2007;24:1336–1344. doi: 10.1111/j.1464-5491.2007.02270.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen S, et al. The long-term effectiveness of metabolic control on cardiovascular disease in patients with diabetes in a real-world health care setting - A prospective diabetes management study. Prim. Care Diabetes. 2020;14:274–281. doi: 10.1016/j.pcd.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 100.Cheng F, et al. Shortened relative leukocyte telomere length is associated with prevalent and incident cardiovascular complications in Type 2 diabetes: analysis from the Hong Kong diabetes register. Diabetes Care. 2020;43:2257–2265. doi: 10.2337/dc20-0028. [DOI] [PubMed] [Google Scholar]
- 101.Christensen PK, et al. QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand J. Clin. Lab Invest. 2000;60:323–332. doi: 10.1080/003655100750046486. [DOI] [PubMed] [Google Scholar]
- 102.Christiansen MS, Hommel E, Magid E, Feldt-Rasmussen B. Orosomucoid in urine predicts cardiovascular and over-all mortality in patients with Type II diabetes. Diabetologia. 2002;45:115–120. doi: 10.1007/s125-002-8251-3. [DOI] [PubMed] [Google Scholar]
- 103.Christiansen MS, Hommel E, Magid E, Feldt-Rasmussen B. Orosomucoid in urine is a powerful predictor of cardiovascular mortality in normoalbuminuric patients with Type 2 diabetes at five years of follow-up. Diabetologia. 2005;48:386–393. doi: 10.1007/s00125-004-1630-1. [DOI] [PubMed] [Google Scholar]
- 104.Church E, et al. Relationship between estimated glomerular filtration rate and incident cardiovascular disease in an ethnically diverse primary care cohort. N Z Med. J. 2019;132:11–26. [PubMed] [Google Scholar]
- 105.Chyun DA, et al. Autonomic dysfunction independently predicts poor cardiovascular outcomes in asymptomatic individuals with Type 2 diabetes in the DIAD study. SAGE Open Med. 2015;3:2050312114568476. doi: 10.1177/2050312114568476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cioffi G, et al. Usefulness of subclinical left ventricular midwall dysfunction to predict cardiovascular mortality in patients with Type 2 diabetes mellitus. Am. J. Cardiol. 2014;113:1409–1414. doi: 10.1016/j.amjcard.2014.01.415. [DOI] [PubMed] [Google Scholar]
- 107.Clarke PM, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 108.Clarke PM, et al. Using the EQ-5D index score as a predictor of outcomes in patients with Type 2 diabetes. Med. Care. 2009;47:61–68. doi: 10.1097/MLR.0b013e3181844855. [DOI] [PubMed] [Google Scholar]
- 109.Cockcroft JR, et al. Pulse pressure predicts cardiovascular risk in patients with type 2 diabetes mellitus. Am. J. Hypertens. 2005;18:1463–1467. doi: 10.1016/j.amjhyper.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 110.Colombo M, et al. Apolipoprotein CIII and N-terminal prohormone b-type natriuretic peptide as independent predictors for cardiovascular disease in Type 2 diabetes. Atherosclerosis. 2018;274:182–190. doi: 10.1016/j.atherosclerosis.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 111.Cortigiani L, et al. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J. Am. Soc. Echocardiogr. 2014;27:742–748. doi: 10.1016/j.echo.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Cosson E, et al. Cardiovascular risk prediction is improved by adding asymptomatic coronary status to routine risk assessment in type 2 diabetic patients. Diabetes Care. 2011;34:2101–2107. doi: 10.2337/dc11-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cournot M, et al. Circulating concentrations of redox biomarkers do not improve the prediction of adverse cardiovascular events in patients with Type 2 diabetes mellitus. J Am Heart Assoc. 2018;7:e007397. doi: 10.1161/JAHA.117.007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cox AJ, et al. Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study. Cardiovasc. Diabetol. 2014;13:160. doi: 10.1186/s12933-014-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cox AJ, et al. Genetic risk score associations with cardiovascular disease and mortality in the Diabetes Heart Study. Diabetes Care. 2014;37:1157–1164. doi: 10.2337/dc13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cox AJ, et al. Usefulness of biventricular volume as a predictor of mortality in patients with diabetes mellitus (from the Diabetes Heart Study) Am. J. Cardiol. 2013;111:1152–1158. doi: 10.1016/j.amjcard.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui NH, Yang JM, Liu X, Wang XB. Poly(ADP-Ribose) Polymerase activity and coronary artery disease in Type 2 Diabetes mellitus: an observational and bidirectional mendelian randomization study. Arterioscler Thromb. Vasc. Biol. 2020;40:2516–2526. doi: 10.1161/ATVBAHA.120.314712. [DOI] [PubMed] [Google Scholar]
- 118.Daka B, et al. Low concentrations of serum testosterone predict acute myocardial infarction in men with Type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:35. doi: 10.1186/s12902-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davis TM, Coleman RL, Holman RR. Ethnicity and long-term vascular outcomes in Type 2 diabetes: a prospective observational study (UKPDS 83) Diabet Med. 2014;31:200–207. doi: 10.1111/dme.12353. [DOI] [PubMed] [Google Scholar]
- 120.Davis WA, Colagiuri S, Davis TM. Comparison of the Framingham and United Kingdom Prospective Diabetes Study cardiovascular risk equations in Australian patients with Type 2 diabetes from the Fremantle Diabetes Study. Med. J. Aust. 2009;190:180–184. doi: 10.5694/j.1326-5377.2009.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 121.Davis WA, Hellbusch V, Hunter ML, Bruce DG, Davis TME. Contemporary cardiovascular risk assessment for Type 2 diabetes including heart failure as an outcome: The fremantle diabetes study phase ii. J. Clin. Med. 2020;9:1428. doi: 10.3390/jcm9051428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davis WA, Knuiman MW, Davis TM. An Australian cardiovascular risk equation for Type 2 diabetes: the Fremantle Diabetes Study. Intern. Med. J. 2010;40:286–292. doi: 10.1111/j.1445-5994.2009.01958.x. [DOI] [PubMed] [Google Scholar]
- 123.Dayan A, et al. Coronary calcium score, albuminuria and inflammatory markers in Type 2 diabetic patients: associations and prognostic implications. Diabetes Res. Clin. Pract. 2012;98:98–103. doi: 10.1016/j.diabres.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 124.de Galan BE, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with Type 2 diabetes: the action in diabetes and vascular disease: preterax and diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia. 2009;52:2328–2336. doi: 10.1007/s00125-009-1484-7. [DOI] [PubMed] [Google Scholar]
- 125.De Lorenzo A, Lima RS, Siqueira-Filho AG, Pantoja MR. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am. J. Cardiol. 2002;90:827–832. doi: 10.1016/S0002-9149(02)02702-9. [DOI] [PubMed] [Google Scholar]
- 126.de Santiago A, García-Lledó A, Ramos E, Santiago C. Prognostic value of ECGs in patients with Type-2 diabetes mellitus without known cardiovascular disease. Rev. Esp. Cardiol. 2007;60:1035–1041. doi: 10.1157/13111235. [DOI] [PubMed] [Google Scholar]
- 127.de Vries TI, et al. Normal-range thyroid-stimulating hormone levels and cardiovascular events and mortality in Type 2 diabetes. Diabetes Res. Clin. Pract. 2019;157:107880. doi: 10.1016/j.diabres.2019.107880. [DOI] [PubMed] [Google Scholar]
- 128.Djaileb L, et al. Prognostic value of SPECT myocardial perfusion entropy in high-risk Type 2 diabetic patients. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1813–1821. doi: 10.1007/s00259-020-05110-4. [DOI] [PubMed] [Google Scholar]
- 129.Doney AS, et al. The FTO gene is associated with an atherogenic lipid profile and myocardial infarction in patients with Type 2 diabetes: a genetics of diabetes audit and research study in Tayside Scotland (Go-DARTS) study. Circ. Cardiovasc. Genet. 2009;2:255–259. doi: 10.1161/CIRCGENETICS.108.822320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality in Type 2 diabetes is associated with the glutathione S transferase theta-null genotype: a Go-DARTS study. Circulation. 2005;111:2927–2934. doi: 10.1161/CIRCULATIONAHA.104.509224. [DOI] [PubMed] [Google Scholar]
- 131.Doney ASF, et al. Association of common variation in the PPARA gene with incident myocardial infarction in individuals with Type 2 diabetes: A Go-DARTS study. Nuclear Receptor. 2005;3:4. doi: 10.1186/1478-1336-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Donnan PT, Donnelly L, New JP, Morris AD. Derivation and validation of a prediction score for major coronary heart disease events in a U.K. Type 2 diabetic population. Diabetes Care. 2006;29:1231–1236. doi: 10.2337/dc05-1911. [DOI] [PubMed] [Google Scholar]
- 133.Drinkwater JJ, et al. Retinopathy predicts stroke but not myocardial infarction in Type 2 diabetes: the Fremantle Diabetes Study Phase II. Cardiovasc. Diabetol. 2020;19:43. doi: 10.1186/s12933-020-01018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drury PL, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in Type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54:32–43. doi: 10.1007/s00125-010-1854-1. [DOI] [PubMed] [Google Scholar]
- 135.Duan JG, et al. Long-term risk of cardiovascular disease among Type 2 diabetic patients with asymptomatic intracranial atherosclerosis: a prospective cohort study. PLoS One. 2014;9:e106623. doi: 10.1371/journal.pone.0106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan JG, et al. Sex differences in epidemiology and risk factors of acute coronary syndrome in Chinese patients with Type 2 diabetes: a long-term prospective cohort study. PLoS One. 2015;10:e0122031. doi: 10.1371/journal.pone.0122031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eguchi K, Hoshide S, Kario K. Target home morning SBP be below 125 mmHg in Type 2 diabetes patients. J. Hypertens. 2018;36:1284–1290. doi: 10.1097/HJH.0000000000001683. [DOI] [PubMed] [Google Scholar]
- 138.Eguchi K, et al. Differential impact of left ventricular mass and relative wall thickness on cardiovascular prognosis in diabetic and nondiabetic hypertensive subjects. Am. Heart J. 2007;154:79.e79–15. doi: 10.1016/j.ahj.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 139.Eguchi K, et al. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with Type 2 diabetes. Am. J. Hypertens. 2009;22:46–51. doi: 10.1038/ajh.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eguchi K, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without Type 2 diabetes. Am. J. Hypertens. 2008;21:443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eguchi K, et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with Type 2 diabetes. Hypertens. Res. 2010;33:737–742. doi: 10.1038/hr.2010.61. [DOI] [PubMed] [Google Scholar]
- 142.Eijkelkamp WB, et al. Renal function and risk for cardiovascular events in Type 2 diabetic patients with hypertension: the RENAAL and LIFE studies. J. Hypertens. 2007;25:871–876. doi: 10.1097/HJH.0b013e328014953c. [DOI] [PubMed] [Google Scholar]
- 143.Eliasson B, et al. Clinical usefulness of different lipid measures for prediction of coronary heart disease in Type 2 diabetes: a report from the Swedish National Diabetes Register. Diabetes Care. 2011;34:2095–2100. doi: 10.2337/dc11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Elkeles RS, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with Type 2 diabetes: the PREDICT study. Eur. Heart J. 2008;29:2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
- 145.Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with Type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–1301. doi: 10.1111/j.1464-5491.2008.02581.x. [DOI] [PubMed] [Google Scholar]
- 146.Elley CR, Robinson E, Kenealy T, Bramley D, Drury PL. Derivation and validation of a new cardiovascular risk score for people with Type 2 diabetes: the new zealand diabetes cohort study. Diabetes Care. 2010;33:1347–1352. doi: 10.2337/dc09-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Estacio RO, Dale RA, Schrier R, Krantz MJ. Relation of reduction in urinary albumin excretion to ten-year cardiovascular mortality in patients with Type 2 diabetes and systemic hypertension. Am. J. Cardiol. 2012;109:1743–1748. doi: 10.1016/j.amjcard.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 148.Everett BM, et al. Troponin and cardiac events in stable ischemic heart disease and diabetes. N. Engl. J. Med. 2015;373:610–620. doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fadini GP, et al. p66Shc gene expression in peripheral blood mononuclear cells and progression of diabetic complications. Cardiovasc. Diabetol. 2018;17:16. doi: 10.1186/s12933-018-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fadini GP, Rigato M, Cappellari R, Bonora BM, Avogaro A. Long-term prediction of cardiovascular outcomes by circulating CD34+ and CD34+CD133+ stem cells in patients with Type 2 diabetes. Diabetes Care. 2017;40:125–131. doi: 10.2337/dc16-1755. [DOI] [PubMed] [Google Scholar]
- 151.Faghihi-Kashani S, et al. Fasting hyperinsulinaemia and 2-h glycaemia predict coronary heart disease in patients with Type 2 diabetes. Diabetes Metab. 2016;42:55–61. doi: 10.1016/j.diabet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 152.Faglia E, et al. Cardiac events in 735 type 2 diabetic patients who underwent screening for unknown asymptomatic coronary heart disease: 5-year follow-up report from the Milan Study on Atherosclerosis and Diabetes (MiSAD) Diabetes Care. 2002;25:2032–2036. doi: 10.2337/diacare.25.11.2032. [DOI] [PubMed] [Google Scholar]
- 153.Ferrarezi DA, et al. Allelic variations of the vitamin D receptor (VDR) gene are associated with increased risk of coronary artery disease in Type 2 diabetics: the DIABHYCAR prospective study. Diabetes Metab. 2013;39:263–270. doi: 10.1016/j.diabet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 154.Filippella M, et al. Ankle brachial pressure index usefulness as predictor factor for coronary heart disease in diabetic patients. J. Endocrinol. Invest. 2007;30:721–725. doi: 10.1007/BF03350808. [DOI] [PubMed] [Google Scholar]
- 155.Florkowski CM, Scott RS, Coope PA, Moir CL. Predictors of mortality from Type 2 diabetes mellitus in Canterbury, New Zealand; a ten-year cohort study. Diabetes Res Clin Pract. 2001;53:113–120. doi: 10.1016/S0168-8227(01)00246-7. [DOI] [PubMed] [Google Scholar]
- 156.Folsom AR, Chambless LE, Duncan BB, Gilbert AC, Pankow JS. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care. 2003;26:2777–2784. doi: 10.2337/diacare.26.10.2777. [DOI] [PubMed] [Google Scholar]
- 157.Fox CS, Sullivan L, D’Agostino RB, Sr, Wilson PW. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704–708. doi: 10.2337/diacare.27.3.704. [DOI] [PubMed] [Google Scholar]
- 158.Fragoso A, Mendes F, Silva AP, Neves PL. Insulin resistance as a predictor of cardiovascular morbidity and end-stage renal disease. J. Diabetes Complications. 2015;29:1098–1104. doi: 10.1016/j.jdiacomp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 159.Freemantle N, Danchin N, Calvi-Gries F, Vincent M, Home PD. Relationship of glycaemic control and hypoglycaemic episodes to 4-year cardiovascular outcomes in people with type 2 diabetes starting insulin. Diabetes Obes. Metab. 2016;18:152–158. doi: 10.1111/dom.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Friedman AN, Hunsicker LG, Selhub J, Bostom AG. Total plasma homocysteine and arteriosclerotic outcomes in Type 2 diabetes with nephropathy. J. Am. Soc. Nephrol. 2005;16:3397–3402. doi: 10.1681/ASN.2004100846. [DOI] [PubMed] [Google Scholar]
- 161.Fukushima H, et al. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J. Am. Coll. Cardiol. 2004;43:2219–2224. doi: 10.1016/j.jacc.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 162.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44:S54–64. doi: 10.1007/PL00002940. [DOI] [PubMed] [Google Scholar]
- 163.Fumisawa Y, et al. Systematic analysis of risk factors for coronary heart disease in Japanese patients with Type 2 diabetes: a matched case-control study. J. Atheroscler. Thromb. 2012;19:918–923. doi: 10.5551/jat.13334. [DOI] [PubMed] [Google Scholar]
- 164.Gasior M, et al. Effect of blood glucose levels on prognosis in acute myocardial infarction in patients with and without diabetes, undergoing percutaneous coronary intervention. Cardiol. J. 2008;15:422–430. [PubMed] [Google Scholar]
- 165.Gazzaruso C, et al. Transcutaneous oxygen tension as a potential predictor of cardiovascular events in Type 2 diabetes: comparison with ankle-brachial index. Diabetes Care. 2013;36:1720–1725. doi: 10.2337/dc12-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gazzaruso C, et al. Lipoprotein(a), apolipoprotein(a) polymorphism and restenosis after intracoronary stent placement in Type 2 diabetic patients. J. Diabetes Complications. 2003;17:135–140. doi: 10.1016/S1056-8727(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 167.Gazzaruso C, et al. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J. Am. Coll. Cardiol. 2008;51:2040–2044. doi: 10.1016/j.jacc.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 168.Georgoulias P, et al. Long-term prognostic value of heart-rate recovery after treadmill testing in patients with diabetes mellitus. Int. J. Cardiol. 2009;134:67–74. doi: 10.1016/j.ijcard.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 169.Gimeno-Orna JA, Lou-Arnal LM, Boned-Juliani B, Molinero-Herguedas E. Mild renal insufficiency as a cardiovascular risk factor in non-proteinuric Type II diabetes. Diabetes Res. Clin. Pract. 2004;64:191–199. doi: 10.1016/j.diabres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 170.Giorda CB, et al. Recurrence of cardiovascular events in patients with Type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31:2154–2159. doi: 10.2337/dc08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giovacchini G, et al. Microalbuminuria predicts silent myocardial ischaemia in Type 2 diabetes patients. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:548–557. doi: 10.1007/s00259-012-2323-5. [DOI] [PubMed] [Google Scholar]
- 172.Group, T. L. A. R. Prospective Association of GLUL rs10911021 with cardiovascular morbidity and mortality among individuals with Type 2 diabetes: The Look AHEAD Study. Diabetes65, 297–302, (2015). [DOI] [PMC free article] [PubMed]
- 173.Guzder RN, Gatling W, Mullee MA, Byrne CD. Impact of metabolic syndrome criteria on cardiovascular disease risk in people with newly diagnosed Type 2 diabetes. Diabetologia. 2006;49:49–55. doi: 10.1007/s00125-005-0063-9. [DOI] [PubMed] [Google Scholar]
- 174.Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD. Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed Type 2 diabetes: results from a United Kingdom study. Diabet Med. 2005;22:554–562. doi: 10.1111/j.1464-5491.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 175.Hadaegh F, et al. Electrocardiography-defined silent CHD and risk of cardiovascular events among diabetic patients in a Middle Eastern population. Eur. J. Prev. Cardiol. 2012;19:1227–1233. doi: 10.1177/1741826711428065. [DOI] [PubMed] [Google Scholar]
- 176.Hadjadj S, et al. Prognostic value of the insertion/deletion polymorphism of the ACE gene in Type 2 diabetic subjects: results from the Non-insulin-dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR), Diabetes. Diabetes Care. 2008;31:1847–1852. doi: 10.2337/dc07-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hage FG, et al. The heart rate response to adenosine: a simple predictor of adverse cardiac outcomes in asymptomatic patients with type 2 diabetes. Int. J. Cardiol. 2013;167:2952–2957. doi: 10.1016/j.ijcard.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Halon DA, et al. Coronary computed tomography (CT) angiography as a predictor of cardiac and noncardiac vascular events in asymptomatic Type 2 diabetics: a 7-year population-based Cohort Study. J. Am. Heart Assoc. 2016;5:e003226. doi: 10.1161/JAHA.116.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Halon DA, et al. Plaque morphology as predictor of late plaque events in patients with asymptomatic Type 2 diabetes. A Long-Term Observational Study. JACC Cardiovasc. Imaging. 2019;12:1353–1363. doi: 10.1016/j.jcmg.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 180.Hamada S, Gulliford MC. Multiple risk factor control, mortality and cardiovascular events in Type 2 diabetes and chronic kidney disease: a population-based cohort study. BMJ Open. 2018;8:e019950. doi: 10.1136/bmjopen-2017-019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hanefeld M, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 182.Hata J, et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with Type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128:1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 183.Hayashi T, et al. Metabolic predictors of ischemic heart disease and cerebrovascular attack in elderly diabetic individuals: difference in risk by age. Cardiovasc Diabetol. 2013;12:10. doi: 10.1186/1475-2840-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 185.He Q, Pan J, Wang L, Fang Y, Hu R. Prospective study: Aldehyde dehydrogenase 2 gene is associated with cardio-cerebrovascular complications in Type 2 diabetes patients. J. Diabetes Investig. 2021;12:1845–1854. doi: 10.1111/jdi.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heidari B, et al. Assessment of serum 25-hydroxy vitamin D improves coronary heart disease risk stratification in patients with Type 2 diabetes. Am. Heart J. 2015;170:573–579.e575. doi: 10.1016/j.ahj.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 187.Heijmans BT, et al. Common paraoxonase gene variants, mortality risk and fatal cardiovascular events in elderly subjects. Atherosclerosis. 2000;149:91–97. doi: 10.1016/S0021-9150(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 188.Ho JS, et al. Association of the PPARG Pro12Ala polymorphism with Type 2 diabetes and incident coronary heart disease in a Hong Kong Chinese population. Diabetes Res. Clin. Pract. 2012;97:483–491. doi: 10.1016/j.diabres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 189.Hoffmann MM, März W, Genser B, Drechsler C, Wanner C. Lack of association between the Trp719Arg polymorphism in kinesin-like protein-6 and cardiovascular risk and efficacy of atorvastatin among subjects with diabetes on dialysis: the 4D study. Atherosclerosis. 2011;219:659–662. doi: 10.1016/j.atherosclerosis.2011.07.126. [DOI] [PubMed] [Google Scholar]
- 190.Hong LF, et al. Predictive value of non-fasting remnant cholesterol for short-term outcome of diabetics with new-onset stable coronary artery disease. Lipids Health Dis. 2017;16:7. doi: 10.1186/s12944-017-0410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Howard BV, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler Thromb Vasc. Biol. 2000;20:830–835. doi: 10.1161/01.ATV.20.3.830. [DOI] [PubMed] [Google Scholar]
- 192.Hsieh YT, et al. Subnormal estimated glomerular filtration rate strongly predict incident cardiovascular events in Type 2 diabetic chinese population with normoalbuminuria. Medicine (Baltimore) 2016;95:e2200. doi: 10.1097/MD.0000000000002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu CP, et al. Platelet distribution width on admission predicts in-stent restenosis in patients with coronary artery disease and Type 2 diabetes mellitus treated with percutaneous coronary intervention. Chin. Med. J. (Engl) 2018;131:757–763. doi: 10.4103/0366-6999.228247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang XH, et al. Angiotensin-converting enzyme gene polymorphism is associated with coronary heart disease in non-insulin-dependent diabetic patients evaluated for 9 years. Metabolism. 1998;47:1258–1262. doi: 10.1016/S0026-0495(98)90333-X. [DOI] [PubMed] [Google Scholar]
- 195.Hunt KJ, et al. Plasma connective tissue growth factor (CTGF/CCN2) levels predict myocardial infarction in the veterans affairs diabetes trial (VADT) Cohort. Diabetes Care. 2018;41:840–846. doi: 10.2337/dc17-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iijima K, et al. Lower physical activity is a strong predictor of cardiovascular events in elderly patients with type 2 diabetes mellitus beyond traditional risk factors: the Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. 2012;12:77–87. doi: 10.1111/j.1447-0594.2011.00815.x. [DOI] [PubMed] [Google Scholar]
- 197.Ikeda Y, et al. Low human paraoxonase predicts cardiovascular events in Japanese patients with type 2 diabetes. Acta Diabetol. 2009;46:239–242. doi: 10.1007/s00592-008-0066-3. [DOI] [PubMed] [Google Scholar]
- 198.Irie Y, et al. The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis. 2013;230:399–405. doi: 10.1016/j.atherosclerosis.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 199.Jeevarethinam A, et al. Usefulness of carotid plaques as predictors of obstructive coronary artery disease and cardiovascular events in asymptomatic individuals with diabetes mellitus. Am. J. Cardiol. 2018;121:910–916. doi: 10.1016/j.amjcard.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 200.Jha D, et al. Prognostic role of soluble ST2 in acute coronary syndrome with diabetes. Eur. J. Clin. Invest. 2018;48:e12994. doi: 10.1111/eci.12994. [DOI] [PubMed] [Google Scholar]
- 201.Jiang R, et al. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27:1991–1997. doi: 10.2337/diacare.27.8.1991. [DOI] [PubMed] [Google Scholar]
- 202.Jimenez-Corona A, et al. Electrocardiographic abnormalities predict deaths from cardiovascular disease and ischemic heart disease in Pima Indians with Type 2 diabetes. Am. Heart J. 2006;151:1080–1086. doi: 10.1016/j.ahj.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 203.Jin JL, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann. Med. 2018;50:576–586. doi: 10.1080/07853890.2018.1523549. [DOI] [PubMed] [Google Scholar]
- 204.Johnston SS, et al. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with Type 2 diabetes. Diabetes Care. 2011;34:1164–1170. doi: 10.2337/dc10-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Juutilainen A, Lehto S, Suhonen M, Rönnemaa T, Laakso M. Thoracoabdominal calcifications predict cardiovascular disease mortality in Type 2 diabetic and nondiabetic subjects: 18-year follow-up study. Diabetes Care. 2010;33:583–585. doi: 10.2337/dc09-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kamoi K, Ito T, Miyakoshi M, Minagawa S. Usefulness of home blood pressure measurement in the morning in patients with type 2 diabetes: long-term results of a prospective longitudinal study. Clin. Exp. Hypertens. 2010;32:184–192. doi: 10.3109/10641960903254513. [DOI] [PubMed] [Google Scholar]
- 207.Katakami N, et al. Accumulation of oxidative stress-related gene polymorphisms and the risk of coronary heart disease events in patients with Type 2 diabetes–an 8-year prospective study. Atherosclerosis. 2014;235:408–414. doi: 10.1016/j.atherosclerosis.2014.05.936. [DOI] [PubMed] [Google Scholar]
- 208.Katakami N, et al. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc. Diabetol. 2014;13:128. doi: 10.1186/s12933-014-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Katakami N, et al. Ultrasonic tissue characterization of carotid plaque improves the prediction of cardiovascular events in diabetic patients: a pilot study. Diabetes Care. 2012;35:2640–2646. doi: 10.2337/dc12-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawasaki R, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan diabetes complications study. Ophthalmology. 2013;120:574–582. doi: 10.1016/j.ophtha.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 211.Keavney BD, et al. UK prospective diabetes study (UKPDS) 14: association of angiotensin-converting enzyme insertion/deletion polymorphism with myocardial infarction in NIDDM. Diabetologia. 1995;38:948–952. doi: 10.1007/BF00400584. [DOI] [PubMed] [Google Scholar]
- 212.Keller T, et al. Prognostic value of high-sensitivity versus conventional cardiac Troponin T assays among patients with Type 2 diabetes mellitus undergoing maintenance hemodialysis. Am. J. Kidney Dis. 2018;71:822–830. doi: 10.1053/j.ajkd.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 213.Kenealy T, et al. An association between ethnicity and cardiovascular outcomes for people with Type 2 diabetes in New Zealand. Diabet. Med. 2008;25:1302–1308. doi: 10.1111/j.1464-5491.2008.02593.x. [DOI] [PubMed] [Google Scholar]
- 214.Kengne AP, et al. The Framingham and UK Prospective Diabetes Study (UKPDS) risk equations do not reliably estimate the probability of cardiovascular events in a large ethnically diverse sample of patients with diabetes: the Action in Diabetes and Vascular Disease: Preterax. Diabetologia. 2010;53:821–831. doi: 10.1007/s00125-010-1681-4. [DOI] [PubMed] [Google Scholar]
- 215.Kengne AP, et al. Contemporary model for cardiovascular risk prediction in people with Type 2 diabetes. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18:393–398. doi: 10.1177/1741826710394270. [DOI] [PubMed] [Google Scholar]
- 216.Khalili S, Hatami M, Hadaegh F, Sheikholeslami F, Azizi F. Prediction of cardiovascular events with consideration of general and central obesity measures in diabetic adults: results of the 8.4-year follow-up. Metab. Syndr. Relat. Disord. 2012;10:218–224. doi: 10.1089/met.2011.0070. [DOI] [PubMed] [Google Scholar]
- 217.Kim MK, et al. Hemoglobin glycation index predicts cardiovascular disease in people with Type 2 diabetes mellitus: A 10-year longitudinal cohort study. J. Diabetes Complications. 2018;32:906–910. doi: 10.1016/j.jdiacomp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 218.Koch M, Kutkuhn B, Grabensee B, Ritz E. Apolipoprotein A, fibrinogen, age, and history of stroke are predictors of death in dialysed diabetic patients: a prospective study in 412 subjects. Nephrol Dial Transplant. 1997;12:2603–2611. doi: 10.1093/ndt/12.12.2603. [DOI] [PubMed] [Google Scholar]
- 219.Koo BK, Chung WY, Moon MK. Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: a prospective cohort study. Cardiovasc. Diabetol. 2020;19:82. doi: 10.1186/s12933-020-01062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kuricová K, et al. NOS3 894G>T polymorphism is associated with progression of kidney disease and cardiovascular morbidity in Type 2 diabetic patients: NOS3 as a modifier gene for diabetic nephropathy? Kidney Blood Press Res. 2013;38:92–98. doi: 10.1159/000355757. [DOI] [PubMed] [Google Scholar]
- 221.Lagani V, et al. Development and validation of risk assessment models for diabetes-related complications based on the DCCT/EDIC data. J. Diabetes Complications. 2015;29:479–487. doi: 10.1016/j.jdiacomp.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 222.Lapin BR, et al. Pain in patients with Type 2 diabetes-related polyneuropathy is associated with vascular events and mortality. J. Clin. Endocrinol. Metab. 2020;105:dgaa394. doi: 10.1210/clinem/dgaa394. [DOI] [PubMed] [Google Scholar]
- 223.Lau KK, et al. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with Type 2 diabetes. Cardiovasc. Diabetol. 2012;11:101. doi: 10.1186/1475-2840-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le Feuvre CL, et al. Stress myocardial scintigraphy and dobutamine echocardiography in the detection of coronary disease in asymptomatic patients with Type 2 diabetes. Diabetes Metab. 2005;31:135–142. doi: 10.1016/S1262-3636(07)70179-9. [DOI] [PubMed] [Google Scholar]
- 225.Lee KY, et al. Computed tomography angiography images of coronary artery stenosis provide a better prediction of risk than traditional risk factors in asymptomatic individuals with Type 2 DIABETES: a long-term study of clinical outcomes. Diabetes Care. 2017;40:1241–1248. doi: 10.2337/dc16-1844. [DOI] [PubMed] [Google Scholar]
- 226.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc. Biol. 1996;16:978–983. doi: 10.1161/01.ATV.16.8.978. [DOI] [PubMed] [Google Scholar]
- 227.Lepojärvi ES, et al. Usefulness of highly sensitive troponin as a predictor of short-term outcome in patients with diabetes mellitus and stable coronary artery disease (from the ARTEMIS Study) Am. J. Cardiol. 2016;117:515–521. doi: 10.1016/j.amjcard.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 228.Levy AP, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J. Am. Coll. Cardiol. 2002;40:1984–1990. doi: 10.1016/S0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 229.Li PI, Wang JN, Guo HR. A long-term quality-of-care score for predicting the occurrence of macrovascular diseases in patients with Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018;139:72–80. doi: 10.1016/j.diabres.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 230.Li WP, et al. Pregnancy-associated plasma protein-A is a stronger predictor for adverse cardiovascular outcomes after acute coronary syndrome in Type-2 diabetes mellitus. Cardiovasc. Diabetol. 2017;16:45. doi: 10.1186/s12933-017-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liao KM, et al. Risk assessment of macrovascular and microvascular events in patients with Type 2 diabetes by analyzing the amplitude variation of the fourth harmonic component of radial pulse wave. Physiol. Rep. 2019;7:e14252. doi: 10.14814/phy2.14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lièvre MM, et al. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials. 2011;12:23. doi: 10.1186/1745-6215-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lim LL, et al. Sudomotor dysfunction independently predicts incident cardiovascular-renal events and all-cause death in Type 2 diabetes: the Joint Asia Diabetes Evaluation register. Nephrol. Dial. Transplant. 2019;34:1320–1328. doi: 10.1093/ndt/gfy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lim S, et al. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis. 2008;196:398–404. doi: 10.1016/j.atherosclerosis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 235.Lin CH, Li HY, Jiang YD, Chang TJ, Chuang LM. Plasma YKL-40 predicts 10-year cardiovascular and all-cause mortality in individuals with Type 2 diabetes. Clin. Endocrinol. (Oxf) 2013;79:185–191. doi: 10.1111/cen.12015. [DOI] [PubMed] [Google Scholar]
- 236.Lin EH, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33:264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lin LY, et al. The ankle brachial index exhibits better association with cardiovascular outcomes than interarm systolic blood pressure difference in patients with type 2 diabetes. Medicine (Baltimore) 2019;98:e15556. doi: 10.1097/MD.0000000000015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Linnemann B, Janka HU. Prolonged QTc interval and elevated heart rate identify the Type 2 diabetic patient at high risk for cardiovascular death. The Bremen Diabetes Study. Exp. Clin. Endocrinol. Diabetes. 2003;111:215–222. doi: 10.1055/s-2003-40466. [DOI] [PubMed] [Google Scholar]
- 239.Linnemann B, Voigt W, Nobel W, Janka HU. C-reactive protein is a strong independent predictor of death in Type 2 diabetes: association with multiple facets of the metabolic syndrome. Exp. Clin. Endocrinol. Diabetes. 2006;114:127–134. doi: 10.1055/s-2006-924012. [DOI] [PubMed] [Google Scholar]
- 240.Lopes-Virella MF, Hunt KJ, Baker NL, Virella G, Moritz T. The levels of MDA-LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis. 2012;224:526–531. doi: 10.1016/j.atherosclerosis.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lorenzo-Almorós A, et al. Galectin-3 is associated with cardiovascular events in post-acute coronary syndrome patients with Type-2 diabetes. J. Clin. Med. 2020;9:1105. doi: 10.3390/jcm9041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lowe G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with Type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63:1115–1123. doi: 10.2337/db12-1625. [DOI] [PubMed] [Google Scholar]
- 243.Lu TM, Lin SJ, Lin MW, Hsu CP, Chung MY. The association of dimethylarginine dimethylaminohydrolase 1 gene polymorphism with Type 2 diabetes: a cohort study. Cardiovasc. Diabetol. 2011;10:16. doi: 10.1186/1475-2840-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lu W, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in Type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 245.Lutgers HL, et al. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in Type 2 diabetes mellitus. Diabetologia. 2009;52:789–797. doi: 10.1007/s00125-009-1308-9. [DOI] [PubMed] [Google Scholar]
- 246.Masi S, et al. Telomere length, antioxidant status and incidence of ischaemic heart disease in Type 2 diabetes. Int. J. Cardiol. 2016;216:159–164. doi: 10.1016/j.ijcard.2016.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Massardo T, et al. Factors associated with silent myocardial ischemia, autonomic or peripheral neuropathies, and survival in diabetes mellitus type 2 patients without cardiovascular symptoms. Int. J. Diabetes Developing Countries. 2020;40:80–86. doi: 10.1007/s13410-019-00758-7. [DOI] [Google Scholar]
- 248.McEwan P, Bennett H, Ward T, Bergenheim K. Refitting of the UKPDS 68 risk equations to contemporary routine clinical practice data in the UK. Pharmacoeconomics. 2015;33:149–161. doi: 10.1007/s40273-014-0225-z. [DOI] [PubMed] [Google Scholar]
- 249.McMurray JJ, et al. Predictors of fatal and nonfatal cardiovascular events in patients with Type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the trial to reduce cardiovascular events with aranesp (darbepoetin-alfa) therapy (TREAT) Am. Heart J. 2011;162:748–755.e743. doi: 10.1016/j.ahj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 250.Meerwaldt R, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107–112. doi: 10.2337/dc06-1391. [DOI] [PubMed] [Google Scholar]
- 251.Mellbin LG, et al. Copeptin, IGFBP-1, and cardiovascular prognosis in patients with Type 2 diabetes and acute myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care. 2010;33:1604–1606. doi: 10.2337/dc10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mentz RJ, et al. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with Type 2 diabetes mellitus: insights from the EXSCEL trial. J. Am. Heart Assoc. 2018;7:e009304. doi: 10.1161/JAHA.118.009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mohammedi K, et al. Plasma extracellular superoxide dismutase concentration, allelic variations in the SOD3 gene and risk of myocardial infarction and all-cause mortality in people with Type 1 and Type 2 diabetes. Cardiovasc. Diabetol. 2015;14:845. doi: 10.1186/s12933-014-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mohammedi K, et al. Absence of peripheral pulses and risk of major vascular outcomes in patients with Type 2 diabetes. Diabetes Care. 2016;39:2270–2277. doi: 10.2337/dc16-1594. [DOI] [PubMed] [Google Scholar]
- 255.Monseu M, et al. Acute kidney injury predicts major adverse outcomes in diabetes: synergic impact with low glomerular filtration rate and albuminuria. Diabetes Care. 2015;38:2333–2340. doi: 10.2337/dc15-1222. [DOI] [PubMed] [Google Scholar]
- 256.Moosaie F, et al. Lp(a) and Apo-lipoproteins as predictors for micro- and macrovascular complications of diabetes: A case-cohort study. Nutr. Metab. Cardiovasc. Dis. 2020;30:1723–1731. doi: 10.1016/j.numecd.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 257.Morieri ML, et al. Genetic tools for coronary risk assessment in Type 2 diabetes: a Cohort study from the ACCORD clinical trial. Diabetes Care. 2018;41:2404–2413. doi: 10.2337/dc18-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Morrish NJ, Stevens LK, Fuller JH, Jarrett RJ, Keen H. Risk factors for macrovascular disease in diabetes mellitus: the London follow-up to the WHO multinational study of vascular disease in diabetics. Diabetologia. 1991;34:590–594. doi: 10.1007/BF00400279. [DOI] [PubMed] [Google Scholar]
- 259.Mukamal KJ, et al. Prediction and classification of cardiovascular disease risk in older adults with diabetes. Diabetologia. 2013;56:275–283. doi: 10.1007/s00125-012-2772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nag S, et al. All-cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabet Med. 2007;24:10–17. doi: 10.1111/j.1464-5491.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 261.Nagamachi S, et al. Prognostic value of cardiac I-123 metaiodobenzylguanidine imaging in patients with non-insulin-dependent diabetes mellitus. J. Nucl. Cardiol. 2006;13:34–42. doi: 10.1016/j.nuclcard.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 262.Nakamura M, et al. Brachial-ankle pulse wave velocity as a risk stratification index for the short-term prognosis of Type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010;33:1018–1024. doi: 10.1038/hr.2010.126. [DOI] [PubMed] [Google Scholar]
- 263.Nam GE, et al. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with Type 2 diabetes: a nationwide Cohort Study. Diabetes Care. 2020;43:2234–2241. doi: 10.2337/dc19-2552. [DOI] [PubMed] [Google Scholar]
- 264.Nargesi AA, et al. Nonlinear relation between pulse pressure and coronary heart disease in patients with Type 2 diabetes or hypertension. J. Hypertens. 2016;34:974–980. doi: 10.1097/HJH.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 265.Nazimek-Siewniak B, Moczulski D, Grzeszczak W. Risk of macrovascular and microvascular complications in Type 2 diabetes: results of longitudinal study design. J. Diabetes Complications. 2002;16:271–276. doi: 10.1016/S1056-8727(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 266.Ndrepepa G, et al. Prognostic value of uric acid in patients with Type 2 diabetes mellitus and coronary artery disease. Clin. Sci. (Lond) 2013;124:259–268. doi: 10.1042/CS20120336. [DOI] [PubMed] [Google Scholar]
- 267.Nelson RG, et al. Low incidence of fatal coronary heart disease in Pima Indians despite high prevalence of non-insulin-dependent diabetes. Circulation. 1990;81:987–995. doi: 10.1161/01.CIR.81.3.987. [DOI] [PubMed] [Google Scholar]
- 268.Neves AL, et al. Allelic variations in superoxide dismutase-1 (SOD1) gene and renal and cardiovascular morbidity and mortality in Type 2 diabetic subjects. Mol. Genet. Metab. 2012;106:359–365. doi: 10.1016/j.ymgme.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 269.Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J. Gen. Intern. Med. 2013;28:691–697. doi: 10.1007/s11606-012-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nilsson PM, et al. Smoking as an independent risk factor for myocardial infarction or stroke in type 2 diabetes: a report from the Swedish National Diabetes Register. Eur. J. Cardiovasc. Prev. Rehabil. 2009;16:506–512. doi: 10.1097/HJR.0b013e32832ccc50. [DOI] [PubMed] [Google Scholar]
- 271.Ningshen R, Odyuo Moathung, Arvind Z, Deba Singh G, Devi TS. B. A study of QTc interval prolongation as an independent predictor of cardiac mortality in type 2 diabetes mellitus. JMS - J. Med. Soc. 2012;26:21–25. [Google Scholar]
- 272.Niskanen L, Turpeinen A, Penttilä I, Uusitupa MI. Hyperglycemia and compositional lipoprotein abnormalities as predictors of cardiovascular mortality in type 2 diabetes: a 15-year follow-up from the time of diagnosis. Diabetes Care. 1998;21:1861–1869. doi: 10.2337/diacare.21.11.1861. [DOI] [PubMed] [Google Scholar]
- 273.Niskanen LK, Penttilã I, Parviainen M, Uusitupa MI. Evolution, risk factors, and prognostic implications of albuminuria in NIDDM. Diabetes Care. 1996;19:486–493. doi: 10.2337/diacare.19.5.486. [DOI] [PubMed] [Google Scholar]
- 274.Nitenberg A, et al. Cardiovascular outcome of patients with abnormal coronary vasomotion and normal coronary arteriography is worse in type 2 diabetes mellitus than in arterial hypertension: a 10 year follow-up study. Atherosclerosis. 2005;183:113–120. doi: 10.1016/j.atherosclerosis.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 275.Novo-Rodríguez C, et al. Circulating levels of sclerostin are associated with cardiovascular mortality. PLoS One. 2018;13:e0199504. doi: 10.1371/journal.pone.0199504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Odeberg J, Larsson CA, Råstam L, Lindblad U. The Asp298 allele of endothelial nitric oxide synthase is a risk factor for myocardial infarction among patients with Type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2008;8:36. doi: 10.1186/1471-2261-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Oellgaard J, et al. Application of urinary proteomics as possible risk predictor of renal and cardiovascular complications in patients with Type 2-diabetes and microalbuminuria. J. Diabetes Complications. 2018;32:1133–1140. doi: 10.1016/j.jdiacomp.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 278.Oliveira JL, et al. Prognostic value of exercise echocardiography in diabetic patients. Cardiovasc. Ultrasound. 2009;7:24. doi: 10.1186/1476-7120-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ong KL, et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia. 2015;58:464–473. doi: 10.1007/s00125-014-3458-7. [DOI] [PubMed] [Google Scholar]
- 280.Ong KL, et al. Relationships of adipocyte-fatty acid binding protein and lipocalin 2 with risk factors and chronic complications in type 2 diabetes and effects of fenofibrate: A fenofibrate Intervention and event lowering in diabetes sub-study. Diabetes Res. Clin. Pract. 2020;169:108450. doi: 10.1016/j.diabres.2020.108450. [DOI] [PubMed] [Google Scholar]
- 281.Ortega Moreno L, et al. Evidence of a causal relationship between high serum adiponectin levels and increased cardiovascular mortality rate in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016;15:17. doi: 10.1186/s12933-016-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Oshima M, et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE trial. J. Am. Soc. Nephrol. 2020;31:2925–2936. doi: 10.1681/ASN.2020050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Otto S, et al. Microembolization and myonecrosis during elective percutaneous coronary interventions in diabetic patients: an intracoronary Doppler ultrasound study with 2-year clinical follow-up. Basic Res. Cardiol. 2012;107:289. doi: 10.1007/s00395-012-0289-x. [DOI] [PubMed] [Google Scholar]
- 284.Pagidipati NJ, et al. Association of obesity with cardiovascular outcomes in patients with Type 2 diabetes and cardiovascular disease: Insights from TECOS. Am. Heart J. 2020;219:47–57. doi: 10.1016/j.ahj.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 285.Panero F, et al. Uric acid is not an independent predictor of cardiovascular mortality in Type 2 diabetes: a population-based study. Atherosclerosis. 2012;221:183–188. doi: 10.1016/j.atherosclerosis.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 286.Park GM, et al. Coronary computed tomographic angiographic findings in asymptomatic patients with Type 2 diabetes mellitus. Am. J. Cardiol. 2014;113:765–771. doi: 10.1016/j.amjcard.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 287.Peng WH, et al. Decreased serum esRAGE level is associated with angiographically determined coronary plaque progression in diabetic patients. Clin. Biochem. 2009;42:1252–1259. doi: 10.1016/j.clinbiochem.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 288.Peters KE, Chubb SA, Davis WA, Davis TM. The relationship between hypomagnesemia, metformin therapy and cardiovascular disease complicating type 2 diabetes: the Fremantle Diabetes Study. PLoS One. 2013;8:e74355. doi: 10.1371/journal.pone.0074355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Petretta M, et al. Transient ischemic dilation in patients with diabetes mellitus: prognostic value and effect on clinical outcome after coronary revascularization. Circ. Cardiovasc. Imaging. 2013;6:908–915. doi: 10.1161/CIRCIMAGING.113.000497. [DOI] [PubMed] [Google Scholar]
- 290.Pfister R, Cairns R, Erdmann E, Schneider CA. Prognostic impact of electrocardiographic signs in patients with Type 2 diabetes and cardiovascular disease: results from the PROactive study. Diabet. Med. 2011;28:1206–1212. doi: 10.1111/j.1464-5491.2011.03281.x. [DOI] [PubMed] [Google Scholar]
- 291.Pickup JC, Mattock MB. Activation of the innate immune system as a predictor of cardiovascular mortality in Type 2 diabetes mellitus. Diabet Med. 2003;20:723–726. doi: 10.1046/j.1464-5491.2003.00990.x. [DOI] [PubMed] [Google Scholar]
- 292.Piniés JA, et al. Development of a prediction model for fatal and non-fatal coronary heart disease and cardiovascular disease in patients with newly diagnosed type 2 diabetes mellitus: the Basque Country Prospective Complications and Mortality Study risk engine (BASCORE) Diabetologia. 2014;57:2324–2333. doi: 10.1007/s00125-014-3370-1. [DOI] [PubMed] [Google Scholar]
- 293.Pintó, X. et al. [Factors predictive of cardiovascular disease in patients with type-2 diabetes and hypercholesterolemia. ESODIAH study]. Rev Esp Cardiol 60, 251–258 (2007). [PubMed]
- 294.Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between beta1-adrenergic receptor polymorphisms and cardiovascular disease in patients with diabetic nephropathy. Nephrology (Carlton) 2010;15:242–247. doi: 10.1111/j.1440-1797.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 295.Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between HSP70-2 A+1267G polymorphism and cardiovascular events of Chinese peritoneal dialysis patients. Nephron Clin. Pract. 2014;128:153–158. doi: 10.1159/000368237. [DOI] [PubMed] [Google Scholar]
- 296.Porchay-Baldérelli I, et al. The CETP TaqIB polymorphism is associated with the risk of sudden death in Type 2 diabetic patients. Diabetes Care. 2007;30:2863–2867. doi: 10.2337/dc07-0869. [DOI] [PubMed] [Google Scholar]
- 297.Porchay-Baldérelli I, et al. Relationships between common polymorphisms of adenosine triphosphate-binding cassette transporter A1 and high-density lipoprotein cholesterol and coronary heart disease in a population with Type 2 diabetes mellitus. Metabolism. 2009;58:74–79. doi: 10.1016/j.metabol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 298.Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with Type 2 diabetes. Diabetic Med. 2016;33:1640–1648. doi: 10.1111/dme.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Qi L, et al. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes. 2005;54:1607–1610. doi: 10.2337/diabetes.54.5.1607. [DOI] [PubMed] [Google Scholar]
- 300.Qi L, et al. Genetic susceptibility to coronary heart disease in type 2 diabetes: 3 independent studies. J. Am. Coll. Cardiol. 2011;58:2675–2682. doi: 10.1016/j.jacc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Qi L, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA. 2013;310:821–828. doi: 10.1001/jama.2013.276305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of Type 2 diabetes. Eur. Heart J. 2012;33:325–334. doi: 10.1093/eurheartj/ehr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Qin Z, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of Type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc. Diabetol. 2020;19:23. doi: 10.1186/s12933-020-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Quan J, et al. Risk prediction scores for mortality, cerebrovascular, and heart disease among Chinese people with Type 2 diabetes. J. Clin. Endocrinol. Metab. 2019;104:5823–5830. doi: 10.1210/jc.2019-00731. [DOI] [PubMed] [Google Scholar]
- 305.Raghavan S, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a National cohort of adults. J. Am. Heart Assoc. 2019;8:e011295. doi: 10.1161/JAHA.118.011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ramírez-Prado D, et al. A four-year cardiovascular risk score for Type 2 diabetic inpatients. PeerJ. 2015;2015:e984. doi: 10.7717/peerj.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rana BS, et al. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91:44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rasmussen DGK, et al. Higher collagen VI formation is associated with all-cause mortality in patients with Type 2 diabetes and microalbuminuria. Diabetes Care. 2018;41:1493–1500. doi: 10.2337/dc17-2392. [DOI] [PubMed] [Google Scholar]
- 309.Ravassa S, et al. Association of low GLP-1 with oxidative stress is related to cardiac disease and outcome in patients with Type 2 diabetes mellitus: a pilot study. Free Radic. Biol. Med. 2015;81:1–12. doi: 10.1016/j.freeradbiomed.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 310.Rawshani A, et al. Risk factors, mortality, and cardiovascular outcomes in patients with Type 2 diabetes. N. Engl. J. Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 311.Rawshani A, et al. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with Type 2 diabetes. JAMA Intern Med. 2016;176:1146–1154. doi: 10.1001/jamainternmed.2016.2940. [DOI] [PubMed] [Google Scholar]
- 312.Read SH, et al. Performance of cardiovascular disease risk scores in people diagnosed with Type 2 diabetes: external validation using data from the National Scottish diabetes register. Diabetes Care. 2018;41:2010–2018. doi: 10.2337/dc18-0578. [DOI] [PubMed] [Google Scholar]
- 313.Reinhard H, et al. Osteoprotegerin and mortality in type 2 diabetic patients. Diabetes Care. 2010;33:2561–2566. doi: 10.2337/dc10-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Resl M, et al. Serum uric acid is related to cardiovascular events and correlates with N-terminal pro-B-type natriuretic peptide and albuminuria in patients with diabetes mellitus. Diabet. Med. 2012;29:721–725. doi: 10.1111/j.1464-5491.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 315.Resl M, et al. Targeted multiple biomarker approach in predicting cardiovascular events in patients with diabetes. Heart. 2016;102:1963–1968. doi: 10.1136/heartjnl-2015-308949. [DOI] [PubMed] [Google Scholar]
- 316.Rossi MC, et al. Quality of diabetes care predicts the development of cardiovascular events: results of the AMD-QUASAR study. Diabetes Care. 2011;34:347–352. doi: 10.2337/dc10-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rotbain Curovic V, et al. Urinary tubular biomarkers as predictors of kidney function decline, cardiovascular events and mortality in microalbuminuric type 2 diabetic patients. Acta Diabetol. 2018;55:1143–1150. doi: 10.1007/s00592-018-1205-0. [DOI] [PubMed] [Google Scholar]
- 318.Roumeliotis A, et al. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus Type 2 and chronic kidney disease. Ren Fail. 2019;41:131–138. doi: 10.1080/0886022X.2019.1585372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Roumeliotis AK, et al. Association of ALOX12 gene polymorphism with all-cause and cardiovascular mortality in diabetic nephropathy. Int. Urol. Nephrol. 2018;50:321–329. doi: 10.1007/s11255-017-1755-z. [DOI] [PubMed] [Google Scholar]
- 320.Roumeliotis S, et al. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complications. 2017;31:1527–1532. doi: 10.1016/j.jdiacomp.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 321.Rozing MP, et al. Changes in HbA1c during the first six years after the diagnosis of Type 2 diabetes mellitus predict long-term microvascular outcomes. PLoS One. 2019;14:e0225230. doi: 10.1371/journal.pone.0225230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ruggenenti P, et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with Type 2 diabetes. J. Am. Soc. Nephrol. 2012;23:1717–1724. doi: 10.1681/ASN.2012030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Russo GT, et al. Mild hyperhomocysteinemia, C677T polymorphism on methylenetetrahydrofolate reductase gene and the risk of macroangiopathy in type 2 diabetes: a prospective study. Acta Diabetol. 2011;48:95–101. doi: 10.1007/s00592-009-0169-5. [DOI] [PubMed] [Google Scholar]
- 324.Rutter MK, Wahid ST, McComb JM, Marshall SM. Significance of silent ischemia and microalbuminuria in predicting coronary events in asymptomatic patients with Type 2 diabetes. J. Am. Coll. Cardiol. 2002;40:56–61. doi: 10.1016/S0735-1097(02)01910-1. [DOI] [PubMed] [Google Scholar]
- 325.Rådholm K, et al. The impact of using sagittal abdominal diameter to predict major cardiovascular events in European patients with Type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017;27:418–422. doi: 10.1016/j.numecd.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 326.Rørth R, et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur. J. Heart Fail. 2019;21:40–49. doi: 10.1002/ejhf.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Saely CH, et al. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J. Clin. Endocrinol. Metab. 2005;90:5698–5703. doi: 10.1210/jc.2005-0799. [DOI] [PubMed] [Google Scholar]
- 328.Saely CH, et al. Lipoprotein(a), type 2 diabetes and vascular risk in coronary patients. Eur. J. Clin. Invest. 2006;36:91–97. doi: 10.1111/j.1365-2362.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 329.Saito I, et al. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann. Intern. Med. 2000;133:81–91. doi: 10.7326/0003-4819-133-2-200007180-00007. [DOI] [PubMed] [Google Scholar]
- 330.Sakai K, et al. Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr. Gerontol. Int. 2018;18:965–972. doi: 10.1111/ggi.13287. [DOI] [PubMed] [Google Scholar]
- 331.Salles GF, Leite NC, Pereira BB, Nascimento EM, Cardoso CR. Prognostic impact of clinic and ambulatory blood pressure components in high-risk Type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. J. Hypertens. 2013;31:2176–2186. doi: 10.1097/HJH.0b013e328364103f. [DOI] [PubMed] [Google Scholar]
- 332.Satirapoj B, Tasanavipas P, Supasyndh O. Role of TCF7L2 and PPARG2 gene polymorphisms in renal and cardiovascular complications among patients with Type 2 diabetes: a Cohort Study. Kidney Dis. 2019;5:220–227. doi: 10.1159/000497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Saulnier PJ, et al. Urinary sodium concentration is an independent predictor of all-cause and cardiovascular mortality in a Type 2 diabetes Cohort Population. J. Diabetes Res. 2017;2017:5327352. doi: 10.1155/2017/5327352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Savonitto S, et al. Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. Diabetes Vasc. Dis. Res. 2018;15:14–23. doi: 10.1177/1479164117735493. [DOI] [PubMed] [Google Scholar]
- 335.Schimke K, Chubb SAP, Davis WA, Davis TME. Helicobacter pylori cytotoxin-associated gene-A antibodies do not predict complications or death in Type 2 diabetes: The Fremantle Diabetes Study. Atherosclerosis. 2010;212:321–326. doi: 10.1016/j.atherosclerosis.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 336.Schulze MB, et al. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27:889–894. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- 337.Schulze MB, et al. Joint role of non-HDL cholesterol and glycated haemoglobin in predicting future coronary heart disease events among women with Type 2 diabetes. Diabetologia. 2004;47:2129–2136. doi: 10.1007/s00125-004-1593-2. [DOI] [PubMed] [Google Scholar]
- 338.Scirica BM, et al. Prognostic implications of biomarker assessments in patients with Type 2 diabetes at high cardiovascular risk: A secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:989–998. doi: 10.1001/jamacardio.2016.3030. [DOI] [PubMed] [Google Scholar]
- 339.Scirica BM, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with Type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol. 2018;3:155–163. doi: 10.1001/jamacardio.2017.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Seferovic JP, et al. Retinopathy, neuropathy, and subsequent cardiovascular events in patients with Type 2 diabetes and acute coronary syndrome in the ELIXA: the importance of disease duration. J. Diabetes Res. 2018;2018:1631263. doi: 10.1155/2018/1631263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Seyoum B, Estacio RO, Berhanu P, Schrier RW. Exercise capacity is a predictor of cardiovascular events in patients with Type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2006;3:197–201. doi: 10.3132/dvdr.2006.030. [DOI] [PubMed] [Google Scholar]
- 342.Shao H, Fonseca V, Stoecker C, Liu S, Shi L. Novel risk engine for diabetes progression and mortality in USA: building, relating, assessing, and validating outcomes (BRAVO) Pharmacoeconomics. 2018;36:1125–1134. doi: 10.1007/s40273-018-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shao H, Shi L, Fonseca VA. Using the BRAVO risk engine to predict cardiovascular outcomes in clinical trials with sodium-glucose transporter 2 inhibitors. Diabetes Care. 2020;43:1530–1536. doi: 10.2337/dc20-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sharma A, et al. Causes of death in a contemporary Cohort of Patients With Type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care. 2017;40:1763–1770. doi: 10.2337/dc17-1091. [DOI] [PubMed] [Google Scholar]
- 345.Sharma A, et al. Clinical and biomarker predictors of expanded heart failure outcomes in patients with Type 2 diabetes mellitus after a recent acute coronary syndrome: insights from the EXAMINE Trial. J. Am. Heart Assoc. 2020;9:e012797. doi: 10.1161/JAHA.119.012797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Shin SH, et al. Hyperglycaemia, ejection fraction and the risk of heart failure or cardiovascular death in patients with Type 2 diabetes and a recent acute coronary syndrome. Eur. J. Heart Fail. 2020;22:1133–1143. doi: 10.1002/ejhf.1790. [DOI] [PubMed] [Google Scholar]
- 347.Siddique A, et al. Relationship of mildly increased albuminuria and coronary artery revascularization outcomes in patients with diabetes. Catheter Cardiovasc. Interv. 2019;93:E217–e224. doi: 10.1002/ccd.27890. [DOI] [PubMed] [Google Scholar]
- 348.Sidorenkov G, Voorham J, de Zeeuw D, Haaijer-Ruskamp FM, Denig P. Do treatment quality indicators predict cardiovascular outcomes in patients with diabetes? PLoS One. 2013;8:e78821. doi: 10.1371/journal.pone.0078821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Silva AP, et al. Phosphorus as an early marker of morbidity and mortality in Type 2 chronic kidney disease diabetic patients. J. Diabetes Complications. 2013;27:328–332. doi: 10.1016/j.jdiacomp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 350.Silva AP, et al. What is the role of apelin regarding cardiovascular risk and progression of renal disease in Type 2 diabetic patients with diabetic nephropathy? Biomed. Res. Int. 2013;2013:247649. doi: 10.1155/2013/247649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Simmons RK, et al. Performance of the UK prospective diabetes study risk engine and the Framingham risk equations in estimating cardiovascular disease in the EPIC- Norfolk Cohort. Diabetes Care. 2009;32:708–713. doi: 10.2337/dc08-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Smáradóttir MI, et al. Copeptin and insulin-like growth factor binding protein-1 during follow-up after an acute myocardial infarction in patients with type 2 diabetes: a report from the diabetes mellitus insulin-glucose infusion in acute myocardial infarction 2 cohort. Diab. Vasc. Dis. Res. 2019;16:22–27. doi: 10.1177/1479164118804451. [DOI] [PubMed] [Google Scholar]
- 353.So WY, et al. Aldose reductase genotypes and cardiorenal complications: an 8-year prospective analysis of 1074 Type 2 diabetic patients. Diabetes Care. 2008;31:2148–2153. doi: 10.2337/dc08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Soejima H, et al. Proteinuria is independently associated with the incidence of primary cardiovascular events in diabetic patients. J. Cardiol. 2020;75:387–393. doi: 10.1016/j.jjcc.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 355.Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. Elevated plasma homocysteine level is an independent predictor of coronary heart disease events in patients with Type 2 diabetes mellitus. Ann. Intern. Med. 2004;140:94–100,. doi: 10.7326/0003-4819-140-2-200401200-00009. [DOI] [PubMed] [Google Scholar]
- 356.Sone H, et al. Waist circumference as a cardiovascular and metabolic risk in Japanese patients with Type 2 diabetes. Obesity (Silver Spring) 2009;17:585–592. doi: 10.1038/oby.2008.481. [DOI] [PubMed] [Google Scholar]
- 357.Sone H, et al. Comparison of various lipid variables as predictors of coronary heart disease in Japanese men and women with Type 2 diabetes: subanalysis of the Japan Diabetes Complications Study. Diabetes Care. 2012;35:1150–1157. doi: 10.2337/dc11-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sone H, et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with Type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS) J. Clin. Endocrinol. Metab. 2011;96:3448–3456. doi: 10.1210/jc.2011-0622. [DOI] [PubMed] [Google Scholar]
- 359.Sone H, et al. Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with Type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS) Diabetologia. 2013;56:1021–1030. doi: 10.1007/s00125-012-2810-z. [DOI] [PubMed] [Google Scholar]
- 360.Spoelstra-de Man AM, Brouwer CB, Stehouwer CD, Smulders YM. Rapid progression of albumin excretion is an independent predictor of cardiovascular mortality in patients with Type 2 diabetes and microalbuminuria. Diabetes Care. 2001;24:2097–2101. doi: 10.2337/diacare.24.12.2097. [DOI] [PubMed] [Google Scholar]
- 361.Standl E, et al. Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich General Practitioner Project. Diabetologia. 1996;39:1540–1545. doi: 10.1007/s001250050612. [DOI] [PubMed] [Google Scholar]
- 362.Stehouwer CD, Gall MA, Hougaard P, Jakobs C, Parving HH. Plasma homocysteine concentration predicts mortality in non-insulin-dependent diabetic patients with and without albuminuria. Kidney Int. 1999;55:308–314. doi: 10.1046/j.1523-1755.1999.00256.x. [DOI] [PubMed] [Google Scholar]
- 363.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin. Sci. (Lond) 2001;101:671–679. doi: 10.1042/cs1010671. [DOI] [PubMed] [Google Scholar]
- 364.Strojek K, et al. Factors associated with cardiovascular events in patients with Type 2 diabetes and acute myocardial infarction. J. Clin. Endocrinol. Metab. 2016;101:243–253. doi: 10.1210/jc.2015-1962. [DOI] [PubMed] [Google Scholar]
- 365.Sultan A, et al. Myocardial perfusion imaging and cardiac events in a cohort of asymptomatic patients with diabetes living in southern France. Diabet. Med. 2006;23:410–418. doi: 10.1111/j.1464-5491.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 366.Svendstrup M, Christiansen MS, Magid E, Hommel E, Feldt-Rasmussen B. Increased orosomucoid in urine is an independent predictor of cardiovascular and all-cause mortality in patients with Type 2 diabetes at 10 years of follow-up. J. Diabetes Complications. 2013;27:570–575. doi: 10.1016/j.jdiacomp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 367.Svensson E, et al. Early glycemic control and magnitude of HbA(1c) reduction predict cardiovascular events and mortality: population-based Cohort Study of 24,752 metformin initiators. Diabetes Care. 2017;40:800–807. doi: 10.2337/dc16-2271. [DOI] [PubMed] [Google Scholar]
- 368.Svensson MK, Cederholm J, Eliasson B, Zethelius B, Gudbjörnsdottir S. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with Type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. Diab. Vasc. Dis. Res. 2013;10:520–529. doi: 10.1177/1479164113500798. [DOI] [PubMed] [Google Scholar]
- 369.Takao T, Suka M, Yanagisawa H, Iwamoto Y. Impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular events and all-cause mortality in patients with Type 2 diabetes. J. Diabetes Investig. 2017;8:600–608. doi: 10.1111/jdi.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tan X, Benedict C. Increased risk of myocardial infarction among patients with type 2 diabetes who carry the common rs10830963 variant in the MTNR1B gene. Diabetes Care. 2020;43:2289–2292. doi: 10.2337/dc20-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tanaka S, et al. Predicting macro- and microvascular complications in Type 2 diabetes: the Japan diabetes complications study/the Japanese elderly diabetes intervention trial risk engine. Diabetes Care. 2013;36:1193–1199. doi: 10.2337/dc12-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Thanyasiri P, Celermajer DS, Adams MR. Predictors of long-term outcome following percutaneous coronary intervention in patients with type 2 diabetes mellitus. Coron Artery Dis. 2006;17:131–138. doi: 10.1097/00019501-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 373.The Look ARG. Prospective association of a genetic risk score and lifestyle intervention with cardiovascular morbidity and mortality among individuals with type 2 diabetes: the Look AHEAD randomised controlled trial. Diabetologia. 2015;58:1803–1813. doi: 10.1007/s00125-015-3610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Theilade S, et al. Pulse pressure is not an independent predictor of outcome in type 2 diabetes patients with chronic kidney disease and anemia–the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) J. Hum. Hypertens. 2016;30:46–52. doi: 10.1038/jhh.2015.22. [DOI] [PubMed] [Google Scholar]
- 375.Thomas MC, et al. Relationship Between Plasma 8-OH-Deoxyguanosine and Cardiovascular Disease and Survival in Type 2 Diabetes Mellitus: Results From the ADVANCE Trial. J. Am. Heart Assoc. 2018;7:e008226. doi: 10.1161/JAHA.117.008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tian P, Zheng X, Li M, Li W, Niu Q. Long-term prognostic value of coronary computed tomography angiography for asymptomatic patients with cad in type 2 diabetes mellitus. Exp. Therap. Med. 2019;18:747–754. doi: 10.3892/etm.2019.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ting RZ, et al. Lipid control and use of lipid-regulating drugs for prevention of cardiovascular events in Chinese type 2 diabetic patients: a prospective cohort study. Cardiovasc. Diabetol. 2010;9:77. doi: 10.1186/1475-2840-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tobias DK, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective Cohort of US women. Circ. Genom. Precis. Med. 2018;11:e002157. doi: 10.1161/CIRCGEN.118.002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Turner RC, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Twito O, et al. New-onset diabetes in elderly subjects: association between HbA1c levels, mortality, and coronary revascularization. Diabetes Care. 2013;36:3425–3429. doi: 10.2337/dc12-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Umamahesh K, Vigneswari A, Surya Thejaswi G, Satyavani K, Viswanathan V. Incidence of cardiovascular diseases and associated risk factors among subjects with type 2 diabetes - an 11-year follow up study. Indian Heart J. 2014;66:5–10. doi: 10.1016/j.ihj.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch. Intern. Med. 2000;160:1093–1100. doi: 10.1001/archinte.160.8.1093. [DOI] [PubMed] [Google Scholar]
- 383.Valoti E, et al. Impact of a complement factor H Gene variant on renal dysfunction, cardiovascular events, and response to ACE inhibitor therapy in Type 2 diabetes. Front Genet. 2019;10:681. doi: 10.3389/fgene.2019.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: The Hoorn Study. Diabetes Care. 2009;32:2094–2098. doi: 10.2337/dc09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.van der Leeuw J, et al. Novel biomarkers to improve the prediction of cardiovascular event risk in Type 2 diabetes mellitus. J. Am. Heart Assoc. 2016;5:e003048. doi: 10.1161/JAHA.115.003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.van der Leeuw J, et al. The validation of cardiovascular risk scores for patients with Type 2 diabetes mellitus. Heart. 2015;101:222–229. doi: 10.1136/heartjnl-2014-306068. [DOI] [PubMed] [Google Scholar]
- 387.Vanzetto G, et al. Prediction of cardiovascular events in clinically selected high-risk NIDDM patients. Prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care. 1999;22:19–26. doi: 10.2337/diacare.22.1.19. [DOI] [PubMed] [Google Scholar]
- 388.Vavruch C, et al. Using proximity extension proteomics assay to discover novel biomarkers associated with circulating leptin levels in patients with Type 2 diabetes. Sci. Rep. 2020;10:13097. doi: 10.1038/s41598-020-69473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Velho G, et al. Plasma copeptin, kidney disease, and risk for cardiovascular morbidity and mortality in two cohorts of Type 2 diabetes. Cardiovasc. Diabetol. 2018;17:110. doi: 10.1186/s12933-018-0753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Vengen IT, Dale AC, Wiseth R, Midthjell K, Videm V. Lactoferrin is a novel predictor of fatal ischemic heart disease in diabetes mellitus type 2: long-term follow-up of the HUNT 1 study. Atherosclerosis. 2010;212:614–620. doi: 10.1016/j.atherosclerosis.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 391.Venskutonyte L, Brismar K, Öhrvik J, Rydén L, Kjellström B. Self-rated health predicts outcome in patients with Type 2 diabetes and myocardial infarction: a DIGAMI 2 quality of life sub-study. Diab. Vasc. Dis. Res. 2013;10:361–367. doi: 10.1177/1479164113482694. [DOI] [PubMed] [Google Scholar]
- 392.Venskutonyte L, Malmberg K, Norhammar A, Wedel H, Rydén L. Effect of gender on prognosis in patients with myocardial infarction and type 2 diabetes. J. Intern. Med. 2010;268:75–82. doi: 10.1111/j.1365-2796.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 393.Venuraju SM, et al. Duration of Type 2 diabetes mellitus and systolic blood pressure as determinants of severity of coronary stenosis and adverse events in an asymptomatic diabetic population: PROCEED study. Cardiovasc. Diabetol. 2019;18:51. doi: 10.1186/s12933-019-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Venuraju SM, et al. Association of epicardial fat volume with the extent of coronary atherosclerosis and cardiovascular adverse events in asymptomatic patients with diabetes. Angiology. 2021;72:442–450. doi: 10.1177/0003319720984607. [DOI] [PubMed] [Google Scholar]
- 395.Venäläinen MS, Klén R, Mahmoudian M, Raitakari OT, Elo LL. Easy-to-use tool for evaluating the elevated acute kidney injury risk against reduced cardiovascular disease risk during intensive blood pressure control. J. Hypertens. 2020;38:511–518. doi: 10.1097/HJH.0000000000002282. [DOI] [PubMed] [Google Scholar]
- 396.Vepsäläinen T, et al. Proteinuria modifies the effect of systolic blood pressure on total and cardiovascular disease mortality in patients with type 2 diabetes. J. Intern. Med. 2012;272:611–619. doi: 10.1111/j.1365-2796.2012.02581.x. [DOI] [PubMed] [Google Scholar]
- 397.von Scholten BJ, et al. Additive prognostic value of plasma N-terminal pro-brain natriuretic peptide and coronary artery calcification for cardiovascular events and mortality in asymptomatic patients with Type 2 diabetes. Cardiovasc. Diabetol. 2015;14:59. doi: 10.1186/s12933-015-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.von Scholten BJ, et al. Urinary biomarkers are associated with incident cardiovascular disease, all-cause mortality and deterioration of kidney function in Type 2 diabetic patients with microalbuminuria. Diabetologia. 2016;59:1549–1557. doi: 10.1007/s00125-016-3937-0. [DOI] [PubMed] [Google Scholar]
- 399.von Scholten BJ, et al. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J Diabetes Complications. 2016;30:248–255. doi: 10.1016/j.jdiacomp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 400.Wallander M, et al. IGF binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes. Diabetes Care. 2007;30:2343–2348. doi: 10.2337/dc07-0825. [DOI] [PubMed] [Google Scholar]
- 401.Wan EY, Fong DY, Fung CS, Lam CL. Incidence and predictors for cardiovascular disease in Chinese patients with Type 2 diabetes mellitus - a population-based retrospective cohort study. J. Diabetes Complications. 2016;30:444–450. doi: 10.1016/j.jdiacomp.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 402.Wan EYF, et al. Development of a cardiovascular diseases risk prediction model and tools for Chinese patients with Type 2 diabetes mellitus: A population-based retrospective cohort study. Diabetes Obes. Metab. 2018;20:309–318. doi: 10.1111/dom.13066. [DOI] [PubMed] [Google Scholar]
- 403.Wang Y, et al. Independent predictive roles of eotaxin Ala23Thr, paraoxonase 2 Ser311Cys and beta-adrenergic receptor Trp64Arg polymorphisms on cardiac disease in Type 2 Diabetes–an 8-year prospective cohort analysis of 1297 patients. Diabet Med. 2010;27:376–383. doi: 10.1111/j.1464-5491.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 404.Wang Y, et al. Prognostic effect of insertion/deletion polymorphism of the ace gene on renal and cardiovascular clinical outcomes in Chinese patients with Type 2 diabetes. Diabetes Care. 2005;28:348–354. doi: 10.2337/diacare.28.2.348. [DOI] [PubMed] [Google Scholar]
- 405.Watson C, et al. Investigation of association of genetic variant rs3918242 of matrix metalloproteinase-9 with hypertension, myocardial infarction and progression of ventricular dysfunction in Irish Caucasian patients with diabetes: a report from the STOP-HF follow-up prog. BMC Cardiovasc. Disord. 2021;21:87. doi: 10.1186/s12872-021-01860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 407.Wells BJ, et al. Prediction of morbidity and mortality in patients with Type 2 diabetes. PeerJ. 2013;2013:e87. doi: 10.7717/peerj.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wijkman M, Länne T, Östgren CJ, Nystrom FH. Aortic pulse wave velocity predicts incident cardiovascular events in patients with type 2 diabetes treated in primary care. J. Diabetes Complications. 2016;30:1223–1228. doi: 10.1016/j.jdiacomp.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 409.Wijkman M, Länne T, Östgren CJ, Nystrom FH. Diastolic orthostatic hypertension and cardiovascular prognosis in Type 2 diabetes: a prospective cohort study. Cardiovasc. Diabetol. 2016;15:83. doi: 10.1186/s12933-016-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Winkler K, et al. Apolipoprotein E genotype predicts cardiovascular endpoints in dialysis patients with Type 2 diabetes mellitus. Atherosclerosis. 2010;208:197–202. doi: 10.1016/j.atherosclerosis.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 411.Wolsk E, et al. Role of B-Type natriuretic peptide and N-Terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and Type 2 diabetes mellitus. J. Am. Heart Assoc. 2017;6:e004743. doi: 10.1161/JAHA.116.004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Wong YK, et al. High-sensitivity troponin I and B-type natriuretic peptide biomarkers for prediction of cardiovascular events in patients with coronary artery disease with and without diabetes mellitus. Cardiovasc. Diabetol. 2019;18:171. doi: 10.1186/s12933-019-0974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Woodward M, et al. Prediction of 10-year vascular risk in patients with diabetes: the AD-ON risk score. Diabetes Obes. Metab. 2016;18:289–294. doi: 10.1111/dom.12614. [DOI] [PubMed] [Google Scholar]
- 414.Yamada T, Itoi T, Kiuchi Y, Nemoto M, Yamashita S. Proliferative diabetic retinopathy is a predictor of coronary artery disease in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2012;96:e4–6. doi: 10.1016/j.diabres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 415.Yamasaki Y, et al. Carotid intima-media thickness in Japanese Type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care. 2000;23:1310–1315. doi: 10.2337/diacare.23.9.1310. [DOI] [PubMed] [Google Scholar]
- 416.Yang F, Ye J, Pomerantz K, Stewart M. Potential modification of the UKPDS risk engine and evaluation of macrovascular event rates in controlled clinical trials. Diabetes, Metab. Syndrome Obesity: Targets Ther. 2013;6:247–256. doi: 10.2147/DMSO.S43724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Yang GR, et al. Association between neck circumference and the occurrence of cardiovascular events in Type 2 diabetes: Beijing Community Diabetes Study 20 (BCDS-20) Biomed. Res. Int. 2019;2019:4242304. doi: 10.1155/2019/4242304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yang SH, et al. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am. J. Med. Sci. 2017;354:117–124. doi: 10.1016/j.amjms.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 419.Yang SH, et al. Serum fibrinogen and cardiovascular events in Chinese patients with Type 2 diabetes and stable coronary artery disease: a prospective observational study. BMJ Open. 2017;7:e015041. doi: 10.1136/bmjopen-2016-015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Yang X, et al. Impacts of chronic kidney disease and albuminuria on associations between coronary heart disease and its traditional risk factors in type 2 diabetic patients - the Hong Kong diabetes registry. Cardiovasc. Diabetol. 2007;6:37. doi: 10.1186/1475-2840-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yang X, et al. Development and validation of a total coronary heart disease risk score in Type 2 diabetes mellitus. Am. J. Cardiol. 2008;101:596–601. doi: 10.1016/j.amjcard.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 422.Yang ZK, et al. Elevated glycated albumin and reduced endogenous secretory receptor for advanced glycation endproducts levels in serum predict major adverse cardio-cerebral events in patients with Type 2 diabetes and stable coronary artery disease. Int. J. Cardiol. 2015;197:241–247. doi: 10.1016/j.ijcard.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 423.Yeboah J, et al. Development of a new diabetes risk prediction tool for incident coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;236:411–417. doi: 10.1016/j.atherosclerosis.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Yeboah P, Hsu FC, Bertoni AG, Yeboah J. Body mass index, change in weight, body weight variability and outcomes in Type 2 diabetes mellitus (from the ACCORD Trial) Am. J. Cardiol. 2019;123:576–581. doi: 10.1016/j.amjcard.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 425.Yiu KH, et al. Predictive value of high-sensitivity troponin-I for future adverse cardiovascular outcome in stable patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2014;13:63. doi: 10.1186/1475-2840-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Yoshida M, et al. Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with Type 2 diabetes. Diabetes Care. 2012;35:178–180. doi: 10.2337/dc11-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Yoshimura T, et al. Low blood flow estimates in lower-leg arteries predict cardiovascular events in Japanese patients with Type 2 diabetes with normal ankle-brachial indexes. Diabetes Care. 2006;29:1884–1890. doi: 10.2337/dc06-0142. [DOI] [PubMed] [Google Scholar]
- 428.Young JB, et al. Development of predictive risk models for major adverse cardiovascular events among patients with Type 2 diabetes mellitus using health insurance claims data. Cardiovasc. Diabetol. 2018;17:118. doi: 10.1186/s12933-018-0759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Yu D, et al. Development and external validation of risk scores for cardiovascular hospitalization and rehospitalization in patients with diabetes. J. Clin. Endocrinol. Metab. 2018;103:1122–1129. doi: 10.1210/jc.2017-02293. [DOI] [PubMed] [Google Scholar]
- 430.Yu D, et al. Total/high density lipoprotein cholesterol and cardiovascular disease (re)hospitalization nadir in type 2 diabetes. J. Lipid Res. 2018;59:1745–1750. doi: 10.1194/jlr.P084269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yu D, et al. Derivation and external validation of a risk prediction algorithm to estimate future risk of cardiovascular death among patients with Type 2 diabetes and incident diabetic nephropathy: prospective cohort study. BMJ Open Diabetes Res Care. 2019;7:e000735. doi: 10.1136/bmjdrc-2019-000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yu D, Simmons D. Relationship between HbA1c and risk of all-cause hospital admissions among people with Type 2 diabetes. Diabetic Medicine. 2013;30:1407–1411. doi: 10.1111/dme.12235. [DOI] [PubMed] [Google Scholar]
- 433.Yu D, Simmons D. Association between blood pressure and risk of cardiovascular hospital admissions among people with type 2 diabetes. Heart. 2014;100:1444–1449. doi: 10.1136/heartjnl-2013-304799. [DOI] [PubMed] [Google Scholar]
- 434.Yun JS, Park YM, Cha SA, Ahn YB, Ko SH. Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc. Diabetol. 2018;17:109. doi: 10.1186/s12933-018-0752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zafrir B, et al. Low cardiorespiratory fitness and coronary artery calcification: Complementary cardiovascular risk predictors in asymptomatic Type 2 diabetics. Atherosclerosis. 2015;241:634–640. doi: 10.1016/j.atherosclerosis.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 436.Zafrir B, et al. Resting heart rate and measures of effort-related cardiac autonomic dysfunction predict cardiovascular events in asymptomatic type 2 diabetes. Eur. J. Prev. Cardiol. 2016;23:1298–1306. doi: 10.1177/2047487315624747. [DOI] [PubMed] [Google Scholar]
- 437.Zethelius B, et al. A new model for 5-year risk of cardiovascular disease in Type 2 diabetes, from the Swedish National Diabetes Register (NDR) Diabetes Res. Clin. Pract. 2011;93:276–284. doi: 10.1016/j.diabres.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 438.Zhang C, et al. Genetic variation in the hepatic lipase gene and the risk of coronary heart disease among US diabetic men: potential interaction with obesity. Diabetologia. 2006;49:1552–1559. doi: 10.1007/s00125-006-0235-2. [DOI] [PubMed] [Google Scholar]
- 439.Zhang XL, et al. Improved framingham risk scores of patients with Type 2 Diabetes Mellitus in the Beijing Community: A 10-Year prospective study of the effects of multifactorial interventions on cardiovascular risk factors (The Beijing Communities Diabetes Study 22) Diabetes Therapy. 2020;11:885–903. doi: 10.1007/s13300-020-00782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhao Q, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc. Diabetol. 2020;19:108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zhou JB, et al. Prediction of proliferative diabetic retinopathy to asymptomatic obstructive coronary artery disease in Chinese Type 2 diabetes individuals: an exploratory study. Metab. Syndr. Relat. Disord. 2019;17:367–373. doi: 10.1089/met.2018.0140. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 442.Zimering MB, Anderson RJ, Ge L, Moritz TE. Increased plasma basic fibroblast growth factor is associated with coronary heart disease in adult type 2 diabetes mellitus. Metabolism. 2011;60:284–291. doi: 10.1016/j.metabol.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 443.Zimering MB, Anderson RJ, Ge L, Moritz TE, Duckworth WC. Basic fibroblast growth factor predicts cardiovascular disease occurrence in participants from the veterans affairs diabetes trial. Fronti. Endocrinol. 2013;4:183. doi: 10.3389/fendo.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zobel EH, et al. Toe-brachial index as a predictor of cardiovascular disease and all-cause mortality in people with type 2 diabetes and microalbuminuria. Diabetologia. 2017;60:1883–1891. doi: 10.1007/s00125-017-4344-x. [DOI] [PubMed] [Google Scholar]
- 445.Zoppini G, et al. Usefulness of the triglyceride to high-density lipoprotein cholesterol ratio for predicting mortality risk in type 2 diabetes: role of kidney dysfunction. Atherosclerosis. 2010;212:287–291. doi: 10.1016/j.atherosclerosis.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 446.Østergaard HB, et al. Limited benefit of haemoglobin glycation index as risk factor for cardiovascular disease in type 2 diabetes patients. Diabetes Metab. 2019;45:254–260. doi: 10.1016/j.diabet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 447.Fitipaldi, H. hugofitipaldi/T2DCVD_Prognostics: T2DCVD-Prognostics Shiny app. Zenodo, 10.5281/zenodo.10277173 (2023).
- 448.Fitipaldi H, Franks PW. Ethnic, gender and other sociodemographic biases in genome-wide association studies for the most burdensome non-communicable diseases: 2005-2022. Hum. Mol. Genet. 2023;32:520–532. doi: 10.1093/hmg/ddac245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Misra S, et al. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: a population-based study. Lancet Diabetes Endocrinol. 2021;9:671–680. doi: 10.1016/S2213-8587(21)00208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Di Angelantonio E, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 451.Tam CHT, et al. Identification of a common variant for coronary heart disease at PDE1A contributes to individualized treatment goals and risk stratification of cardiovascular complications in Chinese patients with Type 2 diabetes. Diabetes Care. 2023;46:1271–1281. doi: 10.2337/dc22-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Chan J, et al. The Joint Asia Diabetes Evaluation (JADE) Program: a web-based program to translate evidence to clinical practice in Type 2 diabetes. Diabet Med. 2009;26:693–699. doi: 10.1111/j.1464-5491.2009.02751.x. [DOI] [PubMed] [Google Scholar]
- 453.Chan JCN, et al. Effect of a web-based management guide on risk factors in patients with Type 2 diabetes and diabetic kidney disease: A JADE randomized clinical trial. JAMA Netw. Open. 2022;5:e223862. doi: 10.1001/jamanetworkopen.2022.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Lim LL, et al. Association of technologically assisted integrated care with clinical outcomes in Type 2 diabetes in Hong Kong using the prospective JADE program: a retrospective cohort analysis. PLoS Med. 2020;17:e1003367. doi: 10.1371/journal.pmed.1003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Chan JC, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med. 2014;174:972–981. doi: 10.1001/jamainternmed.2014.655. [DOI] [PubMed] [Google Scholar]
- 456.Lim LL, et al. Effects of a technology-assisted integrated diabetes care program on cardiometabolic risk factors among patients with Type 2 Diabetes in the Asia-Pacific Region: the JADE program randomized clinical trial. JAMA Netw. Open. 2021;4:e217557. doi: 10.1001/jamanetworkopen.2021.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The protocol for this systematic review and meta-analysis is publicly available through the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD42021262843. Comprehensive search strategies that can be reproduced are outlined in Supplemental Note 1. Any further details required are available from the corresponding author upon reasonable request. Complete lists of the publications where data were extracted for this study are provided as Excel files in Supplemental Data 1 (list of studies on non-genetic biomarkers), Supplemental Data 2 (list of studies on genetic biomarkers), and Supplemental Data 3 (list of studies on non-genetic risk scores). Supplemental Data 4, 6, and 7 provide source data used to generate forest plots and meta-analyses. The data presented in Fig. 3 is also available as online interactive figures (https://hugofitipaldi.shinyapps.io/T2D_prognostic/) and in a data repository (https://zenodo.org/records/10277173)447.