Abstract
About 15–40% of patients with schizophrenia are treatment resistance (TR) and require clozapine. Identifying individuals who have higher risk of development of TR early in the course of illness is important to provide personalized intervention. A total of 1400 patients with FEP enrolled in the early intervention for psychosis service or receiving the standard psychiatric service between July 1, 1998, and June 30, 2003, for the first time were included. Clozapine prescriptions until June 2015, as a proxy of TR, were obtained. Premorbid information, baseline characteristics, and monthly clinical information were retrieved systematically from the electronic clinical management system (CMS). Training and testing samples were established with random subsampling. An automated machine learning (autoML) approach was used to optimize the ML algorithm and hyperparameters selection to establish four probabilistic classification models (baseline, 12-month, 24-month, and 36-month information) of TR development. This study found 191 FEP patients (13.7%) who had ever been prescribed clozapine over the follow-up periods. The ML pipelines identified with autoML had an area under the receiver operating characteristic curve ranging from 0.676 (baseline information) to 0.774 (36-month information) in predicting future TR. Features of baseline information, including schizophrenia diagnosis and age of onset, and longitudinal clinical information including symptoms variability, relapse, and use of antipsychotics and anticholinergic medications were important predictors and were included in the risk calculator. The risk calculator for future TR development in FEP patients (TRipCal) developed in this study could support the continuous development of data-driven clinical tools to assist personalized interventions to prevent or postpone TR development in the early course of illness and reduce delay in clozapine initiation.
Subject terms: Schizophrenia, Prognostic markers
Introduction
About 15–40% of patients with schizophrenia are considered to have treatment-resistant schizophrenia (TRS) [1–3] and were found to have 3- to 11-fold higher direct healthcare costs [4, 5], as well as poorer functional outcomes [1, 6]. Clozapine is among the most effective antipsychotics for TRS patients [7] and is considered the first-line pharmacological treatment for TRS in many countries [8]. Despite its efficacy, there are often years of delays in clozapine initiation with multiple antipsychotic trials prior to the clozapine initiation [9, 10], which was found to be related to poor response to clozapine [1, 11]. Identification of patients who are at higher risk of developing treatment resistance (TR) may reduce the delay of clozapine initiation. Though about 22% of patients are considered to be TR in their first-episode of illness [12], which is likely to have distinctly different mechanisms than those who develop TR after multiple episodes [13], the median time of TR development is up to 10 years [14, 15]. Dopamine hypersensitivity has been suggested as a possible mechanism in the development of TR [16]. Therefore, identification of individuals who have higher risk of developing TR, particularly in the early stage of the illness, would be the first step to facilitate personalized and targeted interventions to prevent or postpone the development of TR.
Though multiple factors have been explored in prospective studies as possible predictors of TRS, only 12 studies have been identified in a recent systematic review and found early age of onset as the most consistent predictor reported [17, 18]. About half of the included studies had five years or less follow-up period. Use of integrated prediction models in TRS prediction has been advocated [19]. However, there are only four studies attempting to develop a prognostic prediction model to predict TR development using machine learning (ML) methods [20–23] with three being in patients with first-episode psychosis (FEP) [20, 21, 23]. Most studies used LASSO logistic regression or forced-entry models with area under curve (AUC) ranging from 0.59 [21] to 0.67 [23]. These studies are initial attempts to establish a predictive model using ML approaches, and results suggest that more advanced ML models may be needed to improve prediction performance. Most of these studies had a moderate follow-up period (<5 years) that might have restricted the predictive performance of the established model. Furthermore, these studies and other general studies on the predictors of TRS, only included demographics and baseline information without considering treatment outcomes and clinical characteristics during the early stage of the illness, which have been related to the development of TR [1, 17]. With few previous studies, it is difficult to determine the optimal ML model to be used. Therefore, to develop a data-assisted clinical tool to estimate individual risks of TRS development, a larger pool of state-of-the-art ML models should be considered. Automated machine learning (autoML) is a process that automates the tasks of applying machine learning, including optimizing algorithm selection and hyperparameter optimization, to maximize the predictive performance of the model.
Clozapine prescription is only recommended for TRS patients in most countries and regions, including Hong Kong [8], and has been considered a proxy for TR status in many population-based studies [21, 24]. Therefore, the current study used clozapine initiation as a proxy of treatment resistance status. The aims of the current study are to establish a prediction model of future clozapine use, a proxy of TR development, among the FEP population over 12–17 years of follow-up using clinical information at baseline and over the initial three years of the treatment with autoML. Prediction models with baseline, 12-month, 24-month, and 36-month information were established separately. An individualized risk calculator for treatment resistance development of FEP patients (TRipCal) was established using the significant features identified with the autoML model. Results of the current study may provide support to the development of personalized interventions in improving outcomes of patients with FEP.
Methods
Data source and study sample
The sample of this study was originally included in a study comparing three-year outcomes of patients with first-episode psychosis (FEP) who were treated by early intervention services (EIS) for psychosis and those who received standard care services (SCS) [25]. A total of 700 FEP patients who were consecutively enrolled in the EIS [26] between July 1, 2001 and June 30, 2003 in all public psychiatric units in Hong Kong and age, gender and diagnosis-matched FEP patients (n = 700) who received the SCS between July 1, 1998 and June 30, 2001 provided by the Hospital Authority (HA) in Hong Kong were included. Patients with diagnosis of substance-induced psychosis, organic disorders, or intellectual disability, and those who had received prior antipsychotic medication for more than a month were excluded from the initial case identification. Detailed medication history of all patients (N = 1400) from their first service visit (EIS or SCS) to June 2015 (follow-up period: 12–17 years) were retrieved from the centralized electronic hospital database (Clinical Management System [CMS]). After excluding 2 patients with missing data for clozapine use, we identified 191 out of 1398 patients (13.7%) who have ever received clozapine prescriptions during this follow-up period. The CMS is an electronic clinical record system of the HA in Hong Kong which covers over 90% of the psychiatric care of severe mental illness patients [27]. All inpatient and outpatient clinical information including hospitalization, consultation records, medication prescription were included in the CMS. Institutional ethical approval was obtained from all Hong Kong hospital clusters for the current study. Data analysis and development of the calculator was conducted between December 2022 and March 2023.
Outcomes and features
Clozapine use was considered as a proxy indicator of TR and the outcome in the current study. All features were obtained from case notes of each enrolled patient using a standardized CMS data entry form [28]. Features of interest at baseline included age at first service contact to the EIS or SCS, sex, years of education, any life events prior to the service entry, smoking status, diagnoses, age of illness onset, received EIS or not, duration of first episode, length of hospitalization at first-episode, duration of untreated psychosis (DUP), suicidal attempts (SA), non-suicidal self-injury (NSSI) during DUP and presence of psychiatric comorbidities. Furthermore, the clinical notes of patients were examined to summarize monthly clinical features including symptoms, functioning, other clinical information, and medication use for the first three years of clinical services. Symptom features included positive and negative psychotic symptoms assessed by the Clinical Global Impressions-Schizophrenia (CGI-SCH) scale [29] and depressive symptoms measured by the Clinical Global Impression scale (CGIS) [30]. Social functioning of patients was assessed by the Social and Occupational Functioning Assessment Scale (SOFAS) [31]. These variables were further summarized into mean and mean of the squared successive differences (MSSD) [32]. Other clinical information included SA, NSSI, substance abuse, Accident & Emergency visit, out patient departments visit, hospitalization, default from outpatient appointments, and relapse. Medication and intervention features included daily defined dose (DDD) of antipsychotic medication [33], and whether anticholinergic, antidepressant, benzodiazepine, mood stabilizer, or electroconvulsive therapy were prescribed. Information on types of antipsychotics and daily dose were used for DDD calculation and monthly average DDD of antipsychotics were determined. Operational definition of the features and the quality assurance of the data including interrater reliability are in the supplementary documents.
Model development and validation followed the guidelines of Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD, Table S1). An automated machine learning (autoML) approach was implemented to automate the selection of ML algorithms and hyperparameters using the Tree-based Pipeline Optimization Tool (TPOT) package in Python [34, 35]. TPOT optimizes the search process for prediction models through employing genetic programming and evolutionary algorithms (see http://epistasislab.github.io/tpot/ for more details).
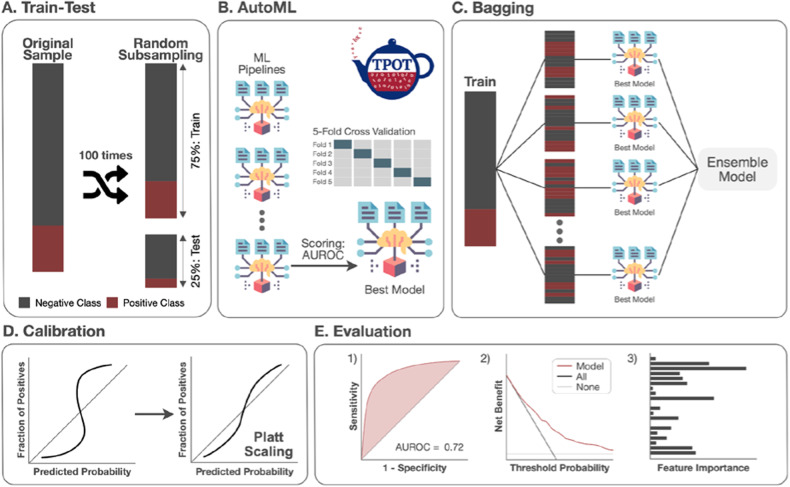
To develop the probabilistic classification models, the sampling process was stratified based on the outcome variable (i.e., clozapine use) with the sample being randomly divided into training (75%) and testing (25%) sets (Fig. 1A). Missing data were imputed using median replace and the features were standardized by subtracting the mean and scaling to the unit variance in the training data. The derived preprocessing steps were then applied to test data. TPOT was set to run for 10 generations with a population size of 50 pipelines (Fig. 1B). For each generation, the best model was selected based on the area under the receiver operating characteristic (AUROC), evaluated through a 5-fold cross-validation (CV) within the training data. The training data was re-fitted with the best model plus bagging and calibration procedures (Fig. 1C, D) to minimize overfitting and improve out-of-sample model performance. To obtain a stable performance and avoid overfitting to a particular subsample, we repeated the above procedure 100 times. For each repeat, the AUROC, calibration performance measured by Brier score, decision curve analysis and feature importance were calculated (Fig. 1E). Detailed approaches of decision curve analysis are in the supplementary methods.
Fig. 1. Overview of probabilistic classification model workflow.
A We split our data using random subsampling. The former approach was the main analysis of the current study. We randomly split the participants into train (75%) and test data sets and repeat this procedure 100 times to obtain a stable performance. The latter approach aims to examine the generalization of our models. B AutoML was implemented in Python using the TPOT package. C Bagging procedure is added when re-training the best model from autoML. D Calibration was performed using Platt scaling. E Evaluation of the performance of test data includes area under the receiver operating characteristic curve (AUROC), decision curve analysis and feature importance.
The overall model performance was obtained by averaging AUROC and Brier scores across 100 repetitions. We ranked the feature importance for each repetition as different algorithms may be selected for each repetition. In scikit-learn, feature importance represents the relative importance of each feature in a trained model for predicting a target variable. Average feature importance rank for each variable was calculated across 100 repetitions for further interpretation. Four probabilistic classification models were developed by incorporating features of different duration (i.e., baseline or first month, 12, 24 and 36-month). For each model, we removed patients with clozapine use within the period of the features (Baseline n = 1398; 12-month n = 1387; 24-month n = 1379; 36-month n = 1363).
Finally, the features were reduced to a reasonable number by refitting the data using the above procedures with top 10, 15 or 20 features and comparing their performance to determine an optimal number of features. A risk calculator was developed with the optimal number of features to calculate predicted probabilities of future clozapine use of FEP patients.
Results
Sample characteristics
Table 1 displays the comparison of basic demographics between patients with and without clozapine use. In general, patients with clozapine use compared to their counterparts had younger age of first service contact, with a lower education level, were more likely to have a schizophrenia diagnosis, and younger age of illness onset. The mean duration of first use of clozapine from the first service contact was 83.9 months (7 years) (SD = 48.9, median = 76.7, range = [2.17, 201.2]).
Table 1.
Sample characteristics of first-episode psychosis (FEP) patients with clozapine use compared to those without.
| Clozapine Use | |||||
|---|---|---|---|---|---|
| Characteristic | No, N = 1207a | Yes, N = 191a | T/ | p-valueb | q-valuec |
| Age at first service contact | 21.91 (3.41) | 20.27 (3.30) | 6.4 | <0.001 | <0.001 |
| Sex | 0.10 | 0.8 | 0.8 | ||
| Male | 617 (51%) | 100 (52%) | |||
| Female | 590 (49%) | 91 (48%) | |||
| Years of education | 10.82 (2.39) | 10.14 (2.19) | 3.9 | <0.001 | <0.001 |
| Diagnosis | 39 | <0.001 | <0.001 | ||
| Schizophrenia | 775 (64%) | 166 (87%) | |||
| Other diagnoses of psychotic disorders | 432 (36%) | 25 (13%) | |||
| Age of illness onset | 20.76 (3.47) | 19.26 (3.38) | 5.7 | <0.001 | <0.001 |
| Treatment | 3.8 | 0.052 | 0.072 | ||
| Early Intervention | 616 (51%) | 83 (43%) | |||
| Standard Care | 591 (49%) | 108 (57%) | |||
| DUP days (log) | 4.13 (1.87) | 4.25 (1.60) | −0.93 | 0.4 | 0.4 |
aMean (SD); n (%).
bWelch Two Sample t-test; Pearson’s Chi-squared test.
cFalse discovery rate correction for multiple testing.
The bold values are significant value after the false discovery rate correction for multiple testing.
Probability classification and predicted probability
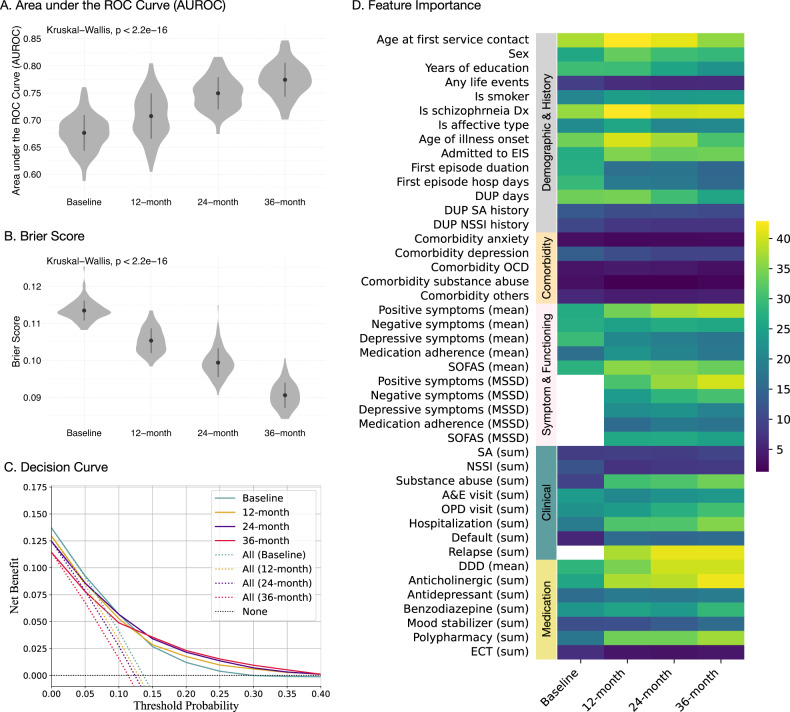
Figure 2A shows distribution of the AUROC with a mean and standard deviation (SD) over 100 repeated random subsampling. The autoML model discriminated between patients with and without future clozapine use with a baseline AUROC of 0.676 (SD = 0.033, 95% CI = [0.670, 0.683]), a 12-month AUROC of 0.707 (SD = 0.042, 95% CI = [0.699, 0.716]), a 24-month AUROC of 0.749 (SD = 0.030, 95% CI = [0.744, 0.755]), and a 36-month AUROC of 0.774 (SD = 0.031, 95% CI = [0.768, 0.780]). The model with longer longitudinal information had a better discrimination ability (Kruskal–Wallis χ2 = 227.9, df = 3, p < 2.2e-16).
Fig. 2. Performance of the baseline, 12-, 24- and 36-month probability classification models.
A Area under the receiver operating characteristic curve (AUROC). B Brier score. C Decision curve. The results indicate that models with longer longitudinal information performed better. D The average feature importance rank was calculated across 100 repetitions of the autoML procedures, where a higher value indicates greater importance of a feature. Dx diagnosis, EIS early intervention service, DUP duration of untreated psychosis, SA suicide attempts, NSSI non-suicidal self-injury, OCD obsessive-compulsive disorder, SOFAS Social and Occupational Functioning Assessment Scale, A&E accident & emergency, OPD out patient departments, DDD daily defined dose, ECT electroconvulsive therapy.
Figure 2B shows that each model had a low Brier score (<0.12), suggesting moderate to good agreement between observed and expected risk. The Brier scores were 0.113 (SD = 0.0027, 95% CI = [0.113, 0.114]) for the baseline model, 0.105 (SD = 0.0033, 95% CI = [0.105, 0.106]) for the 12-month model, 0.0994 (SD = 0.0039, 95% CI = [0.0986, 0.100]) for the 24-month model, and 0.0906 (SD = 0.0034, 95% CI = [0.0899, 0.0913]) for the 36-month model. Longer longitudinal information improves the Brier scores of probabilistic predictions (Kruskal–Wallis χ2 = 346.1, df = 3, p < 2.2e-16).
Figure 2C displays that all models outperformed the two extreme strategies of intervening in all or none of the patients, as indicated by the higher net benefits. Generally, the models with longer longitudinal information had better performance in terms of net benefits. The performance of the models at various thresholds is presented in Table 2 (Supplementary material for examples).
Table 2.
Performance measures for a range of dichotomous risk score cutoffs.
| Model | Threshold | Sensitivity | Specificity | PPV | NPV | NB | sNB |
|---|---|---|---|---|---|---|---|
| Baseline | 0.05 | 0.99 ± 0.01 | 0.04 ± 0.05 | 0.14 ± 0.01 | 0.97 ± 0.04 | 0.09 ± 0.00 | 0.42 ± 0.01 |
| 0.10 | 0.84 ± 0.06 | 0.39 ± 0.06 | 0.18 ± 0.01 | 0.94 ± 0.02 | 0.06 ± 0.01 | 0.26 ± 0.03 | |
| 0.15 | 0.54 ± 0.08 | 0.69 ± 0.04 | 0.22 ± 0.03 | 0.90 ± 0.01 | 0.03 ± 0.01 | 0.12 ± 0.04 | |
| 0.20 | 0.33 ± 0.07 | 0.85 ± 0.03 | 0.26 ± 0.05 | 0.89 ± 0.01 | 0.01 ± 0.01 | 0.05 ± 0.04 | |
| 0.25 | 0.18 ± 0.06 | 0.93 ± 0.03 | 0.29 ± 0.07 | 0.88 ± 0.01 | 0.00 ± 0.01 | 0.02 ± 0.03 | |
| 0.30 | 0.08 ± 0.05 | 0.97 ± 0.02 | 0.31 ± 0.15 | 0.87 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.02 | |
| 0.35 | 0.03 ± 0.03 | 0.99 ± 0.01 | 0.36 ± 0.31 | 0.87 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.02 | |
| 0.40 | 0.01 ± 0.01 | 1.00 ± 0.01 | 0.31 ± 0.38 | 0.86 ± 0.00 | 0.00 ± 0.00 | −0.01 ± 0.02 | |
| 12-month | 0.05 | 0.96 ± 0.04 | 0.15 ± 0.06 | 0.14 ± 0.01 | 0.97 ± 0.03 | 0.09 ± 0.00 | 0.39 ± 0.02 |
| 0.10 | 0.78 ± 0.08 | 0.49 ± 0.04 | 0.19 ± 0.01 | 0.94 ± 0.02 | 0.05 ± 0.01 | 0.24 ± 0.04 | |
| 0.15 | 0.55 ± 0.08 | 0.72 ± 0.03 | 0.23 ± 0.03 | 0.92 ± 0.01 | 0.03 ± 0.01 | 0.13 ± 0.05 | |
| 0.20 | 0.38 ± 0.06 | 0.85 ± 0.03 | 0.28 ± 0.05 | 0.90 ± 0.01 | 0.02 ± 0.01 | 0.08 ± 0.05 | |
| 0.25 | 0.24 ± 0.06 | 0.93 ± 0.02 | 0.34 ± 0.08 | 0.89 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.04 | |
| 0.30 | 0.15 ± 0.05 | 0.96 ± 0.01 | 0.40 ± 0.12 | 0.88 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.03 | |
| 0.35 | 0.09 ± 0.04 | 0.98 ± 0.01 | 0.45 ± 0.18 | 0.88 ± 0.00 | 0.00 ± 0.01 | 0.01 ± 0.02 | |
| 0.40 | 0.05 ± 0.03 | 0.99 ± 0.01 | 0.50 ± 0.27 | 0.88 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 | |
| 24-month | 0.05 | 0.97 ± 0.03 | 0.23 ± 0.05 | 0.15 ± 0.01 | 0.98 ± 0.02 | 0.09 ± 0.00 | 0.39 ± 0.02 |
| 0.10 | 0.79 ± 0.07 | 0.57 ± 0.03 | 0.21 ± 0.01 | 0.95 ± 0.01 | 0.06 ± 0.01 | 0.26 ± 0.03 | |
| 0.15 | 0.56 ± 0.07 | 0.77 ± 0.03 | 0.26 ± 0.03 | 0.93 ± 0.01 | 0.03 ± 0.01 | 0.16 ± 0.04 | |
| 0.20 | 0.40 ± 0.06 | 0.87 ± 0.02 | 0.31 ± 0.04 | 0.91 ± 0.01 | 0.02 ± 0.01 | 0.10 ± 0.04 | |
| 0.25 | 0.29 ± 0.06 | 0.92 ± 0.02 | 0.35 ± 0.07 | 0.90 ± 0.01 | 0.01 ± 0.01 | 0.06 ± 0.04 | |
| 0.30 | 0.20 ± 0.05 | 0.95 ± 0.02 | 0.38 ± 0.09 | 0.89 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.04 | |
| 0.35 | 0.14 ± 0.04 | 0.97 ± 0.01 | 0.42 ± 0.14 | 0.89 ± 0.01 | 0.00 ± 0.01 | 0.01 ± 0.04 | |
| 0.40 | 0.09 ± 0.04 | 0.98 ± 0.01 | 0.45 ± 0.17 | 0.88 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.03 | |
| 36-month | 0.05 | 0.96 ± 0.03 | 0.32 ± 0.05 | 0.15 ± 0.01 | 0.98 ± 0.01 | 0.08 ± 0.00 | 0.35 ± 0.01 |
| 0.10 | 0.74 ± 0.08 | 0.64 ± 0.03 | 0.21 ± 0.02 | 0.95 ± 0.01 | 0.05 ± 0.01 | 0.22 ± 0.03 | |
| 0.15 | 0.57 ± 0.07 | 0.81 ± 0.03 | 0.28 ± 0.03 | 0.94 ± 0.01 | 0.04 ± 0.01 | 0.16 ± 0.04 | |
| 0.20 | 0.42 ± 0.08 | 0.89 ± 0.02 | 0.33 ± 0.05 | 0.92 ± 0.01 | 0.02 ± 0.01 | 0.11 ± 0.04 | |
| 0.25 | 0.31 ± 0.07 | 0.93 ± 0.02 | 0.37 ± 0.07 | 0.91 ± 0.01 | 0.02 ± 0.01 | 0.07 ± 0.04 | |
| 0.30 | 0.23 ± 0.06 | 0.96 ± 0.01 | 0.41 ± 0.09 | 0.91 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.04 | |
| 0.35 | 0.16 ± 0.06 | 0.97 ± 0.01 | 0.44 ± 0.11 | 0.90 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.03 | |
| 0.40 | 0.10 ± 0.05 | 0.98 ± 0.01 | 0.42 ± 0.17 | 0.89 ± 0.01 | 0.00 ± 0.01 | 0.01 ± 0.03 |
Average feature importance rank of each variable over 100 repeated random subsampling is displayed in Fig. 2D. For the baseline model, the most important features were age at first service contact, schizophrenia diagnosis, age of onset, duration of first episode, days of hospitalization during first episode, days of DUP and DDD at baseline. For the 12-, 24- and 36-month models, longitudinal features, including number of months with relapse (Relapse [sum]), mean DDD, and number of months of anticholinergic use (Anticholinergic [sum]) were the most important features. Mean and MSSD of positive symptoms and SOFAS as well as polypharmacy were also important features.
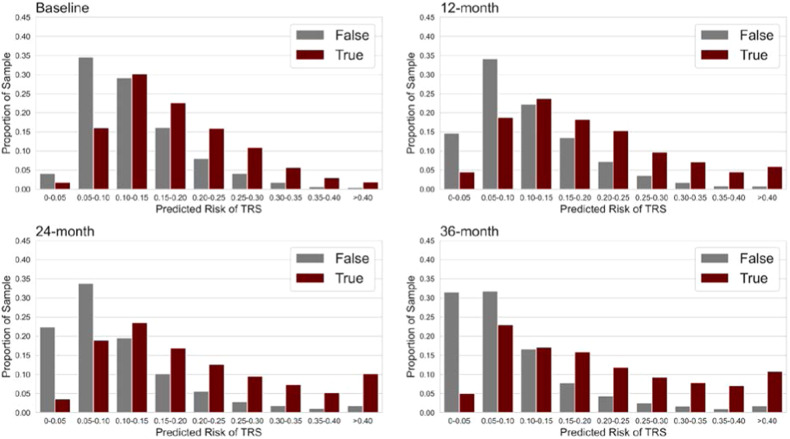
Figure 3 presents that patients with higher predicted probability had a higher chance of clozapine use after a threshold of 0.1 for all the models. With a progressively higher predicted probability, the proportion of clozapine use in patients increased. These patterns again suggested that our models were able to differentiate patients with and without future clozapine use.
Fig. 3. Frequency distributions of predicted risks of an individual whether prescribed with clozapine (true or false) of the baseline, 12-, 24- and 36-month probability classification models.
As the predicted risk increases from 0.10 or higher, there is a proportional increase in the number of individuals with clozapine use compared to those without in each subsequent risk class.
We evaluated our models with only top 10, top 15, or top 20 features selected by feature importance. Results suggested that models with top 10 features performed similarly in terms of AUROC and Brier score compared to the model with all features (Fig. S1). The baseline model with top 10 features performed slightly better than that with all features. Therefore, the final probability calculator was developed using only top 10 features with all our samples. A description of the calculator program can be found in the supplementary materials.
Discussion
In this population-based cohort study using intensively collected clinical data over 12 years in Hong Kong, we developed an individualized risk calculator to predict clozapine prescription, a proxy for TR status, using autoML. About 13.7% of FEP patients were prescribed clozapine, similar as in a previous population-based cohort study using Danish registry data (13.2%) [24]. The ML models identified future clozapine users with AUROC ranging from 0.676 (with baseline information) to 0.774 (with 36-month information). The AUROC of models with information across more than 12 months were all over 0.7, suggesting that the models with longitudinal clinical information have an acceptable prediction ability. Models with the top 10 features were found to have similar performance in terms of AUROC and Brier score compared to the full model and were thus used to establish the individualized risk calculator for development of TR in FEP (TRipCal).
Our models performed better than the previous attempts of using machine learning approaches in predicting the development of TR in psychosis [20, 21]. It is likely that the autoML allows for the optimal selection of the machine learning pipelines and hyperparameter optimization, and thus better handles the more complex real-life prediction needs of the psychiatric population. Furthermore, models with longitudinal clinical information performed better. These highlighted that longitudinal clinical information reflecting the dynamics of clinical characteristics and medication treatment over time may be more powerful in predicting the development of TR. The increased use of electronic health records (eHR) and the development of technology such as natural language processing in extracting relevant clinical information from the eHR would allow the automated use of longitudinal clinical information in individual risk calculators. This effort could develop into a data-driven clinical assistant system to support clinicians in tailoring individual patient interventions to postpone or prevent TR development as well as reduce delay in clozapine initiation.
Some of the predictive features identified in the current study are in line with the previous studies [17, 18], such as younger age of onset, schizophrenia diagnosis and relapse [1, 17, 18]. Duration and hospitalization of the first episode as well as the average DDD were found to be prominent features of the baseline prediction model. Having poor response to first-line antipsychotics early in treatment may reflect a different dopamine system function and would be an important indicator in predicting future TR development. This is aligned with findings of neuroimaging studies that patients with TRS have normal dopamine synthesis capacity [36, 37]. The significant role of DDD of antipsychotics and the number of relapses in predicting future TR status suggest the possibility of dopaminergic hypersensitivity as one of the mechanisms of development of TR [16, 38]. One notable finding of the current study is the increasing significance of the use of anticholinergic drugs in predicting future TR status. This may reflect the use of high antipsychotic dose leading to more extrapyramidal side effects, thus more use of anticholinergic medications. On the other hand, the loss of cholinergic neurons has been hypothesized as a possible pathogenesis of tardive dyskinesias, antipsychotic hypersensitivity and refractory status to antipsychotic treatment in patients with schizophrenia in earlier reports [39, 40]. Studies of other cohorts would be needed to replicate these risk factors of TR.
One of the key limitations of the study is the use of clozapine as a proxy for TR. Clozapine may be used to alleviate other conditions such as recurrent suicidality [41] and tardive dyskinesia [42]. However, over 90% of patients who were prescribed with clozapine were considered to have fulfilled the criteria of TRS [1, 43]. Furthermore, there are also individuals who had TR but were not on clozapine, that was estimated to be about 4% in our previous study of similar follow-up duration [1]. This group might impact the performance of the model development. Patients with a wide range of baseline FEP diagnosis were included in this study though 87% of patients on clozapine had a baseline diagnosis of schizophrenia. This approach, though not able to focus specifically on schizophrenia diagnosis and limit the interpretation of the results from a theoretical perspective, may have better translational value as results could be more readily integrated into the current practice of FEP service. Future larger sample studies could focus on the examination of predictors of treatment-resistant schizophrenia, particularly the possible differential predictors of TRS in the first episode and those after multiple episodes. Quality of the data retrieval, particularly the clinical symptoms, depending on the quality of the clinical record, could have contributed to information bias. Third, this study cohort has a limited age range and has a relatively low rate of comorbid substance use. Therefore, results might not be generalizable to other populations, validation studies with cohorts of different countries and characteristics are needed. Finally, a lack of external validation may limit the generalizability of the trained models. Future effort should focus on collecting additional data from diverse sources to validate the model’s performance and ensure its robustness and applicability in real-world scenarios.
In conclusion, our study presented the development of a risk calculator of future clozapine use, a proxy of TR, in FEP patients (TRipCal) over 12–17 years, using both baseline and longitudinal clinical information in the first 36 months of treatment. This work demonstrated the importance of longitudinal clinical information in predicting development of future TR with acceptable accuracy using the AutoML approach and thus the possibility of establishing data-driven tools assisting clinicians for earlier detection of individuals with higher risk of future TR development. The individual calculator developed using the top 10 features identified in the current study could be used to personalize the interventions to prevent, postpone TR development and reduce the delay of clozapine use. Future validation studies in different populations and settings are required.
Supplementary information
Acknowledgements
The current work is partly funded by the Health and Medical Research Fund Hong Kong (grant number:11121721); Health and Health Services Research Fund of Hong Kong (grant number: 03040141). The funder had no role in design and conduct of the study, no role collection, management, analysis, and interpretation of the data, no role in preparation, review or approval of the manuscript, no role in decision to submit the manuscript for publication.
Author contributions
SKWC designed the study, obtained the funding for the study, responsible for data collection, data analysis plan, and manuscript draft. TYW responsible for data analysis and manuscript preparation. HL, JT, TM and RG supported data analysis and were involved in manuscript preparation. YNS, CH responsible for data management and collection. WCC, EL, WCY, AL, EC, CKK, LTP, KMC responsible for data collection and clinical supervision, EYHC obtained research funding and oversees the data collection and supported manuscript preparation. All authors involved in finalizing the manuscript.
Data availability
Data of the study is available at the reasonable request to the corresponding author for research purpose.
Code availability
Data was preprocessed in R (version 4.1.3). All the probabilistic classification models were developed in Python (version 3.8.13) with scikit-learn and TPOT modules. Visualization was done in Python using the matplotlib module and R using the ggplot2 package. The calculator program was implemented using the tinker module in Python. All our analysis scripts are available at https://github.com/kamione/clozapineuse_prediction and source codes of our probability calculator application can be found at https://github.com/kamione/prob_calculator_clozapine.
Competing interests
EYHC is the convener of EASY service at the Hospital Authority EASY working group at a voluntary basis. SKWC, WCC and EHML were all members of the working group as a voluntary position. All authors have no specific financial interest relating to the subject.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-02754-w.
References
- 1.Chan SKW, Chan HYV, Honer WG, Bastiampillai T, Suen YN, Yeung WS, et al. Predictors of treatment-resistant and clozapine-resistant schizophrenia: a 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull. 2021;47:485–94. doi: 10.1093/schbul/sbaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 3.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/S0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer HY, Cola P, Way L, Thompson PA, Bastani B, Davies MA, et al. Cost effectiveness of clozapine in neuroleptic-resistant schizophrenia. Am J Psychiatry. 1993;150:1630–8. doi: 10.1176/ajp.150.11.1630. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76. doi: 10.1097/YIC.0b013e32836508e6. [DOI] [PubMed] [Google Scholar]
- 6.Iasevoli F, Giordano S, Balletta R, Latte G, Formato MV, Prinzivalli E, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:34–48. doi: 10.1016/j.pnpbp.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74:686–93. doi: 10.1001/jamapsychiatry.2017.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102. doi: 10.1186/1471-244X-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201:481–5. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 10.John AP, Ko EKF, Dominic A. Delayed initiation of clozapine continues to be a substantial clinical concern. Can J Psychiatry. 2018;63:526–31. doi: 10.1177/0706743718772522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah P, Iwata Y, Plitman E, Brown EE, Caravaggio F, Kim J, et al. The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: a systematic review. Psychiatry Res. 2018;268:114–22. doi: 10.1016/j.psychres.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 12.Siskind D, Orr S, Sinha S, Yu O, Brijball B, Warren N, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. 2022;220:115–120. doi: 10.1192/bjp.2021.61. [DOI] [PubMed] [Google Scholar]
- 13.Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–40. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- 14.Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull. 1998;24:75–85. doi: 10.1093/oxfordjournals.schbul.a033315. [DOI] [PubMed] [Google Scholar]
- 15.Meltzer HY. Treatment-resistant schizophrenia-the role of clozapine. Curr Med Res Opin. 1997;14:1–20. doi: 10.1185/03007999709113338. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka H, Kanahara N, Suzuki T, Takase M, Moriyama T, Watanabe H, et al. Impact of dopamine supersensitivity psychosis in treatment-resistant schizophrenia: An analysis of multi-factors predicting long-term prognosis. Schizophr Res. 2016;170:252–8. doi: 10.1016/j.schres.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Smart SE, Kępińska AP, Murray RM, MacCabe JH. Predictors of treatment resistant schizophrenia: a systematic review of prospective observational studies. Psychol Med. 2021;51:44–53. doi: 10.1017/S0033291719002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47:1981–9. doi: 10.1017/S0033291717000435. [DOI] [PubMed] [Google Scholar]
- 19.Leung CC-Y, Gadelrab R, Ntephe CU, McGuire PK, Demjaha A. Clinical course, neurobiology and therapeutic approaches to treatment resistant schizophrenia. Toward an integrated view. Front Psychiatry. 2019;10:601. doi: 10.3389/fpsyt.2019.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajnakina O, Agbedjro D, Lally J, Forti MD, Trotta A, Mondelli V, et al. Predicting onset of early- and late-treatment resistance in first-episode schizophrenia patients using advanced shrinkage statistical methods in a small sample. Psychiatry Res. 2020;294:113527. doi: 10.1016/j.psychres.2020.113527. [DOI] [PubMed] [Google Scholar]
- 21.Smart SE, Agbedjro D, Pardiñas AF, Ajnakina O, Alameda L, Andreassen OA, et al. Clinical predictors of antipsychotic treatment resistance: development and internal validation of a prognostic prediction model by the STRATA-G consortium. Schizophr Res. 2022;250:1–9. doi: 10.1016/j.schres.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legge SE, Dennison CA, Pardiñas AF, Rees E, Lynham AJ, Hopkins L, et al. Clinical indicators of treatment-resistant psychosis. Br J Psychiatry. 2020;216:259–266. doi: 10.1192/bjp.2019.120. [DOI] [PubMed] [Google Scholar]
- 23.Osimo EF, Perry BI, Mallikarjun P, Pritchard M, Lewis J, Katunda A, et al. Predicting treatment resistance from first-episode psychosis using routinely collected clinical information. Nat Ment Health. 2023;1:25–35. doi: 10.1038/s44220-022-00001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wimberley T, Støvring H, Sørensen HJ, Horsdal HT, MacCabe JH, Gasse C. Predictors of treatment resistance in patients with schizophrenia: a population-based cohort study. Lancet Psychiatry. 2016;3:358–66. doi: 10.1016/S2215-0366(15)00575-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen EYH, Tang JYM, Hui CLM, Chiu CPY, Lam MML, Law CW, et al. Three-year outcome of phase-specific early intervention for first-episode psychosis: a cohort study in Hong Kong. Early Inter Psychiatry. 2011;5:315–23. doi: 10.1111/j.1751-7893.2011.00279.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang JYM, Wong GHY, Hui CLM, Lam MML, Chiu CPY, Chan SKW, et al. Early intervention for psychosis in Hong Kong-the EASY programme. Early Inter Psychiatry. 2010;4:214–9. doi: 10.1111/j.1751-7893.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan SKW, So HC, Hui CLM, Chang WC, Lee EHM, Chung DWS, et al. 10-year outcome study of an early intervention program for psychosis compared with standard care service. Psychol Med. 2015;45:1181–93. doi: 10.1017/S0033291714002220. [DOI] [PubMed] [Google Scholar]
- 28.Chan SKW, Chan SWY, Pang HH, Yan KK, Hui CLM, Chang WC, et al. Association of an early intervention service for psychosis with suicide rate among patients with first-episode schizophrenia-spectrum disorders. JAMA Psychiatry. 2018;75:458–64. doi: 10.1001/jamapsychiatry.2018.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, et al. The Clinical Global Impression–Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand. 2003;107:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 30.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 31.Diagnostic A. Statistical manual of mental disorders DSM-IV-TR. American Psychiatric Association Task Force on DSM-IV; Washington DC; 2000.
- 32.von Neumann J, Kent RH, Bellinson HR, Hart BI. The mean square successive difference. Ann Math Stat. 1941;12:153–62. doi: 10.1214/aoms/1177731746. [DOI] [Google Scholar]
- 33.Nosè M, Tansella M, Thornicroft G, Schene A, Becker T, Veronese A, et al. Is the Defined Daily Dose system a reliable tool for standardizing antipsychotic dosages? Int Clin Psychopharmacol. 2008;23:287–90. doi: 10.1097/YIC.0b013e328303ac75. [DOI] [PubMed] [Google Scholar]
- 34.Olson RS, Bartley N, Urbanowicz RJ, Moore JH. Evaluation of a tree-based pipeline optimization tool for automating data science. In: Proceedings of the genetic and evolutionary computation conference 2016. New York, NY, USA: Association for Computing Machinery; 2016. p. 485–92.
- 35.Olson RS, Urbanowicz RJ, Andrews PC, Lavender NA, Kidd LC, Moore JH. Automating Biomedical Data Science Through Tree-Based Pipeline Optimization. In: Squillero, G., Burelli, P. (eds) Applications of Evolutionary Computation. EvoApplications 2016. Lecture Notes in Computer Science, vol 9597. Springer, Cham. 10.1007/978-3-319-31204-0_9.
- 36.Kim E, Howes OD, Veronese M, Beck K, Seo S, Park JW, et al. Presynaptic dopamine capacity in patients with treatment-resistant schizophrenia taking clozapine: An [18F]DOPA PET study. Neuropsychopharmacology. 2016;42:941–50. doi: 10.1038/npp.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 38.Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135:1409–10. doi: 10.1176/ajp.135.11.1409. [DOI] [PubMed] [Google Scholar]
- 39.Miller R, Chouinard G. Loss of striatal cholinergic neurons as a basis for tardive and L-dopa-induced dyskinesias, neuroleptic-induced supersensitivity psychosis and refractory schizophrenia. Biol Psychiatry. 1993;34:713–38. doi: 10.1016/0006-3223(93)90044-E. [DOI] [PubMed] [Google Scholar]
- 40.Miller R. Schizophrenia as a progressive disorder: relations to EEG, CT, neuropathological and other evidence. Prog Neurobiol. 1989;33:17–44. doi: 10.1016/0301-0082(89)90034-8. [DOI] [PubMed] [Google Scholar]
- 41.Masdrakis VG, Baldwin DS. Prevention of suicide by clozapine in mental disorders: systematic review. Eur Neuropsychopharmacol. 2023;69:4–23. doi: 10.1016/j.euroneuro.2022.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Wong J, Pang T, Cheuk NKW, Liao Y, Bastiampillai T, Chan SKW. A systematic review on the use of clozapine in treatment of tardive dyskinesia and tardive dystonia in patients with psychiatric disorders. Psychopharmacology. 2022;239:3393–420. doi: 10.1007/s00213-022-06241-2. [DOI] [PubMed] [Google Scholar]
- 43.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–29. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of the study is available at the reasonable request to the corresponding author for research purpose.
Data was preprocessed in R (version 4.1.3). All the probabilistic classification models were developed in Python (version 3.8.13) with scikit-learn and TPOT modules. Visualization was done in Python using the matplotlib module and R using the ggplot2 package. The calculator program was implemented using the tinker module in Python. All our analysis scripts are available at https://github.com/kamione/clozapineuse_prediction and source codes of our probability calculator application can be found at https://github.com/kamione/prob_calculator_clozapine.