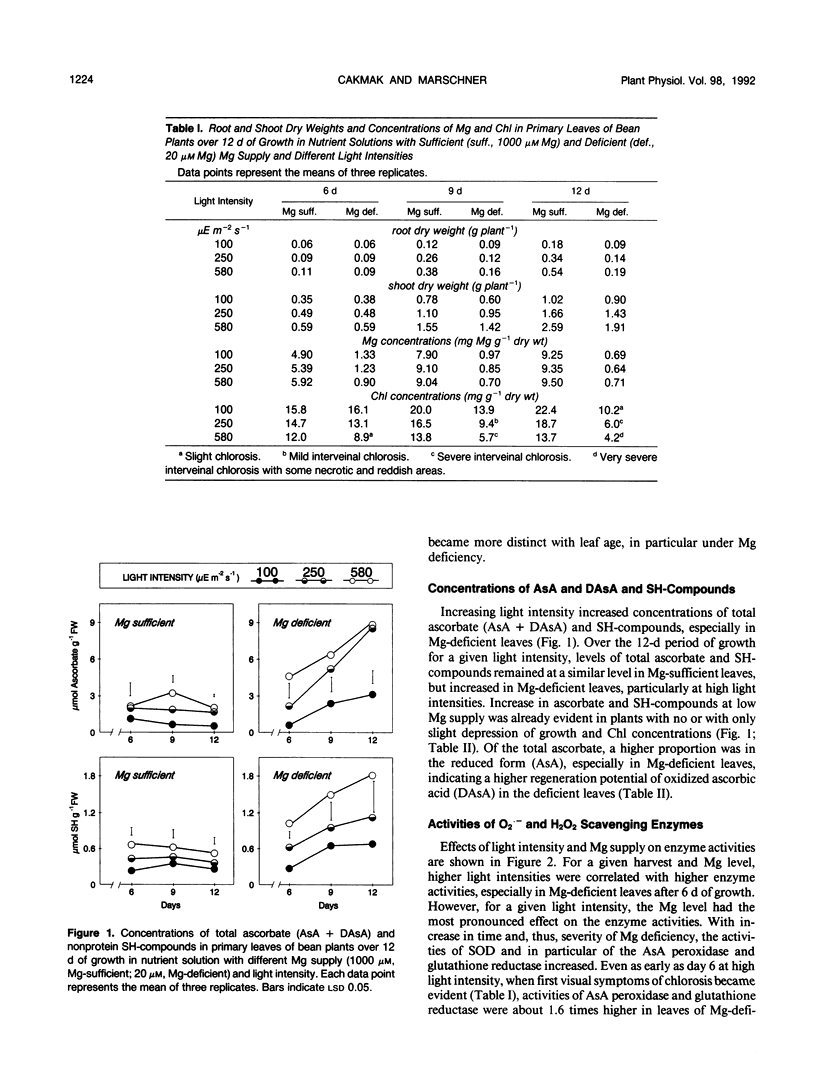
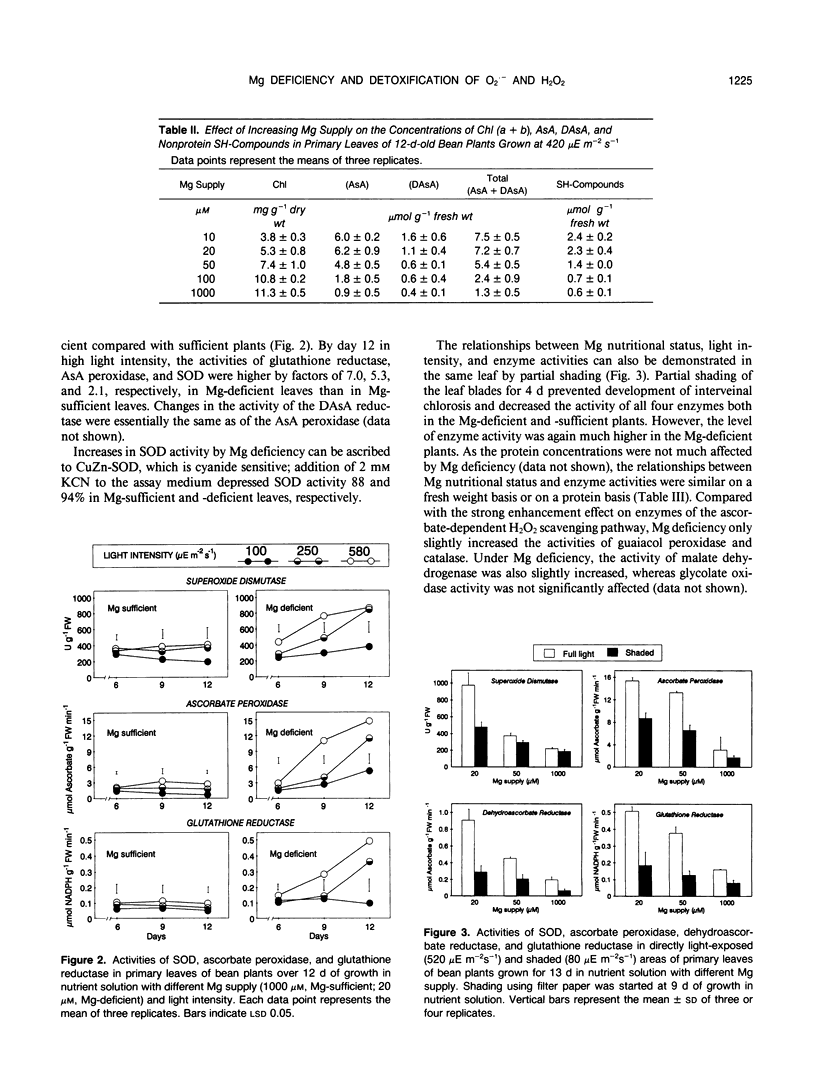
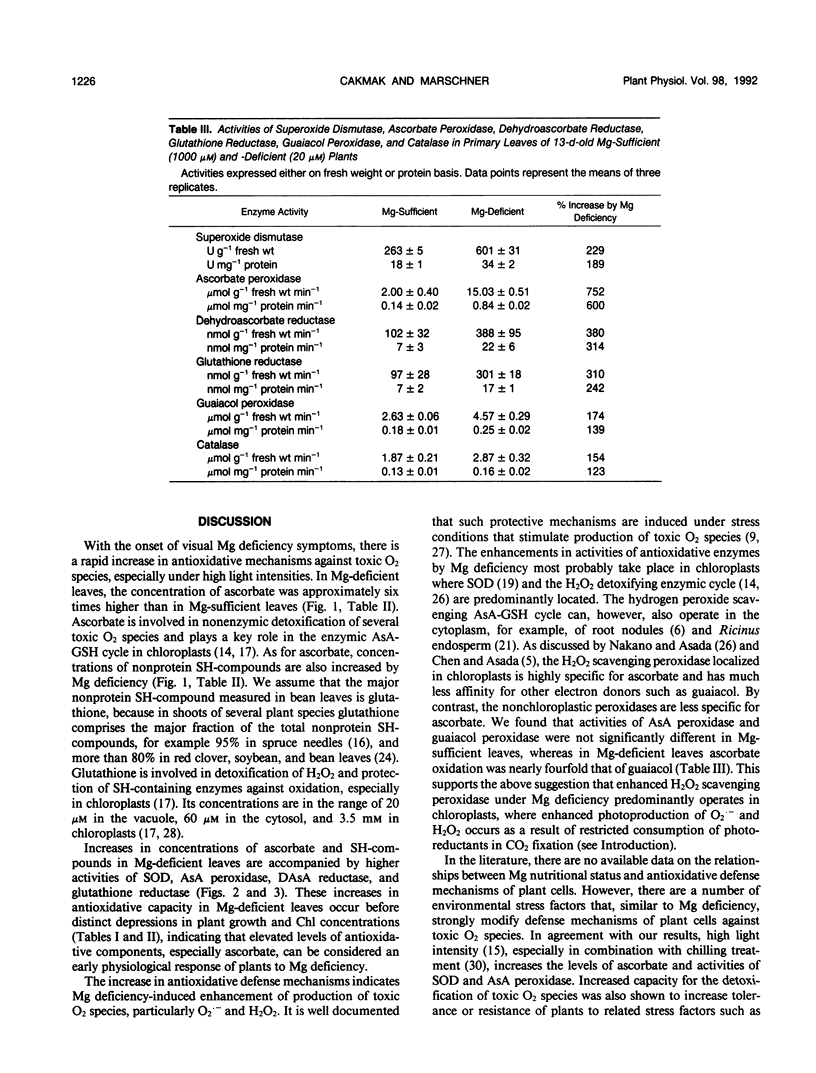
Abstract
The influence of varied Mg supply (10-1000 micromolar) and light intensity (100-580 microeinsteins per square meter per second) on the concentrations of ascorbate (AsA) and nonprotein SH-compounds and the activities of superoxide dismutase (SOD; EC 1.15.11) and the H2O2 scavenging enzymes, AsA peroxidase (EC 1.11.1.7), dehydroascorbate reductase (EC 1.8.5.1), and glutathione reductase (EC 1.6.4.2) were studied in bean (Phaseolus vulgaris L.) leaves over a 13-day period. The concentrations of AsA and SH-compounds and the activities of SOD and H2O2 scavenging enzymes increased with light intensity, in particular in Mg-deficient leaves. Over the 12-day period of growth for a given light intensity, the concentrations of AsA and SH-compounds and the activities of these enzymes remained more or less constant in Mg-sufficient leaves. In contrast, in Mg-deficient leaves, a progressive increase was recorded, particularly in concentrations of AsA and activities of AsA peroxidase and glutathione reductase, whereas the activities of guaiacol peroxidase and catalase were only slightly enhanced. Partial shading of Mg-deficient leaf blades for 4 days prevented chlorosis, and the activities of the O2.− and H2O2 scavenging enzymes remained at a low level. The results demonstrate the role of both light intensity and Mg nutritional status on the regulation of O2.− and H2O2 scavenging enzymes in chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton D. A., Russell S. A., Hanus F. J., Pascoe G. A., Evans H. J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Foster J. G., Hess J. L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980 Sep;66(3):482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopolitis C. N., Ries S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977 Feb;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor R. E., Bahr J. T., Jensen R. G. Measurement of the Enzyme-CO(2)-Mg Form of Spinach Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1986 Feb;80(2):599–600. doi: 10.1104/pp.80.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1974 Sep;54(3):379–381. doi: 10.1104/pp.54.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]


