Abstract
Copper is an essential trace element for the human body. The epidemiological evidence for the association of dietary intake of copper with the risk of Parkinson’s disease (PD) is limited. We conducted an evaluation of the cross-sectional data gathered from the National Health and Nutrition Examination Surveys spanning from 2007 to 2018, which comprised a total of 17,948 participants. To discern the distinct characteristics of the participants, we performed a univariate analysis and utilized a 1:2 ratio propensity score matching method to minimize the effects of selection bias. We employed weighted univariate as well as three multivariate logistic regression models both prior to and following matching, with the aim of examining the association between dietary copper intake and PD risk. Finally, we used the restricted cubic spline (RCS) methodology in order to investigate possible non-linear relationships. Furthermore, subgroup analysis was undertaken to elicit further understanding concerning the association between copper intake and PD. A negative correlation resulted between dietary copper intake and PD risk in both univariate and multivariate logistic regression models, prior to and following matching. Our findings demonstrate that there is a nonlinear, dose-dependent relationship between copper intake and PD, according to our RCS analysis. In subgroup analysis, copper intake was identified as an important protective factor for individuals who were non-Hispanic White, unmarried, and had completed higher education. Dietary copper intake was associated with the risk of PD. Supplementation of dietary copper may have potentially beneficial effects.
Keywords: Parkinson’s disease (PD), Dietary copper intake, Propensity score matching (PSM), The National Health and Nutritional Examination Surveys (NHANES)
Introduction
Parkinson’s disease (PD) is a progressive neurological disorder characterized by the degeneration of dopaminergic neurons [1]. At present, it is believed that the pathogenesis of PD is the result of the interaction between genetic factors and the environment [2]. Epidemiological studies have shown that there is a clear association between the occurrence of PD and exposure to some heavy metals such as lead, mercury, copper, and zinc [3]. After entering the body, these trace metals cross the blood-brain barrier and disrupt the redox equilibrium, causing free radical formation and reducing antioxidant enzyme levels [3, 4]. They may inhibit mitochondrial ATP synthesis and impair metabolic processes, which in turn destroy dopaminergic neurons [3]. However, they are equally indispensable trace elements involved in the proper functioning of many cellular enzymes and proteins [5–8]. In conclusion, the imbalance of heavy metal levels is closely related to the pathogenesis of PD.
A large number of enzymes rely on copper as a cofactor to function [9]. Copper is also essential for metabolism in the body [9]. Moreover, copper plays a key role in mitochondrial electron transport and is an essential cofactor for oxidoreductases [10, 11]. Copper, on the other hand, is a low-toxic metal, and excessive amounts will damage the nervous system [10]. It contributes to the pathological process of PD [10, 12, 13]. A debate exists regarding the correlation between copper concentration and PD pathogenesis. In some theories, excessive copper toxicity contributes to PD pathology [14], while some studies have found that copper deficiency contributes to PD pathology [15–17]. It has been demonstrated that PD patients have altered copper levels in their brains and serums [16, 18, 19]. A meta-analysis confirmed the presence of copper deficiency in the substantia nigra of patients with PD; however, no significant difference was observed in the levels of copper in their cerebrospinal fluid and serum [20]. Although a few epidemiological studies have suggested that there is no association between dietary copper intake and the risk of PD [21, 22], as mentioned above, there is a subtle association between dietary copper intake and PD, and the relationship between copper and PD risk is worthy of further investigation.
Given these facts, there is a requirement to clarify the associations between dietary copper intake and the risk of PD. Here, we conducted a cross-sectional study based on the National Health and Nutrition Examination Survey (NHANES) database aiming to evaluate dietary intake of copper and the risk of PD, which can be used as a reference for the prevention of PD.
Methods
Database and Survey Populations
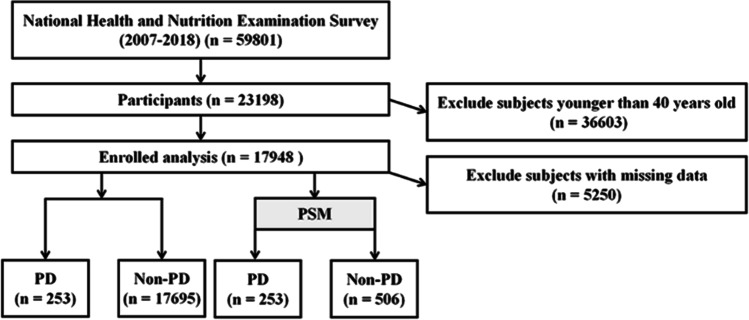
Data for this study were obtained from the National Health and Nutrition Examination Survey website (https://www.cdc.gov/nchs/nhanes/index.htm). We constructed a dataset for this study by using publicly available NHANES responses from 2007 to 2018. A total of 17,948 NHANES participants over 40 years old were included in this study. The entire data integration process is shown in Fig. 1.
Fig. 1.
Flow chart of study inclusion. Flowchart of the participants’ selection from NHANES 2007–2018
Assessment of Parkinson’s Disease
In the NHANES database, combined with previous literature [23–25], participants with Parkinson’s disease were identified by using “ANTIPAKINSON AGENTS”; this determination was made based on the responses to prescription medication questions. Due to the limitations of medications and codes included in the NHANES, an individual had to be receiving treatment for PD to be classified as having it, and others were identified with non-Parkinson’s disease.
Dietary Copper Intake
Copper intake data was obtained from the NHANES database, and participants were asked to recall their copper intake twice in 24 h. During the first recall interview, the respondent completed a test at the NHANES Mobile Testing Center, while respondents were asked to complete a telephone interview 3–10 days in the second recall interview. In our study, to calculate dietary copper intake, we averaged data from two dietary recalls; otherwise, the single diet data will be deleted.
Covariates Assessment
The main covariates were demographic characteristics and chronic comorbidities. Demographic characteristics including age, gender, race, education level, and marital status were reported by interviewees. Chronic comorbidities included diabetes, coronary heart disease, hypertension, stroke, and viral hepatitis, which were diagnosed based either by the doctor or by a self-report questionnaire. Weight and height were measured by well-trained health technologists, and BMI was calculated as weight (kg) divided by height squared (m2).
Statistical Analysis
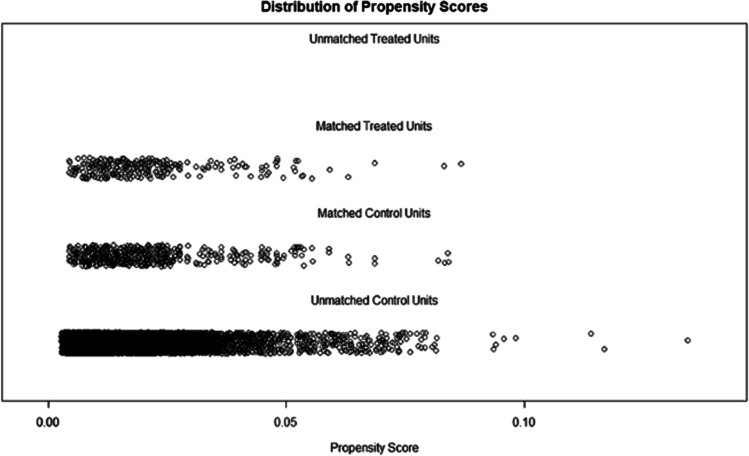
Data extraction and analyses were performed with the use of the “nhanesR” package of R software (4.2.1). Categorical variables are expressed as frequency and percentages, while continuous variables are expressed as means and standard deviations (SD). The analysis was based on the non-parametric Mann-Whitney U test or the t-test for continuous variables. During the comparisons of categorical variables, chi-square tests and Fisher’s exact tests were used. Propensity score matching (PSM) is a method that can reduce selection bias to strengthen causal arguments in observational studies (as shown in Fig. 2). A 1:2 rule is used in PSM to perform nearest-neighbor matching. An analysis of the relationship between dietary copper intake and PD risk was conducted using weighted univariate and three multivariate logistic regression models and possible non-linear relationships are determined by restricted cubic splines (RCS). Finally, we conducted subgroup analyses according to age, gender, race, marital status, and education level.
Fig. 2.
“Jitter” diagram of PSM. Before undergoing propensity score matching (PSM) at a 1:2 ratio, unmatched treated units, and unmatched non-treated units represented the distribution of individuals in the PD and non-PD groups, respectively. After matching, the two groups were represented by matched treated units and matched non-treated units, respectively. The figure illustrates the balanced distribution of individuals in the matched groups following PSM
Results
Characteristics of Included Participants Before Matching by PD
As shown in Table 1, there were significant differences in gender (p = 0.027), age (p = 0.003), race (p = 0.017), coronary heart disease (p = 0.038), and stroke (p < 0.0001) between the PD and non-PD groups. It seems that the participants were older than those of the non-PD group (61.262±1.103) vs. 57.938(±0.174) years). Most importantly, there was a significant difference in dietary copper intake between PD and non-PD with 1.069 (±0.047) and 1.276 (±0.014) (p<0.0001).
Table 1.
Baseline characteristics of participants with or without PD
| Characters | Total | Non-Parkinson | Parkinson | p-value |
|---|---|---|---|---|
| Gender | 0.027 | |||
| Male | 8571 (47.757) | 8458 (46.819) | 113 (35.969) | |
| Female | 9376 (52.243) | 9236 (53.181) | 140 (64.031) | |
| Age | 57.983 (0.175) | 57.938 (0.174) | 61.262 (1.103) | 0.003 |
| Race | 0.017 | |||
| Non-Hispanic White | 8103 (45.15) | 7937 (71.541) | 166 (81.310) | |
| Non-Hispanic Black | 3956 (22.043) | 3918 (10.478) | 38 (8.874) | |
| Mexican American | 2364 (13.172) | 2342 (6.333) | 22 (3.382) | |
| Other race | 3524 (19.636) | 3497 (11.649) | 27 (6.434) | |
| Marital | 0.060 | |||
| Married | 13,062 (72.781) | 12,880 (74.529) | 182 (64.728) | |
| Unmarried | 4885 (27.219) | 4814 (25.471) | 71 (35.272) | |
| Education | 0.089 | |||
| Lower high school | 4464 (24.873) | 4390 (15.212) | 74 (22.804) | |
| High school | 4158 (23.168) | 4100 (23.535) | 58 (25.054) | |
| Over high school | 9325 (51.959) | 9204 (61.253) | 121 (52.142) | |
| BMI | 29.532 (0.097) | 29.518 (0.097) | 30.564 (0.557) | 0.062 |
| Copper | 1.273 (0.014) | 1.276 (0.014) | 1.069 (0.047) | < 0.0001 |
| Coronary heart disease | 0.038 | |||
| No | 16,846 (93.865) | 16,617 (94.624) | 229 (89.155) | |
| Yes | 1101 (6.135) | 1077 (5.376) | 24 (10.845) | |
| Diabetes | 0.215 | |||
| No | 12,306 (68.569) | 12,154 (75.277) | 152 (69.177) | |
| Pre-diabetes | 1133 (6.313) | 1116 (5.635) | 17 (6.775) | |
| Diabetes | 4508 (25.118) | 4424 (19.088) | 84 (24.048) | |
| Hypertension | 0.004 | |||
| No | 7769 (43.289) | 7692 (49.248) | 77 (34.917) | |
| Yes | 10,178 (56.711) | 10,002 (50.752) | 176 (65.083) | |
| Stroke | < 0.0001 | |||
| No | 16,926 (94.311) | 16,710 (95.664) | 216 (84.270) | |
| Yes | 1021 (5.689) | 984 (4.336) | 37 (15.730) | |
| Viral hepatitis | 0.475 | |||
| No | 17,449 (97.225) | 17,206 (97.571) | 243 (96.759) | |
| Yes | 498 (2.775) | 488 (2.429) | 10 (3.241) |
Data are presented as N% (χ2 test) or mean (SD) (independent t-test). *p < 0.05; **p < 0.01; ***p < 0.001
The use of bold font indicates that the corresponding p-values are less than 0.05, signifying statistical significance
Characteristics of Participants After Matching by PD
Our study established a comparable control group using closest-neighbor PSM (1:2) to help confirm the association between dietary copper intake and the risk of Parkinson’s disease. After PSM, data sets with 253 participants in the PD group and 506 participants in the non-PD group were constructed. Table 2 shows that dietary copper intake (p = 0.001) was also significantly different between the PD group and the non-PD group after matching, and other covariates were not statistically different (Table 2).
Table 2.
Characteristics of participants after matching by PD
| Characters | Total | Non-Parkinson | Parkinson | p-value |
|---|---|---|---|---|
| Gender | 0.689 | |||
| Male | 328 (43.215) | 215 (43.192) | 113 (35.969) | |
| Female | 431 (56.785) | 291 (56.808) | 140 (64.031) | |
| Age | 62.052 (0.594) | 62.383 (0.641) | 61.262 (1.103) | 0.35 |
| Race | 0.984 | |||
| Non-Hispanic White | 500 (65.876) | 334 (86.016) | 166 (81.310) | |
| Non-Hispanic Black | 113 (14.888) | 75 (5.611) | 38 (8.874) | |
| Mexican American | 68 (8.959) | 46 (2.791) | 22 (3.382) | |
| Other race | 78 (10.277) | 51 (5.581) | 27 (6.434) | |
| Marital | 0.55 | |||
| Married | 563 (74.177) | 381 (74.463) | 182 (64.728) | |
| Unmarried | 196 (25.823) | 125 (25.537) | 71 (35.272) | |
| Education | 0.986 | |||
| Lower high school | 226 (29.776) | 152 (19.764) | 74 (22.804) | |
| High school | 173 (22.793) | 115 (20.500) | 58 (25.054) | |
| Over high school | 360 (47.431) | 239 (59.736) | 121 (52.142) | |
| BMI | 30.409 (0.392) | 30.344 (0.488) | 30.564 (0.557) | 0.758 |
| Copper | 1.235 (0.037) | 1.293 (0.048) | 1.096 (0.036) | 0.001 |
| Coronary heart disease | 0.705 | |||
| No | 680 (89.592) | 451 (88.165) | 229 (89.155) | |
| Yes | 79 (10.408) | 55 (11.835) | 24 (10.845) | |
| Diabetes | 0.957 | |||
| No | 455 (59.947) | 303 (70.250) | 152 (69.177) | |
| Pre-diabetes | 48 (6.324) | 31 (4.944) | 17 (6.775) | |
| Diabetes | 256 (33.729) | 172 (24.806) | 84 (24.048) | |
| Hypertension | 0.878 | |||
| No | 227 (29.908) | 150 (36.458) | 77 (34.917) | |
| Yes | 532 (70.092) | 356 (63.542) | 176 (65.083) | |
| Stroke | 0.836 | |||
| No | 652 (85.903) | 436 (89.776) | 216 (84.270) | |
| Yes | 107 (14.097) | 70 (10.224) | 37 (15.730) | |
| Viral hepatitis | 0.759 | |||
| No | 732 (96.443) | 489 (97.087) | 243 (96.759) | |
| Yes | 27 (3.557) | 17 (2.913) | 10 (3.241) |
Data are presented as N% (χ2 test) or mean (SD) (independent t-test). *p < 0.05; **p < 0.01; ***p < 0.001
The use of bold font indicates that the corresponding p-values are less than 0.05, signifying statistical significance
Correlation Analysis of Copper Intake and PD Before and After Matching
Analysis of Univariate Logistics Regressions of Copper Intake and Physical Activity Before and After Matching
Table 3 shows that age and BMI were positively correlated with PD occurrence, and the effect value OR and 95% confidence interval were 1.024 (1.009, 1.040) (p=0.003) and 1.022 (1.001, 1.044) (p=0.043). Females were more likely to develop PD than males, with an OR of 1.567 (1.049, 2.342) (p = 0.029). Coronary heart disease, hypertension, and strokes were associated with a higher risk of PD than those without coronary heart disease, hypertension, and strokes, with odds ratios and 95% confidence intervals of 2.141 (1.027, 4.464) (p=0.042), 1.809 (1.202, 2.722) (p=0.005), and 4.119 (2.351, 7.214) (p<0.0001), respectively. Most importantly, we found that copper intake was negatively associate with the occurrence of PD (OR: 0.574 (0.404, 0.816) (p=0.002) and 0.498 (0.337, 0.735) (p<0.001)) before and after matching, respectively (Table 3).
Table 3.
Univariate logistics regression analysis of the association between copper intake and PD before and after matching
| Characters | Unmatching | Matching | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Gender | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 1.567 (1.049, 2.342) | 0.029 | 1.353 (0.852, 2.150) | 0.197 |
| Age | 1.024 (1.009, 1.040) | 0.003 | 0.992 (0.976, 1.009) | 0.350 |
| Race | ||||
| Non-Hispanic White | Ref | Ref | Ref | Ref |
| Non-Hispanic Black | 0.745 (0.420, 1.323) | 0.312 | 1.673 (0.934, 2.997) | 0.083 |
| Mexican American | 0.470 (0.270, 0.817) | 0.008 | 1.282 (0.651, 2.525) | 0.468 |
| Other race | 0.486 (0.271, 0.870) | 0.016 | 1.219 (0.555, 2.678) | 0.617 |
| Marital | ||||
| Married | Ref | Ref | Ref | Ref |
| Unmarried | 1.594 (0.977, 2.603) | 0.062 | 1.589 (0.903, 2.797) | 0.107 |
| Education | ||||
| Lower high school | Ref | Ref | Ref | Ref |
| High school | 0.710 (0.381, 1.322) | 0.277 | 1.059 (0.511, 2.193) | 0.876 |
| Over high school | 0.568 (0.321, 1.004) | 0.052 | 0.757 (0.397, 1.441) | 0.392 |
| BMI | 1.022 (1.001, 1.044) | 0.043 | 1.004 (0.979, 1.030) | 0.760 |
| Copper | 0.574 (0.404, 0.816) | 0.002 | 0.498 (0.337, 0.735) | <0.001 |
| Coronary heart disease | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 2.141 (1.027, 4.464) | 0.042 | 0.906 (0.416, 1.976) | 0.802 |
| DM | ||||
| No | Ref | Ref | Ref | Ref |
| Pre-diabetes | 1.308 (0.675, 2.536) | 0.422 | 1.392 (0.566, 3.424) | 0.468 |
| Diabetes | 1.371 (0.958, 1.961) | 0.084 | 0.984 (0.643, 1.507) | 0.942 |
| Hypertension | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.809 (1.202, 2.722) | 0.005 | 1.069 (0.660, 1.732) | 0.783 |
| Stroke | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 4.119 (2.351, 7.214) | <0.0001 | 1.639 (0.849, 3.163) | 0.139 |
| Viral hepatitis | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.346 (0.590, 3.068) | 0.476 | 1.117 (0.328, 3.805) | 0.858 |
*p < 0.05; ** p < 0.01; *** p < 0.001
The use of bold font indicates that the corresponding p-values are less than 0.05, signifying statistical significance
Multivariable Logistics Regression Analysis of the Relationship Between Copper Intake and PD Before and After Matching
We utilized three logistic regression models (Table 4) to investigate the connection between copper intake and PD. Model 1 was a crude model that did not adjust for any covariates, while model 2 adjusted for age, gender, race, and BMI. Model 3 incorporated adjustments for age, gender, race, education level, BMI, marital status, coronary heart disease, diabetes, hypertension, stroke, and viral hepatitis. In both pre- and post-matching scenarios, our findings indicate that all models displayed significant disparities. Our results suggest that daily copper intake is closely linked to PD, with OR values and 95% confidence intervals of 0.574 (0.404, 0.816), 0.627 (0.437, 0.900), and 0.696 (0.498, 0.973) (p<0.05) before matching, respectively. This implies that an increase in dietary copper intake acts as a protective factor against PD and is negatively associated with the risk of PD. Moreover, a significant correlation between copper intake and PD persisted even after matching was conducted.
Table 4.
Multivariable logistics regression analysis of the association between copper intake and PD before and after matching
| Model | Unmatching | Matching | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | p for trend | OR (95% CI) | p-value | p for trend | |
| Model 1 | 0.574 (0.404, 0.816) | 0.002 | 0.002 | 0.498 (0.337, 0.735) | <0.001 | <0.001 |
| Model 2 | 0.627 (0.437, 0.900) | 0.012 | 0.012 | 0.490 (0.322, 0.745) | 0.001 | 0.001 |
| Model 3 | 0.696 (0.498, 0.973) | 0.034 | 0.034 | 0.498 (0.327, 0.759) | 0.002 | 0.002 |
Model 1: no adjustments made for confounding factors. Model 2: adjustments made for age, gender, race, and BMI. Model 3: adjustments made for age, gender, race, education level, BMI, marital status, coronary heart disease, diabetes, hypertension, stroke, and viral hepatitis. *p < 0.05; **p < 0.01; ***p < 0.001
The use of bold font indicates that the corresponding p-values are less than 0.05, signifying statistical significance
Nonlinear Relationship Between Dietary Copper Intake and the Risk of PD
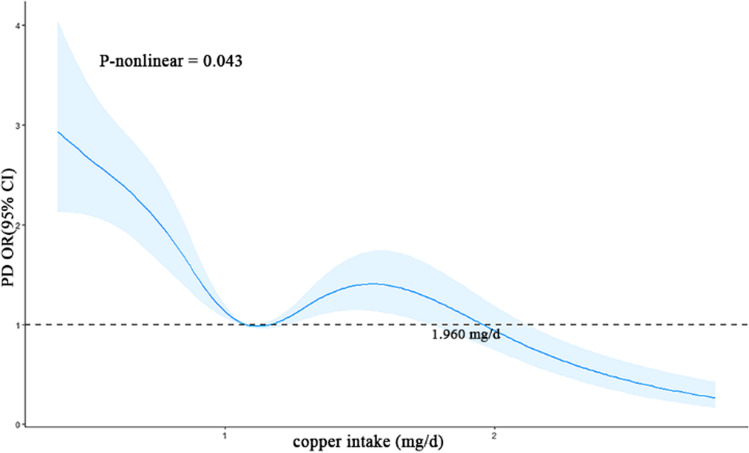
Utilizing RCS, we generated a graphical depiction (Fig. 3) of the connection between dietary copper intake and PD. Our results indicate a non-linear relationship between dietary copper intake and PD risk (p=0.04) after matching had been performed. Specifically, our findings suggest that copper intake is a risk factor for PD when intake levels remain below 1.960 mg/day. However, the risk of developing PD shows a gradual decrease as copper intake surpasses 1.960 mg/day.
Fig. 3.
The RCS plot between copper intake and PD. RCS, restricted cubic spline. RCS plot between copper intake and PD after matching. The x-axis represents copper intake (mg/day), while the y-axis represents the odds ratio (OR) and the 95% confidence interval (CI) for PD (blue area). The dashed line indicates an OR of 1, which represents no association between copper intake and PD risk. The model adjusted by BMI, age, gender, race, marital status, education level, coronary heart disease, diabetes, hypertension, stroke, and viral hepatitis
Subgroup Analysis Before and After Matching
To determine whether the relationship between copper intake and PD varied by age, gender, race, marital, and education, stratified analyses were employed. Based on subgroup analysis, copper intake was correlated with PD more strongly in 50–60-year-olds after matching, with odds ratios of 0.293 (0.133, 0.647), while were no significant differences before matching. Likewise, male, non-Hispanic White, unmarried, and over high school had a stronger correlation between copper intake and PD before matching with odds ratios of 0.548 (0.356, 0.844), 0.539 (0.349, 0.832), 0.390 (0.212, 0.715), and 0.529 (0.331, 0.846), while female, non-Hispanic White, unmarried, and over high school had a stronger correlation between copper intake and PD after matching with odds ratios of 0.443 (0.225, 0.874), 0.442 (0.267, 0.733), 0.277 (0.115, 0.667), and 0.522 (0.283, 0.964), respectively. There is an interaction between copper intake and marital status (p = 0.013) before PSM. In a general way, the consistent results before and after PSM matching are considered to be reliable. Therefore, copper intake was found to be a significant protective factor in people who were non-Hispanic White, unmarried, and over high school (Table 5).
Table 5.
Subgroup analysis before and after matching
| Characters | Unmatching | Matching | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | p for interaction | OR (95% CI) | p-value | p for interaction | |
| Age | 0.683 | 0.118 | ||||
| 40-50 year old | 0.542 (0.284, 1.032) | 0.062 | 0.243 (0.050, 1.166) | 0.069 | ||
| 50-60 year old | 0.576 (0.295, 1.127) | 0.106 | 0.293 (0.133, 0.647) | 0.004 | ||
| 60-70 year old | 0.604 (0.319, 1.143) | 0.119 | 0.404 (0.144, 1.134) | 0.082 | ||
| >70 year old | 0.772 (0.505, 1.178) | 0.227 | 1.046 (0.519, 2.107) | 0.898 | ||
| Gender | 0.268 | 0.584 | ||||
| Male | 0.548 (0.356, 0.844) | 0.007 | 0.635 (0.363, 1.111) | 0.109 | ||
| Female | 0.771 (0.497, 1.196) | 0.242 | 0.443 (0.225, 0.874) | 0.020 | ||
| Race | 0.164 | 0.252 | ||||
| Non-Hispanic White | 0.539 (0.349, 0.832) | 0.006 | 0.442 (0.267, 0.733) | 0.002 | ||
| Non-Hispanic Black | 0.788 (0.455, 1.364) | 0.390 | 0.907 (0.331, 2.488) | 0.802 | ||
| Mexican American | 1.064 (0.944, 1.200) | 0.305 | 0.747 (0.247, 2.263000e+00) | 0.658 | ||
| Other race | 0.910 (0.454, 1.825) | 0.788 | 0.848 (0.335, 2.148) | 0.757 | ||
| Marital | 0.013 | 0.118 | ||||
| Married | 0.784 (0.580, 1.059) | 0.111 | 0.646 (0.390, 1.071) | 0.089 | ||
| Unmarried | 0.390 (0.212, 0.715) | 0.003 | 0.277 (0.115, 0.667) | 0.006 | ||
| Education | 0.546 | 0.641 | ||||
| Lower high school | 0.870 (0.619, 1.222) | 0.416 | 0.709 (0.340, 1.478) | 0.348 | ||
| High school | 0.703 (0.223, 2.214) | 0.543 | 0.465 (0.143, 1.510) | 0.190 | ||
| Over high school | 0.529 (0.331, 0.846) | 0.008 | 0.522 (0.283, 0.964) | 0.038 | ||
Subgroup analysis for the association between copper intake and PD. Weighted univariate logistic regression was used for subgroup analysis. Adjustments were made for BMI, coronary heart disease, diabetes, hypertension, stroke, and viral hepatitis. *p < 0.05; **p < 0.01; ***p < 0.001
The use of bold font indicates that the corresponding p-values are less than 0.05, signifying statistical significance
Discussion
Copper is an essential metal element for proper nervous system function. However, the exact role of metallic copper in PD remains unclear. Therefore, it is crucial from both clinical and societal perspectives to investigate the relationship between dietary copper and PD. In our study, we examined the correlation between dietary copper intake and PD, with multivariate logistic regression revealing a negative association between increased copper intake and PD risk. To minimize confounding variables’ influence, we utilized PSM analysis to account for differences between the PD and non-PD groups. After matching, RCS analysis demonstrated that the relationship between dietary copper intake and PD risk was non-linear.
Copper is an essential metal element for human bodily function, primarily obtained and supplemented through daily dietary intake via the small intestine [26]. Within the body, copper plays a pivotal role in activating various enzyme activities, cell physiological metabolism, oxidative phosphorylation, and electron transport [3, 5–7, 10]. Disruptions to copper homeostasis can lead to nervous system diseases [6, 8, 10], though its association with PD remains controversial. Some studies have suggested that copper concentrations in PD blood are reduced [15, 16, 18], while others have reported increased copper concentration in PD blood [27]. Additionally, meta-analyses have failed to identify significant differences in serum copper concentration between individuals with PD and those without [20].
The results of copper concentration in cerebrospinal fluid of PD are used to be controversial. An earlier study involving 24 PD patients and 34 controls found that PD patients had higher copper concentrations in their CSF [28]. Furthermore, by comparing 215 PD patients with 119 controls, no difference was found in CSF copper levels in PD patients [29]. The findings of a 2019 meta-analysis indicated that there was no significant difference in the levels of copper observed in the cerebrospinal fluid of patients with PD and those without the condition [20]. Interestingly, analysis of copper content in frozen sections of brain parenchyma revealed a significant reduction in copper content, ranging from 45 to 65%, in substantia nigra with severe PD lesions. This finding was further confirmed by electron microscopy imaging, which showed a significant reduction in copper content by 55% in neurons in locus coeruleus [19]. The meta-analysis also confirmed a significant reduction of copper levels in the substantia nigra of patients with PD [20]. The above evidence supports that the imbalance of copper homeostasis is closely related to PD. In fact, copper is involved in the neuropathological changes of PD. In vitro experiments showed that copper could directly bind to α-synuclein and induce its abnormal aggregation [12, 30]. In addition, it can directly participate in the oxidation of dopamine, resulting in oxidative damage to dopamine nerves and aggravating the pathological changes of PD [14, 31]. By increasing the expression of heat shock protein Hsp27 to bind the excess free copper ions, the neurotoxicity caused by copper imbalance can be alleviated to a certain extent. The interaction of α-synuclein with copper reduces the electrostatic repulsion between the negative charges in the polypeptide chain, the partial misfolded conformation of the peptide chain is more stable, and the tendency of α-synuclein to aggregate is increased [17]. Given the observed decrease of copper in the substantia nigra of individuals with PD, it appears that the mechanism of alpha-synuclein aggregation caused by an increase in copper is unlikely to occur in the PD brain. And some studies have shown the opposite. Copper also exerts an indirect regulatory effect on α-synuclein through other copper affinities proteins such as ATOX1 and Ctr1. In vitro experiments confirmed that these copper-avidity proteins could inhibit the folding and aggregation of α-synuclein and alleviate the pathological changes of PD [32, 33]. Besides, the reduced binding of the antioxidant enzyme SOD1 to copper can alter protein conformation, leading to a reduction in antioxidant activity [34]. Copper deficiency-induced abnormal folding of SOD1 is a potential mechanism that may contribute to the development of PD. Evidence of abnormal SOD1 folding and metal deficiency has been demonstrated in the brains of individuals with PD [35, 36]. As copper is almost supplemented by dietary intake, previous studies have also investigated the relationship between dietary copper intake and PD; however, the results showed no significant difference in dietary copper intake between PD and control populations [21, 37].
An analysis was conducted to determine the nonlinear relationship between variables and outcomes. According to RCS analysis, there appears to be a connection between dietary copper intake and PD. Specifically, when it comes to copper intake levels below or above 1.960mg/day, the effect of copper seems to be reversed. Within a certain range of change, dietary copper intake may be a risk factor for PD, but as intake levels increase beyond 1.960 mg/day, the risk of PD decreases. This complex and controversial relationship between copper and PD from dietary intake levels is further explained. To maintain healthy copper intake, it is crucial to control intake within a certain range. Our findings may provide new perspectives for health policymakers in preventing PD, suggesting that people consume more than 1.960 mg/day rather than less than 1.960 mg/day. Furthermore, subgroup analysis revealed that dietary copper intake served as a protective characteristic for individuals non-Hispanic White, unmarried, and with a high school education level. It is worth noting that excessive copper intake can lead to adverse effects such as liver failure. To ensure safety, the National Academy of Sciences recommends that the daily intake of copper should not exceed 10mg [38].
There are several advantages and implications to our study. First, this study is based on the sample survey of the American population, which has the characteristics of high credibility and strong representativeness. Second, by reducing selection bias, PSM strengthens the causal arguments in observational studies. Thirdly, through the use of RCS analysis, we were able to demonstrate the nonlinear associations between dietary copper intake and Parkinson’s disease, illuminating new insights that may be useful for health policymakers through the result of RCS curves and cutoff values. However, this study also has some limitations. First, NHANES relies on the use of anti-Parkinson’s drugs to define Parkinson’s disease patients, which may not accurately diagnose the condition. Additionally, other patient populations, such as those with a history of stroke or psychiatric illness, may also take anti-Parkinson’s drugs, potentially confounding the results in case studies. Second, due to the cross-sectional nature of the study, no causal inference can be made. The confounding factors selected for this study have limitations, and the influence of many unidentified confounders could not be completely excluded from this study. It is necessary to conduct more prospective clinical studies to clarify the relationship between copper intake and PD. In addition, the complex relationship between copper and PD also needs more basic research to clarify.
Conclusions
In conclusion, this study suggests that a higher intake of dietary copper is associated with a lower risk of PD, and that the relationship between copper intake and PD risk is nonlinear. Given the increasing prevalence of PD and the potential neuroprotective effects of copper, this finding has implications for public health and clinical practice, as it suggests that maintaining adequate levels of dietary copper may be important for reducing the risk of PD. However, further research is needed to understand the underlying mechanisms and explore the potential benefits and risks of copper supplementation.
Author Contributions
ZHZ contributed to the study conception and design. Material preparation, data collection, and analysis were performed by ZHZ and LJX. The first draft of the manuscript was written by ZHZ and YMC. GH and YL consulted the literature and examined the manuscript. XGL is responsible for reviewing the literature. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the Science and Technology Planning Project of Shenzhen Municipality (KCXFZ20201221173605013)
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics Approval
This is an observational study. The studies involving human participants were reviewed and approved by NCHS Ethics Review Board (ERB).
Consent to Participate
The patients/participants provided their written informed consent to participate in this study.
Consent to Publish
The authors affirm that no potentially identifiable human images or data is presented in this study. The patients/participants consent to publish this study.
Competing Interests
The authors declare competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhaohao Zeng and Yanmei Cen contributed equally to this work.
References
- 1.Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021;20(5):385–397. doi: 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steece-Collier K, Maries E, Kordower JH. Etiology of Parkinson’s disease: genetics and environment revisited. Proc Natl Acad Sci U S A. 2002;99(22):13972–13974. doi: 10.1073/pnas.242594999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj K, Kaur P, Gupta GD, Singh S. Metals associated neurodegeneration in Parkinson’s disease: insight to physiological, pathological mechanisms and management. Neurosci Lett. 2021;753:135873. doi: 10.1016/j.neulet.2021.135873. [DOI] [PubMed] [Google Scholar]
- 4.Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E. Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol. 2014;232:1–44. doi: 10.1007/978-3-319-06746-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008;13(2):102–108. doi: 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethell DW, Fei Q. Parkinson-linked genes and toxins that affect neuronal cell death through the Bcl-2 family. Antioxid Redox Signal. 2009;11(3):529–540. doi: 10.1089/ars.2008.2228. [DOI] [PubMed] [Google Scholar]
- 7.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13(10):1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies KM, Mercer JF, Chen N, Double KL. Copper dyshomoeostasis in Parkinson’s disease: implications for pathogenesis and indications for novel therapeutics. Clin Sci (Lond) 2016;130(8):565–574. doi: 10.1042/CS20150153. [DOI] [PubMed] [Google Scholar]
- 9.Cass AE, Hill HA. Copper proteins and copper enzymes. Ciba Found Symp. 1980;79:71–91. doi: 10.1002/9780470720622.ch5. [DOI] [PubMed] [Google Scholar]
- 10.Pohanka M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl Lek Listy. 2019;120(6):397–409. doi: 10.4149/BLL_2019_065. [DOI] [PubMed] [Google Scholar]
- 11.Campos-Shimada LB, Hideo Gilglioni E, Fernandes Garcia R, Rizato Martins-Maciel E, Luiza Ishii-Iwamoto E, Luzia S-PC. Superoxide dismutase: a review and a modified protocol for activities measurements in rat livers. Arch Physiol Biochem. 2020;126(4):292–299. doi: 10.1080/13813455.2018.1520891. [DOI] [PubMed] [Google Scholar]
- 12.Miotto MC, Rodriguez EE, Valiente-Gabioud AA, et al. Site-specific copper-catalyzed oxidation of α-synuclein: tightening the link between metal binding and protein oxidative damage in Parkinson's disease. Inorg Chem. 2014;53(9):4350–4358. doi: 10.1021/ic4031377. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Zhang Y, Lu L, et al. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem Toxicol. 2022;168:113369. doi: 10.1016/j.fct.2022.113369. [DOI] [PubMed] [Google Scholar]
- 14.Ha Y, Yang A, Lee S, et al. Dopamine and Cu+/2+ can induce oligomerization of α-synuclein in the absence of oxygen: two types of oligomerization mechanisms for α-synuclein and related cell toxicity studies. J Neurosci Res. 2014;92(3):359–368. doi: 10.1002/jnr.23323. [DOI] [PubMed] [Google Scholar]
- 15.Forte G, Bocca B, Senofonte O, et al. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson’s disease. J Neural Transm (Vienna) 2004;111(8):1031–1040. doi: 10.1007/s00702-004-0124-0. [DOI] [PubMed] [Google Scholar]
- 16.Bocca B, Alimonti A, Senofonte O, et al. Metal changes in CSF and peripheral compartments of parkinsonian patients. J Neurol Sci. 2006;248(1-2):23–30. doi: 10.1016/j.jns.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Bisaglia M, Bubacco L. Copper ions and Parkinson’s disease: why is homeostasis so relevant. Biomolecules. 2020;10(2):195. doi: 10.3390/biom10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younes-Mhenni S, Aissi M, Mokni N, et al. Serum copper, zinc and selenium levels in Tunisian patients with Parkinson’s disease. Tunis Med. 2013;91(6):402–405. [PubMed] [Google Scholar]
- 19.Davies KM, Bohic S, Carmona A, et al. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging. 2014;35(4):858–866. doi: 10.1016/j.neurobiolaging.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Genoud S, Senior AM, Hare DJ, et al. Meta-analysis of copper and iron in Parkinson’s disease brain and biofluids. Mov Disord. 2020;35:662–671. doi: 10.1002/mds.27947. [DOI] [PubMed] [Google Scholar]
- 21.Miyake Y, Tanaka K, Fukushima W, et al. Dietary intake of metals and risk of Parkinson’s disease: a case-control study in Japan. J Neurol Sci. 2011;306(1-2):98–102. doi: 10.1016/j.jns.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Cheng P, Yu J, Huang W, et al. Dietary intake of iron, zinc, copper, and risk of Parkinson’s disease: a meta-analysis. Neurol Sci. 2015;36(12):2269–2275. doi: 10.1007/s10072-015-2349-0. [DOI] [PubMed] [Google Scholar]
- 23.Xu S, Li W, Di Q. Association of dietary patterns with Parkinson’s disease: a cross-sectional study based on the United States National Health and Nutritional Examination Survey Database. Eur Neurol. 2023;86(1):63–72. doi: 10.1159/000527537. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Song J, Zhang Q, et al. Association between organophosphorus pesticide exposure and depression risk in adults: a cross-sectional study with NHANES data. Environ Pollut. 2023;316(Pt 1):120445. doi: 10.1016/j.envpol.2022.120445. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Li F, Wu Q, et al. Association between trichlorophenols and neurodegenerative diseases: a cross-sectional study from NHANES 2003-2010. Chemosphere. 2022;307(Pt 2):135743. doi: 10.1016/j.chemosphere.2022.135743. [DOI] [PubMed] [Google Scholar]
- 26.Lan AP, Chen J, Chai ZF, Hu Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals. 2016;29(4):665–678. doi: 10.1007/s10534-016-9942-4. [DOI] [PubMed] [Google Scholar]
- 27.Hegde ML, Shanmugavelu P, Vengamma B, et al. Serum trace element levels and the complexity of inter-element relations in patients with Parkinson’s disease. J Trace Elem Med Biol. 2004;18(2):163–171. doi: 10.1016/j.jtemb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Pall HS, Williams AC, Blake DR, et al. Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet. 1987;2(8553):238–241. doi: 10.1016/S0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- 29.Mariani S, Ventriglia M, Simonelli I, et al. Fe and Cu do not differ in Parkinson’s disease: a replication study plus meta-analysis. Neurobiol Aging. 2013;34(2):632–633. doi: 10.1016/j.neurobiolaging.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 30.McLeary FA, Rcom-H'cheo-Gauthier AN, Kinder J, et al. Dexamethasone Inhibits copper-induced alpha-synuclein aggregation by a metallothionein-dependent mechanism. Neurotox Res. 2018;33(2):229–238. doi: 10.1007/s12640-017-9825-7. [DOI] [PubMed] [Google Scholar]
- 31.Tavassoly O, Nokhrin S, Dmitriev OY, Lee JS. Cu(II) and dopamine bind to α-synuclein and cause large conformational changes. FEBS J. 2014;281(12):2738–2753. doi: 10.1111/febs.12817. [DOI] [PubMed] [Google Scholar]
- 32.Horvath I, Blockhuys S, Šulskis D, et al. Interaction between copper chaperone Atox1 and Parkinson’s disease protein α-synuclein includes metal-binding sites and occurs in living cells. ACS Chem Neurosci. 2019;10(11):4659–4668. doi: 10.1021/acschemneuro.9b00476. [DOI] [PubMed] [Google Scholar]
- 33.Gou DH, Huang TT, Li W, et al. Inhibition of copper transporter 1 prevents α-synuclein pathology and alleviates nigrostriatal degeneration in AAV-based mouse model of Parkinson's disease. Redox Biol. 2021;38:101795. doi: 10.1016/j.redox.2020.101795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trist BG, Hare DJ, Double KL. A proposed mechanism for neurodegeneration in movement disorders characterized by metal dyshomeostasis and oxidative stress. Cell Chem Biol. 2018;25:807–816. doi: 10.1016/j.chembiol.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Trist BG, Davies KM, Cottam V, et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol. 2017;134:113–127. doi: 10.1007/s00401-017-1726-6. [DOI] [PubMed] [Google Scholar]
- 36.Genoud S, Jones M, Trist BG, et al. Simultaneous structural and elemental nano-imaging of human brain tissue. Chem Sci. 2020;11:8919–8927. doi: 10.1039/D0SC02844D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng WW, Zhu Q, Zhang HY. Mineral nutrition and the risk of chronic diseases: a mendelian randomization study. Nutrients. 2019;11(2):378. doi: 10.3390/nu11020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.