Abstract
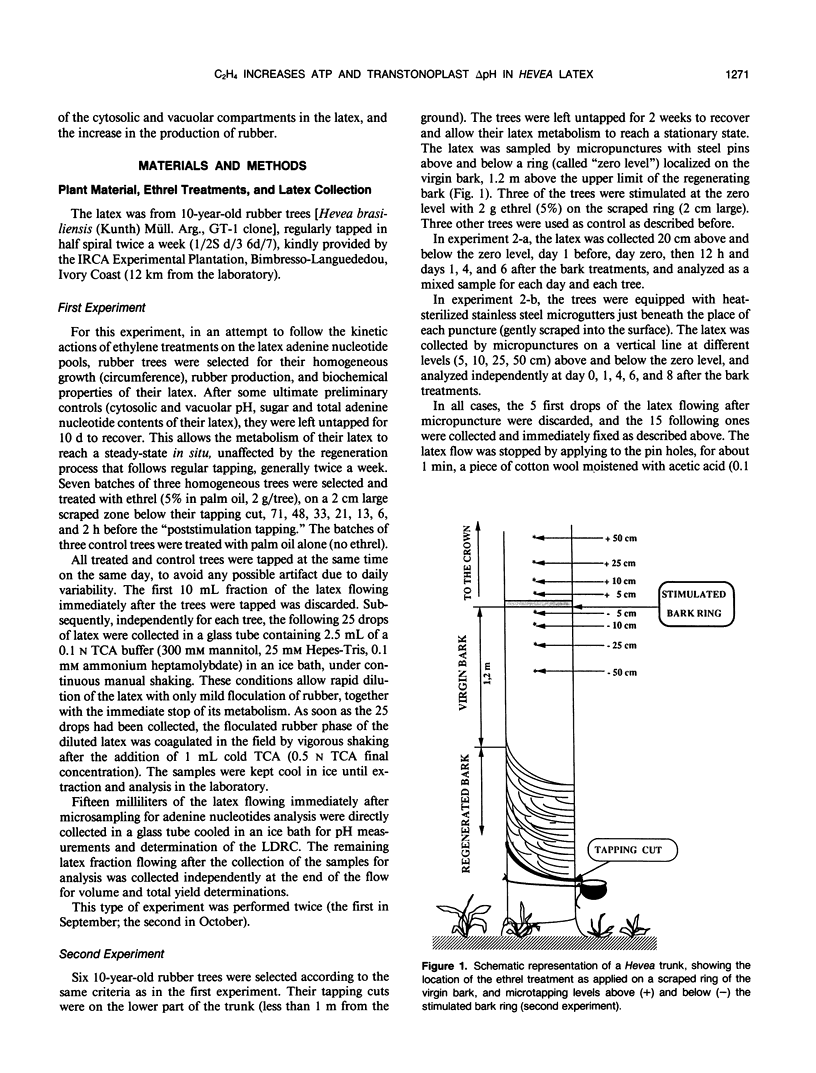
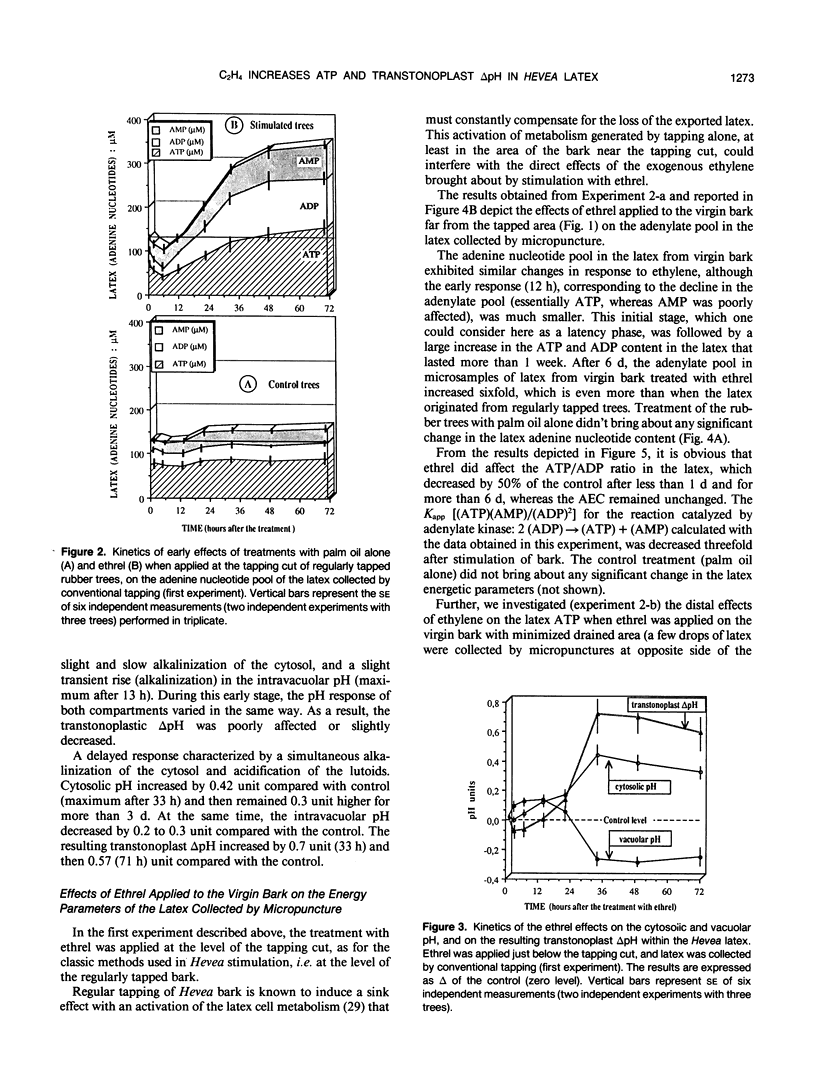
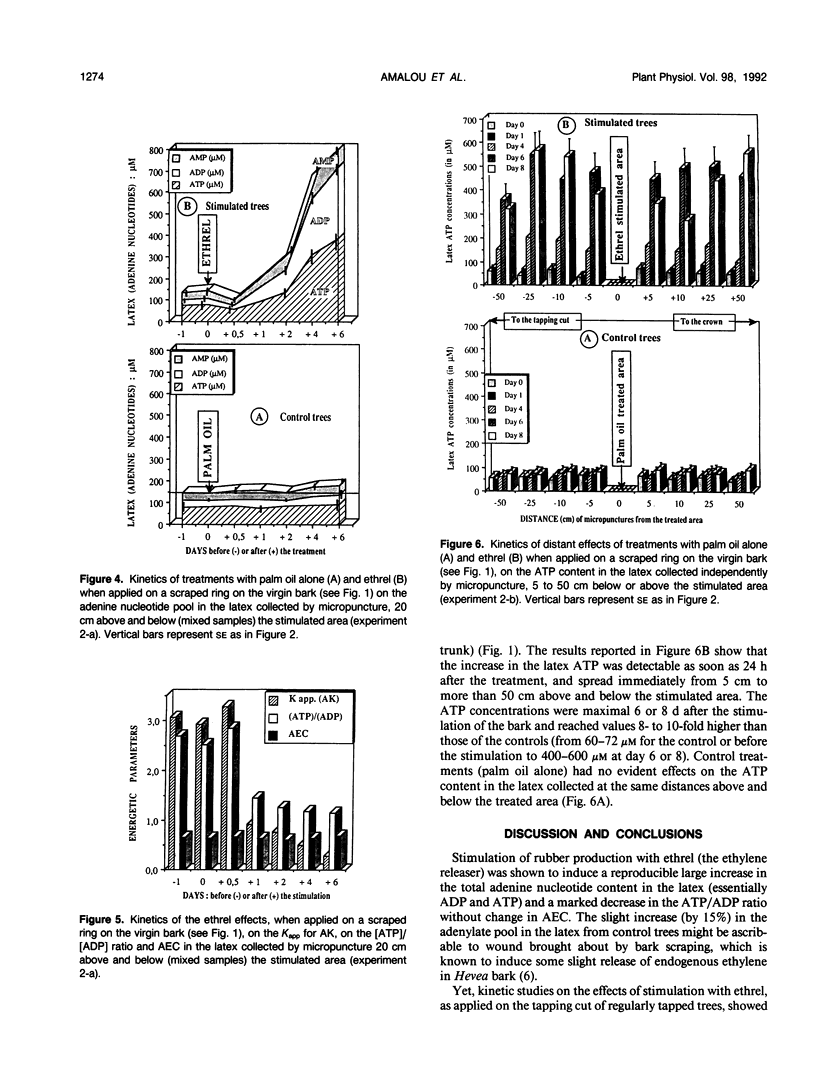
The treatment of rubber tree (Hevea brasiliensis) bark with chloro-2-ethyl phosphonic acid (ethrel), an ethylene-releasing chemical, induced, after a lag period of 13 to 21 hours, a marked increase in the total adenine nucleotides (essentially ATP and ADP) of latex cells. This rise in the latex adenylate pool was concomitant with a marked decrease in the [ATP]/[ADP] ratio without significant changes in the adenylate energy charge. The apparent equilibrium constant for the adenylate kinase, which appeared to behave as a key enzyme in maintaining the adenylate energy charge in the latex, was considerably reduced, probably as a consequence of the alkalinization of the latex cytosol induced by the treatment with ethrel. To reduce the “sink effect” and activation of the metabolism induced in Hevea bark by regular tapping, the latex was collected by micropuncture (few drops) at increasing distance (5-50 centimeters) above and below an ethrel-treated area on the virgin bark of resting trees. The effect of ethrel was shown to spread progressively along the trunk. The increase in the adenylate pool (essentially ATP) was detectable as early as 24 hours after the bark treatment and was maximum after 6 or 8 days, 5 centimeters as well as 50 centimeters above and below the stimulated bark ring. The correlative vacuolar acidification and cytosolic alkalinization, i.e. the increase in the transtonoplast ΔpH, induced in the latex cells by ethrel were shown to be concomitant with the rise in ATP content of the latex. This suggests that the tonoplast H+-pumping ATPase, which catalyzes vacuolar acidification in the latex, is directly and essentially under the control of the availability of its substrate (i.e. ATP) in the latex. The results are discussed in relation to energy-dependent activation of metabolism, and increased rubber production, as induced by the stimulation of rubber trees with ethrel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Cretin H. The proton gradient across the vacuo-lysosomal membrane of lutoids from the latex of Hevea brasiliensis. I. Further evidence for a proton-translocating ATPase on the vacuo-lysosomal membrane of intact lutoids. J Membr Biol. 1982;65(3):175–184. doi: 10.1007/BF01869961. [DOI] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidrol X., Chrestin H., Mounoury G., D'Auzac J. Early Activation by Ethylene of the Tonoplast H-Pumping ATPase in the Latex from Hevea brasiliensis. Plant Physiol. 1988 Mar;86(3):899–903. doi: 10.1104/pp.86.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B., Marin-Lanza M., Komor E. The protonmotive potential difference across the vacuo-lysosomal membrane of Hevea brasiliensis (rubber tree) and its modification by a membrane-bound adenosine triphosphatase. Biochem J. 1981 Aug 15;198(2):365–372. doi: 10.1042/bj1980365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm V. L., Leung H. Regulation of adenosine monophosphate levels as a function of adenosine triphosphate and inorganic phosphate. A proposed metabolic role for adenosine monophosphate nucleosidase from Azotobacter vinelandii. J Biol Chem. 1973 Dec 10;248(23):8313–8315. [PubMed] [Google Scholar]
- d' Auzac J., Jacob J. L. Inhibition par l'ATP de la malate-déshydrogénase, de l'alcool-déshydrogénase et de la lactate-déshydrogénase du latex d'Hevea brasiliensis. Bull Soc Chim Biol (Paris) 1968 Mar 2;50(1):143–156. [PubMed] [Google Scholar]


