Abstract
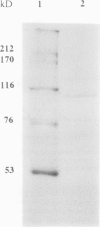
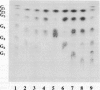
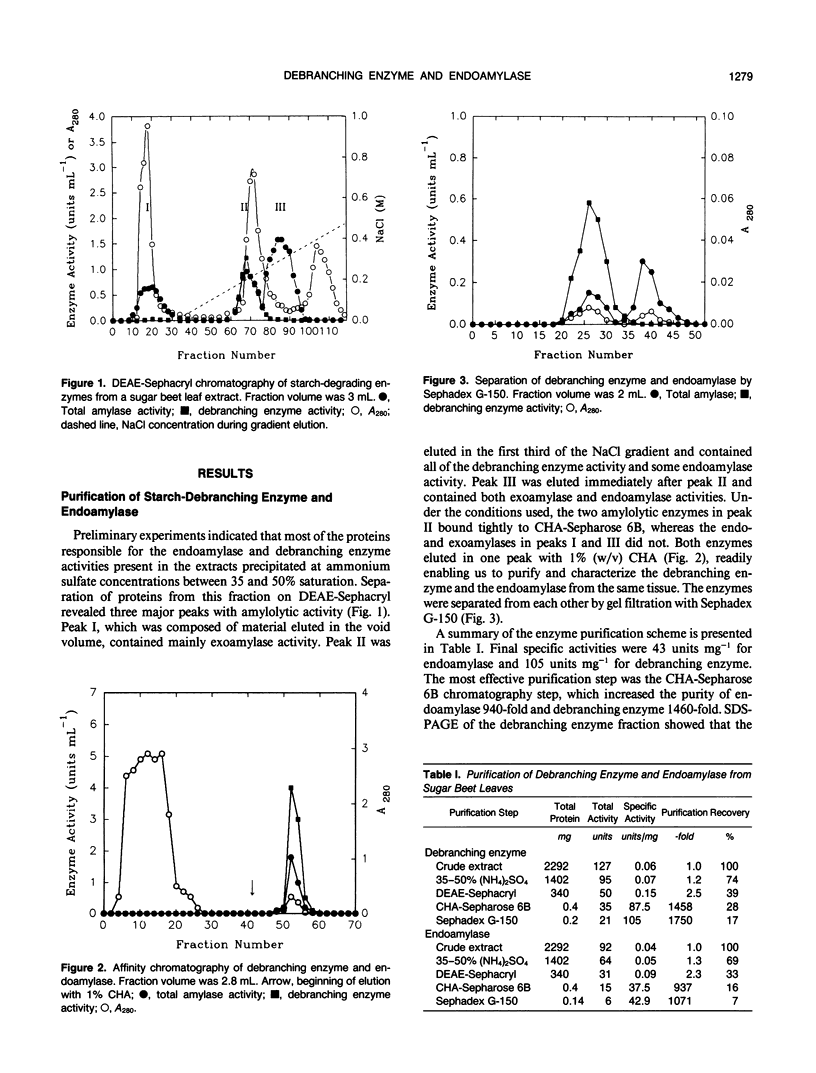
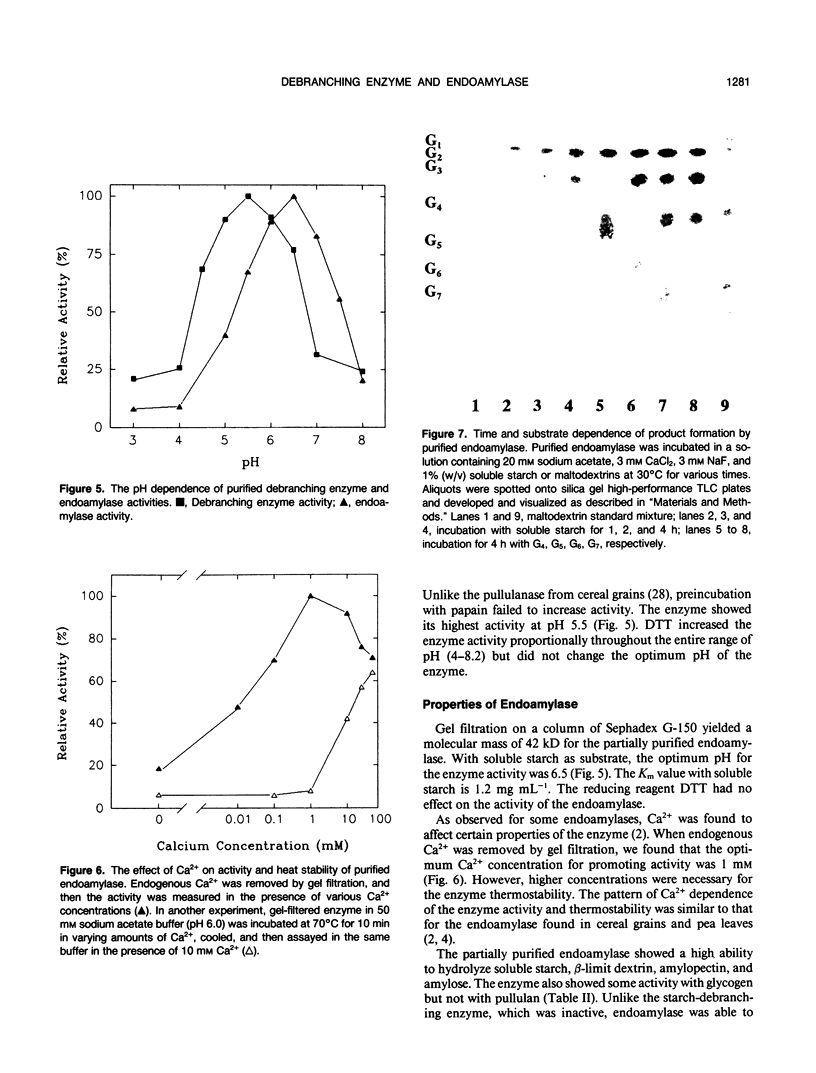
Sugar beet leaves (Beta vulgaris L.) contained up to five endoamylases, two exoamylases, and a single debranching enzyme. Four of the endoamylases and the debranching enzyme were present in the chloroplast. The chloroplastic starch-debranching enzyme and an apoplastic endoamylase were copurified from mature leaves of sugar beet by 35 to 50% ammonium sulfate precipitation and chromatography on diethylaminoethyl-Sephacryl, β-cyclodextrin Sepharose 6B, and Sephadex G-150. The debranching enzyme, which was purified to homogeneity, had a molecular mass of 100 kilodaltons and a pH optimum of 5.5. It showed a high activity with pullulan as a substrate, low activity with soluble starch and amylopectin, and no activity with native starch grains isolated from sugar beet leaves. The endoamylase, which was partially purified, had a molecular mass of 43,000 kilodaltons, a pH optimum of 6.5, required calcium for activity and thermal stability, and showed an ability to hydrolyze native starch grains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beers E. P., Duke S. H. Characterization of alpha-Amylase from Shoots and Cotyledons of Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Apr;92(4):1154–1163. doi: 10.1104/pp.92.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Localization of alpha-Amylase in the Apoplast of Pea (Pisum sativum L.) Stems. Plant Physiol. 1988 Aug;87(4):799–802. doi: 10.1104/pp.87.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz S. J. Regulation of photosynthate partitioning into starch in soybean leaves : response to natural daylight. Plant Physiol. 1990 Sep;94(1):350–356. doi: 10.1104/pp.94.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T., Lin T. P., Kakefuda G., Benbow L., Preiss J., Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol. 1991 Apr;95(4):1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H. Specific Determination of alpha-Amylase Activity in Crude Plant Extracts Containing beta-Amylase. Plant Physiol. 1983 Feb;71(2):229–234. doi: 10.1104/pp.71.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. C., Geiger D. R. Effects of decreased net carbon exchange on carbohydrate metabolism in sugar beet source leaves. Plant Physiol. 1984 Nov;76(3):763–768. doi: 10.1104/pp.76.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Hanson A. D., Chandler P. C. Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 1986 Feb;80(2):350–359. doi: 10.1104/pp.80.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C. R., Preiss J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988 Dec;88(4):1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig I., Ziegler P., Beck E. Purification and properties of spinach leaf debranching enzyme. Plant Physiol. 1984 Apr;74(4):856–861. doi: 10.1104/pp.74.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I., Mizuno Y., Seno N. Activation of Sepharose with epichlorohydrin and subsequent immobilization of ligand for affinity adsorbent. J Biochem. 1979 Apr;85(4):1091–1098. doi: 10.1093/oxfordjournals.jbchem.a132417. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Preiss J. Starch Degradation in Spinach Leaves: ISOLATION AND CHARACTERIZATION OF THE AMYLASES AND R-ENZYME OF SPINACH LEAVES. Plant Physiol. 1980 Nov;66(5):870–876. doi: 10.1104/pp.66.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Henson C. A. Degradation of Native Starch Granules by Barley alpha-Glucosidases. Plant Physiol. 1990 Sep;94(1):320–327. doi: 10.1104/pp.94.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. H., Pollak A., McCurry S. D., Sue J. M., Knowles J. R., Whitesides G. M. Synthesis of ribulose 1,5-bisphosphate: routes from glucose 6-phosphate (via 6-phosphogluconate) and from adenosine monophosphate (via ribose 5-phosphate). Methods Enzymol. 1982;89(Pt 500):108–121. doi: 10.1016/s0076-6879(82)89020-4. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Coleman R. D. Detection of pullulanase in polyacrylamide gels using pullulan-reactive red agar plates. Anal Biochem. 1987 Feb 1;160(2):480–482. doi: 10.1016/0003-2697(87)90079-0. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]