Abstract
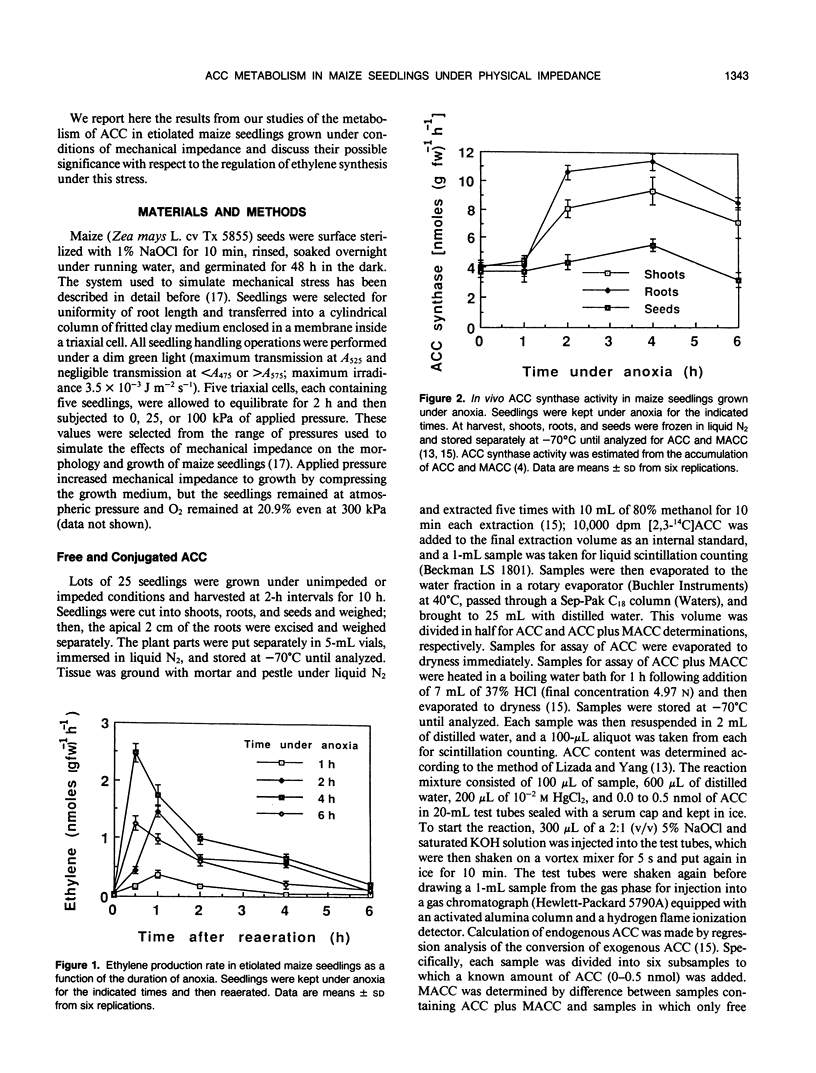
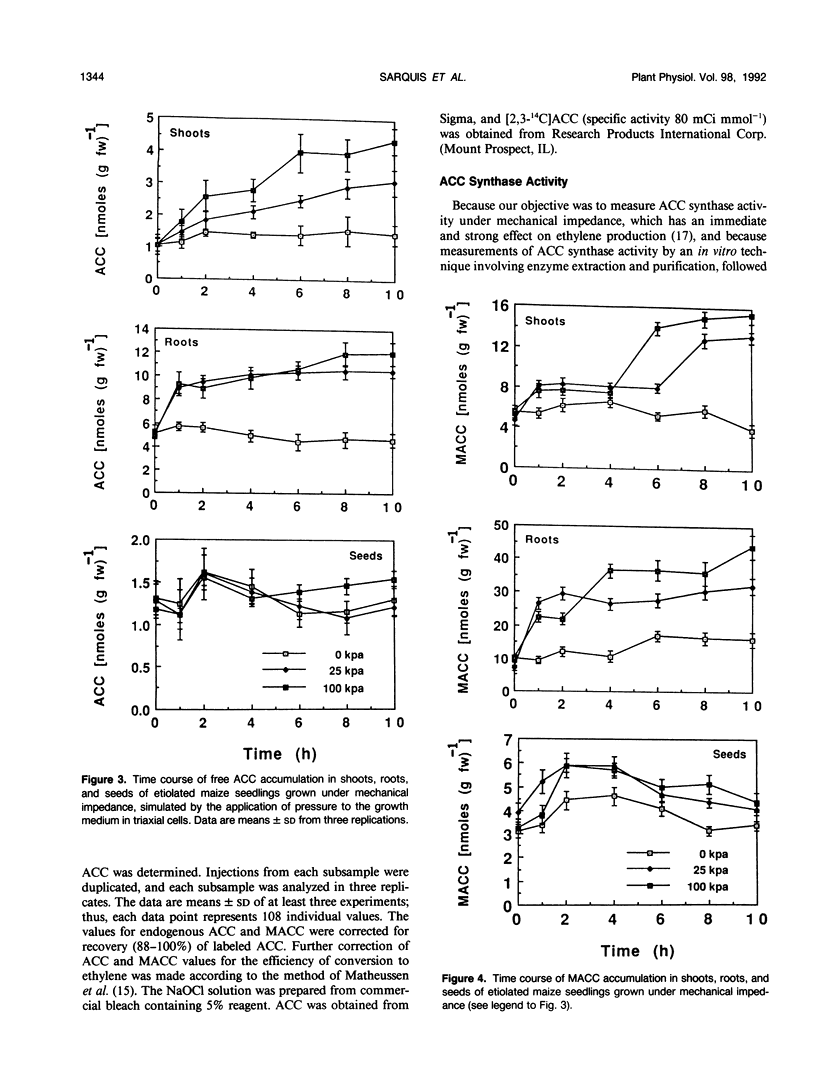
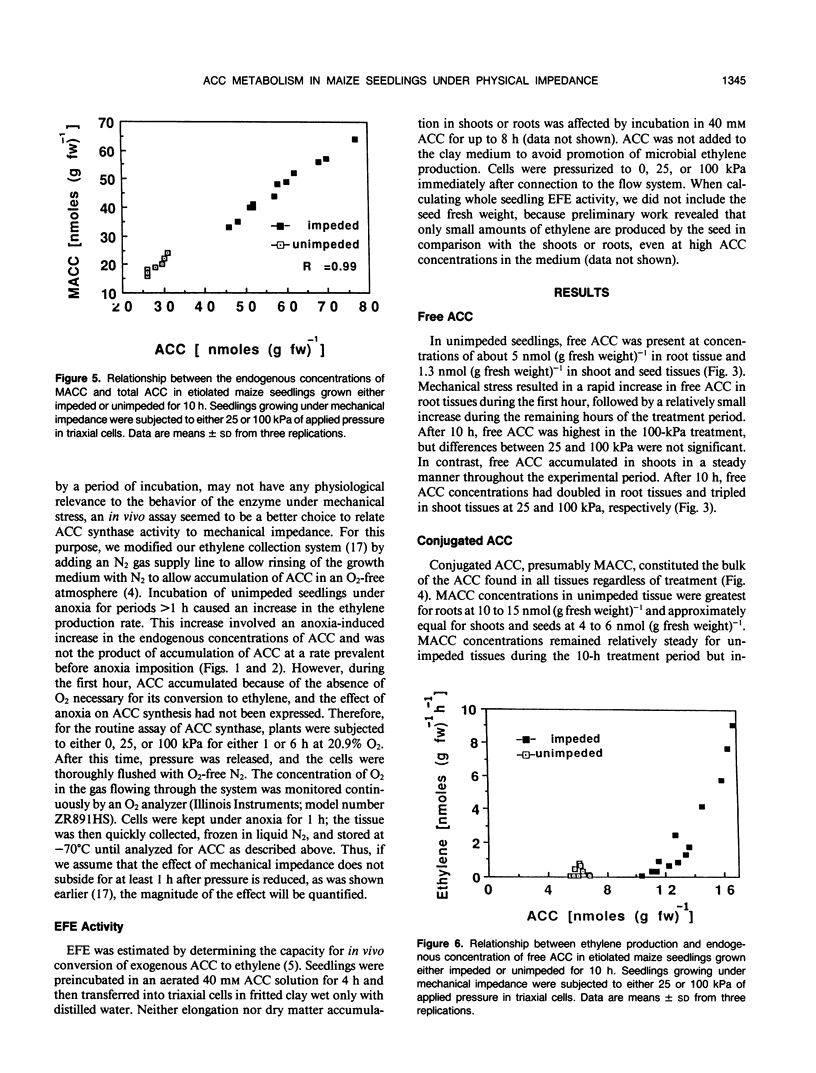
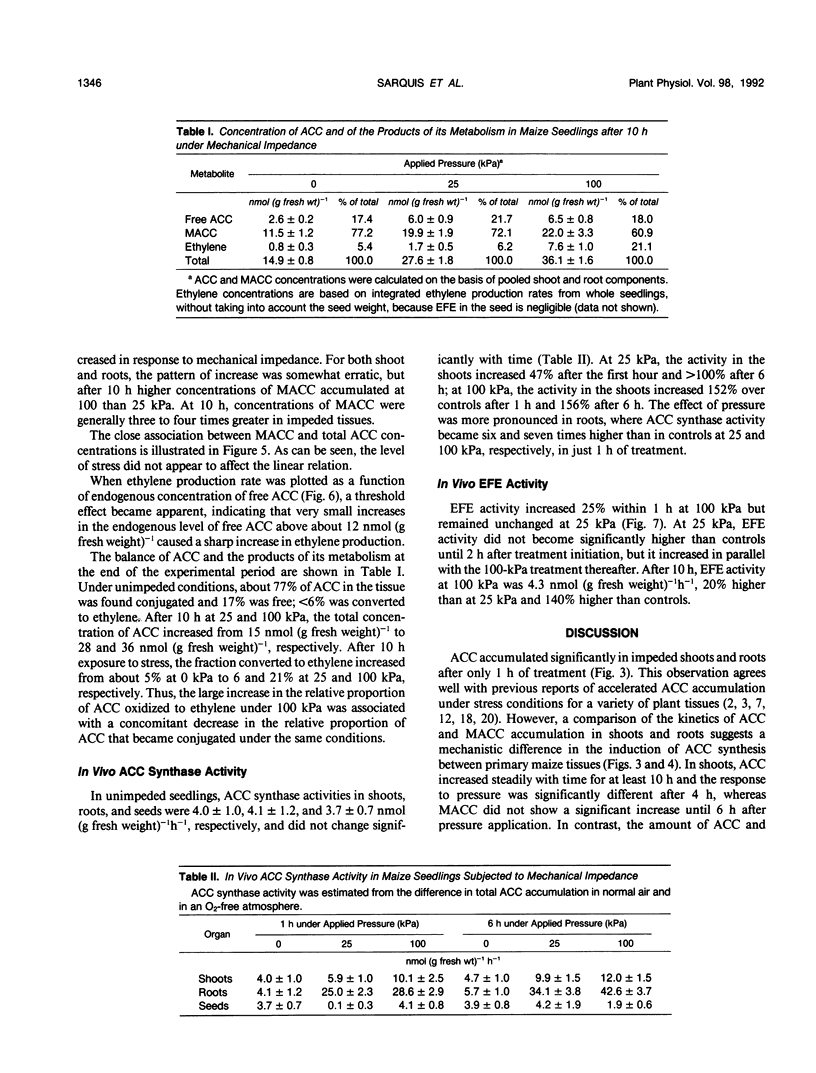
We investigated the metabolism of 1-aminocyclopropane-1-carboxylic acid (ACC) in etiolated maize (Zea mays L.) seedlings subjected to mechanical impedance by applying pressure to the growing medium. Total concentrations of ACC varied little in unimpeded seedlings, but impeded organs accumulated ACC. Roots had consistently higher concentrations of ACC than shoots or seeds, regardless of treatment. The concentration of ACC in the roots increased more than 100% during the first hour of treatment irrespective of the pressure applied; in shoots, total ACC concentration increased 46% at either low or high pressure during the first hour of treatment. The bulk of ACC synthesized under impeded and unimpeded conditions was present in a conjugated form, presumably, 1-(malonylamino)-cyclopropane-1-carboxylic acid. However, 1-(malonylamino)-cyclopropane-1-carboxylic acid increased 73% over controls after 10 hours at 25 kilopascals of pressure. Unimpeded tissue had about 77% ACC as the conjugate and 17% as free ACC, and less than 6% was used in ethylene production. Increased amounts of ACC were converted into ethylene under stress. In vivo ACC synthase activity in roots became six and seven times higher only 1 hour after initiation of treatment at 25 and 100 kilopascals of pressure, respectively, and remained high for at least 6 hours. However, the immediate and massive conjugation of mechanically induced ACC suggests that ACC N-malonyltransferase may play an important role in the regulation of mechanically induced ethylene production. After 8 hours, in vivo activity of the ethylene-forming enzyme complex increased 100 and 50% above normal level at 100 and 25 kilopascals, respectively. Furthermore, ethylene-forming enzyme complex activity was significantly greater at 100 kilopascals than in controls as early as 1 hour after treatment initiation. These data suggest that regulation of ethylene production under mechanical impedance involves the concerted action of ACC synthase, the ethylene-forming enzyme complex, and ACC N-malonyltransferase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Yang S. F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981 Sep;68(3):594–596. doi: 10.1104/pp.68.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Yang S. F. Xylem Transport of 1-Aminocyclopropane-1-carboxylic Acid, an Ethylene Precursor, in Waterlogged Tomato Plants. Plant Physiol. 1980 Feb;65(2):322–326. doi: 10.1104/pp.65.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deepwater rice : enhancement by submergence and low oxygen levels. Plant Physiol. 1987 Jun;84(2):282–286. doi: 10.1104/pp.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew M. C., He C. J., Morgan P. W. Decreased Ethylene Biosynthesis, and Induction of Aerenchyma, by Nitrogen- or Phosphate-Starvation in Adventitious Roots of Zea mays L. Plant Physiol. 1989 Sep;91(1):266–271. doi: 10.1104/pp.91.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Fu J. R., Yang S. F. Identification and Metabolism of 1-(Malonylamino)cyclopropane-1-carboxylic Acid in Germinating Peanut Seeds. Plant Physiol. 1983 Jan;71(1):197–199. doi: 10.1104/pp.71.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X. Z., Philosoph-Hadas S., Su L. Y., Yang S. F. The Conversion of 1-(Malonylamino)cyclopropane-1-Carboxylic Acid to 1-Aminocyclopropane-1-Carboxylic Acid in Plant Tissues. Plant Physiol. 1986 Jun;81(2):637–641. doi: 10.1104/pp.81.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X. Z., Yip W. K., Yang S. F. The effect of light and phytochrome on 1-aminocyclopropane-1-carboxylic Acid metabolism in etiolated wheat seedling leaves. Plant Physiol. 1987 Nov;85(3):643–647. doi: 10.1104/pp.85.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Saltveit M. E., Dilley D. R. Rapidly Induced Wound Ethylene from Excised Segments of Etiolated Pisum sativum L., cv. Alaska: I. Characterization of the Response. Plant Physiol. 1978 Mar;61(3):447–450. doi: 10.1104/pp.61.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarquis J. I., Jordan W. R., Morgan P. W. Ethylene Evolution from Maize (Zea mays L.) Seedling Roots and Shoots in Response to Mechanical Impedance. Plant Physiol. 1991 Aug;96(4):1171–1177. doi: 10.1104/pp.96.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Biosynthesis of wound ethylene. Plant Physiol. 1980 Aug;66(2):281–285. doi: 10.1104/pp.66.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


