Abstract
In response to bacterial entry epithelial cells up-regulate expression and secretion of various proinflammatory cytokines, including interleukin-8 (IL-8). We studied Yersinia enterocolitica O:8-induced IL-8 secretion by intestinal epithelial cells as a function of cell differentiation. For this purpose, human T84 intestinal epithelial cells were grown on permeable supports, which led to the formation of tight monolayers of polarized intestinal epithelial cells. To analyze IL-8 secretion as a function of cell differentiation, T84 monolayers were infected from the apical or basolateral side at different stages of differentiation. Both virulent (plasmid-carrying) and nonvirulent (plasmid-cured) Y. enterocolitica strains invaded nondifferentiated T84 cells from the apical side. Yersinia invasion into T84 cells was followed by secretion of IL-8. After polarized differentiation of T84 cells Y. enterocolitica was no longer able to invade from the apical side or to induce IL-8 secretion by T84 cells. However, Y. enterocolitica invaded and induced IL-8 secretion by polarized T84 cells after infection from the basolateral side. Basolateral invasion required the presence of the Yersinia invasion locus, inv, suggesting β1 integrin-mediated cell invasion. After basolateral infection, Yersinia-induced IL-8 secretion was not strictly dependent on cell invasion. Thus, although the plasmid-carrying Y. enterocolitica strain did not significantly invade T84 cells, it induced significant IL-8 secretion. Taken together, these data show that Yersinia-triggered IL-8 secretion by intestinal epithelial cells depends on cell differentiation and might be induced by invasion as well as by basolateral adhesion, suggesting that invasion is not essential for triggering IL-8 production. Whether IL-8 secretion is involved in the pathogenesis of Yersinia-induced abscess formation in Peyer’s patch tissue remains to be shown.
Pathogenic bacteria that invade the host via the gastrointestinal tract must cross the epithelial surface to gain access to the underlying mucosa. In addition to forming a physical barrier to bacterial infection, intestinal epithelial cells can function as integral components of the host’s mucosal immune system. Thus, intestinal epithelial cells express major histocompatibility complex class I and class II molecules (3, 36). In addition, intestinal epithelial cells can act as sensors of bacterial invasion (16, 29). In response to bacterial invasion, e.g., by Salmonella dublin, Shigella dysenteriae, Yersinia enterocolitica, Listeria monocytogenes, and enteroinvasive Escherichia coli, they rapidly up-regulate expression and secretion of proinflammatory cytokines, including interleukin-8 (IL-8), monocyte chemotactic protein-1, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha (TNF-α) (14, 15, 19, 28).
The enterobacterium Y. enterocolitica causes a broad range of gastrointestinal syndromes, ranging from acute enteritis and enterocolitis to mesenteric lymphadenitis (7, 11). The virulence of Y. enterocolitica is controlled by chromosomal (yst, inv) (12, 26, 40, 42) and plasmid-encoded genes (for reviews, see references 9 and 10). The pYV (for Yersinia virulence) virulence plasmid directs production of the outer membrane protein YadA and secretion of 11 antihost proteins called Yops, 7 of which have been shown to be essential for virulence (10, 20, 55).
Invasion studies in an experimental mouse infection model revealed that Y. enterocolitica selectively invades M cells located in the follicle-associated epithelium overlying Peyer’s patches (1, 21, 22). After transcytosis via M cells, Y. enterocolitica multiplies in Peyer’s patch tissue, accompanied by an influx of polymorphonuclear and mononuclear phagocytes (2). Subsequently, microabscesses can be found beneath the follicle-associated epithelium and transmigrating polymorphonuclear leukocytes (PMNs) can be found within the epithelium (1). The cytokines IL-1, IL-6, TNF-α, and gamma interferon are probably involved in the local host defense in Peyer’s patch tissue infected by Y. enterocolitica (2, 4, 5, 48).
Adherence to and internalization of Y. enterocolitica and Yersinia pseudotuberculosis by epithelial cells (33, 41) depend on a series of surface proteins, namely Inv (25, 45, 58), Ail (39, 40), and YadA (6, 17, 23, 53, 54, 57). Invasion by Y. enterocolitica of HeLa, HEp-2, and T84 cells depends on the interaction between the invasin Inv and β1 integrins on the surface of the eukaryotic cell (25). Infection of HeLa and T84 epithelial cells by Y. enterocolitica induces IL-8 secretion (15, 19, 28, 52). After infection by virulent Yersinia organisms, IL-8 secretion is partially counteracted by the action of a so-far-unknown Yop protein(s) (52).
Most investigations of Y. enterocolitica invasion of epithelial cells and Yersinia-induced IL-8 secretion have employed nonintestinal, or at least nonpolarized, cell types. However, on intestinal epithelial cells β1 integrins are located on the basolateral side (56). Thus, the epithelial ligand for the Yersinia invasin Inv is physically separated from the intestinal lumen. It was demonstrated that entry of Y. pseudotuberculosis into Caco-2 cells is mediated by β1 integrins. After redistribution of β1 integrins to the basolateral surface during differentiation, Y. pseudotuberculosis is not able to invade epithelial cells from the apical side (8). Likewise, polarized epithelial T84 cells are resistant to invasion by Y. pseudotuberculosis (37). However, T84 monolayers become susceptible to Y. pseudotuberculosis invasion in regions where transient microdiscontinuities resulting from neutrophil migration permit access to β1 integrins from the apical side (37).
We wondered whether Y. enterocolitica is able to invade polarized human intestinal epithelial cells and whether this interaction with polarized cells leads to IL-8 secretion. To address these questions, we used an in vitro cell culture system of polarized T84 cells (34). In infection experiments we analyzed the effect of cell differentiation on the apical and basolateral entry of Y. enterocolitica into T84 cells and measured IL-8 secretion by infected cells. Our results suggest that Yersinia invasion of polarized intestinal epithelial cells occurs from the basolateral surfaces rather than from the apical surfaces. In addition, infection by pYV+ or pYV− Y. enterocolitica strains from the basolateral side led to significant IL-8 secretion, although pYV+ bacteria did not significantly invade polarized cells. These results suggest that Y. enterocolitica basolateral adhesion and invasion may trigger IL-8 secretion by polarized epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
Plasmid-harboring (pYV+) and plasmid-cured (pYV−) Y. enterocolitica WA314 strains of serotype O:8 were described by J. Heesemann and R. Laufs (24). The Y. enterocolitica WA314 (pYV−) inv mutant was obtained from K. Ruckdeschel (51). As a control for a noninvasive bacterium we employed a laboratory E. coli HB101 strain.
Bacterial growth conditions.
Bacteria were routinely grown in Luria-Bertani broth (LB). For infection experiments, overnight cultures of Yersinia were diluted to an optical density at 600 nm (OD600) of 0.2 in LB or brain heart infusion (Difco, Detroit, Mich.) and incubated for 3 h at 27 or 37°C, respectively. Antibiotics (Boehringer, Mannheim, Germany) were used at the following final concentrations: gentamicin, 100 μg ml−1; kanamycin, 50 μg ml−1; and nalidixic acid, 35 μg ml−1.
Cell lines.
Human colon epithelial carcinoma T84 cells (ATCC CCL-248) were obtained from the American Type Culture Collection, Rockville, Md. T84 cells (passages 56 to 70) were cultured in 50% Dulbecco’s modified Eagle’s medium (Gibco BRL, Paisley, Scotland)–50% Ham’s F12 (BiochromKG, Berlin, Germany) and 10% fetal bovine serum (Gibco BRL), supplemented with 10 mM HEPES buffer (pH 7.5), 1 mM sodium pyruvate, 2 mM l-glutamine (Gibco BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml) (BiochromKG). The cells were grown in a humidified 5% CO2 atmosphere at 37°C.
Construction of monolayers.
T84 cells were grown and maintained as confluent monolayers as described previously (13, 34, 43) with the following modifications. Approximately 106 cells cm−2 were seeded on 6.5- or 24-mm-diameter polycarbonate permeable supports (Costar Corp., Cambridge, Mass.) with a pore size of 3.0 or 0.4 μm, respectively. Inverted monolayers for basolateral infections were constructed on 6.5-mm-diameter filters coated with rat tail collagen (type 1; Sigma Chemical Co., St. Louis, Mo.). T84 cells were added to filters inverted (underside facing up) onto sterile petri dishes and were allowed to attach overnight; the inserts were then placed right side up into 24-well culture plates. Filters were used when they had reached steady-state transepithelial resistance or at indicated time points.
Measurement of TER.
Transepithelial electrical resistance (TER) measurements were carried out with either a Millicell-ERS volt-ohmmeter (Millipore, Bedford, Mass.) or a commercial voltage clamp (Physiologic Instruments, San Diego, Calif.) interfaced with an equilibrated pair of calomel electrodes along with a pair of Ag-AgCl electrodes submerged in saturated KCl. Agar bridges were used to interface the electrodes with the solutions on either side of the monolayer (one calomel and one Ag-AgCl electrode on each side). Measurements of short-circuit current and resistance were made as detailed elsewhere (34, 35).
Infection protocol.
Bacteria grown for 3 h in LB or brain heart infusion at 27 or 37°C, respectively (see “Bacterial growth conditions”) were collected by centrifugation and washed twice in sterile phosphate-buffered saline (PBS) (pH 7.4). After determination of the OD, appropriate dilutions of the bacteria in PBS were performed before infection. Monolayers were infected at a bacterium-to-cell ratio of 500:1 or as indicated. The actual number of bacteria administered was determined by plating 0.1 ml of 1:10 serial dilutions on Mueller-Hinton agar and counting CFU after 36 h of incubation at 27°C.
T84 monolayers grown on commercially available (Costar) polycarbonate-permeable supports were washed two times with PBS and incubated in medium containing heat-inactivated fetal bovine serum without antibiotics. After 1 to 2 h of equilibration bacterial samples were added. Monolayers and bacteria were incubated for 1 h to allow adherence and entry of the bacteria. After removal of the medium, cultures were washed three times with PBS to remove extracellular bacteria and further incubated for 4 h in the presence of 100 μg of gentamicin per ml to kill remaining extracellular bacteria. Then the culture supernatants (apical and basolateral reservoirs) were removed and centrifuged for 20 min to pellet residual bacteria and cells before IL-8 measurement. Cells of the monolayers were lysed with 1% Triton X-100 in PBS. The number of released viable bacteria was determined by plating serial 10-fold dilutions on Mueller-Hinton agar. For apical infection of inverted monolayers, filters were washed and inverted onto a sterile petri dish containing PBS. The apical surfaces of the monolayers (now facing up) were infected with the appropriate bacterial dilutions in 100 μl of cell culture medium.
TNF-α, a gift from Bender, Vienna, Austria, and lipopolysaccharide from E. coli (Sigma) were used in concentrations of 50 and 100 ng/ml, respectively.
Determination of IL-8 production by ELISA.
The amount of IL-8 secreted into the supernatant was determined by an enzyme-linked immunosorbent assay (ELISA) with optimal concentrations of a mouse anti-human IL-8 monoclonal antibody (MAb) and a biotinylated mouse anti-human IL-8 MAb as detecting antibodies. ELISA microtiter plates (Nunc) were coated overnight with anti-human IL-8 MAb (G265-5; Pharmingen, San Diego, Calif.). After nonspecific binding sites were blocked, supernatants were added to the wells and incubated overnight. After several washing steps, biotin-labeled anti-human IL-8 MAb (G265-8; Pharmingen) was added. Finally, an avidin-biotin-alkaline phosphatase complex (Strept ABC-AP kit; Dako, Glostrup, Denmark) was added. For signal development the wells were incubated with p-nitrophenylphosphate disodium (pNPP; Sigma), and the OD was determined at wavelengths of 405 and 490 nm. IL-8 concentrations were calculated from the straight-line portion of standard curves with recombinant human IL-8 (Pharmingen).
Electron microscopy and immunofluorescence microscopy.
Following incubation with bacteria, T84 monolayers on permeable supports were washed with PBS and fixed at 4°C in 1/2 Karnovsky’s fixative in 0.05 M sodium cacodylate buffer. After fixation, filters were further processed for scanning electron microscopy (SEM) or transmission electron microscopy (TEM) essentially as described by Karunasagar et al. (31, 32). For immunofluorescence microscopy samples were washed twice with PBS and fixed with 3% paraformaldehyde in PBS for 15 min at room temperature. Then the following steps were performed at room temperature. The samples were quenched with 50 mM NH4Cl in PBS for 15 min, washed with PBS, and then permeabilized with 2% Triton X-100 in PBS for 4 min. The samples were washed with PBS and incubated for 20 min with PBS containing 1% bovine serum albumin. Filters were cut out from the ring support with a scalpel and incubated for 1 h with the primary antibody, anti-human β1 integrin clone P4C10 (Gibco BRL). After being washed, the filters were incubated for 1 h with CY2-conjugated anti-mouse immunoglobulin G (Dianova, Hamburg, Germany). To visualize microfilaments, the fixed and permeabilized cells were incubated with tetramethyl rhodamine isocyanate-conjugated phalloidine (2 ng/ml; Sigma). The fluorescence images were obtained with a confocal laser scanning microscope (Leica TCS 4D) equipped with detectors and an argon-krypton laser for simultaneous scanning of different fluorochromes. The samples were analyzed with a 40× (numeric aperture 1.4) PlanApo objective oil immersion lens. The pinhole was set to optimal values according to the manufacturer’s instructions. Images were processed with Graphikkonverter version 2.5 software (LemkeSoft, Peine, Germany).
Statistics.
Differences between mean values were analyzed by the Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
Establishment of an in vitro monolayer system to study Yersinia-induced IL-8 secretion.
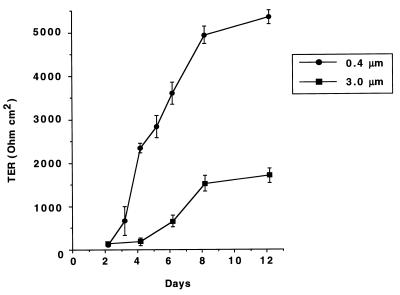
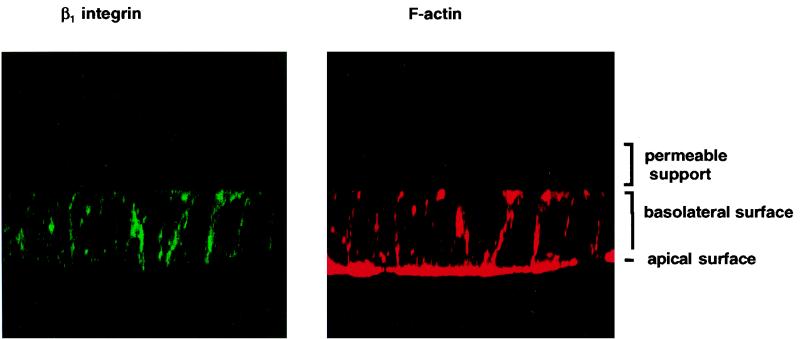
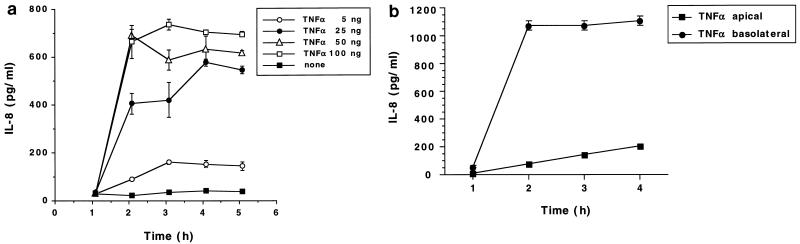
To study bacterial invasion of, and release of IL-8 by, polarized human intestinal epithelial cells after infection with Y. enterocolitica O:8, we established an in vitro system employing T84 cells grown on permeable supports as described previously (13, 34, 43). In order to analyze the effect of Y. enterocolitica infection as a function of the differentiation status of the cells, monolayers were morphologically and functionally characterized over a period of 2 weeks. The tightness of the monolayers was assessed by measuring the TER. Figure 1 shows the TER development of T84 monolayers on permeable supports with 0.4- or 3.0-μm pore size. TER increased continuously and reached steady-state levels after 8 days. However, the actual steady-state levels differed, depending on the pore sizes of the permeable supports. Differentiation of T84 cells was assessed by electron microscopy and immunofluorescence microscopy. SEM and TEM showed that T84 cells grown on permeable supports differentiated from nonpolarized cells on day 2, becoming tall columnar cells with apical microvilli, and finally developing tight or occluding junctions on day 9 (data not shown). Indirect immunofluorescence with an anti-β1 integrin MAb showed basolateral but not apical expression of β1 integrins in differentiated T84 cells (Fig. 2). IL-8 secretion by T84 monolayers was analyzed after stimulation of the monolayers with the physiological agonist TNF-α. T84 monolayers responded to TNF-α stimulation with time- and dose-dependent IL-8 secretion (Fig. 3a). Thus, after 2 h of stimulation T84 cells produced 600 pg of IL-8/ml. About 50 ng of TNF-α induced submaximal IL-8 production, and that amount was used as a control in subsequent experiments. Moreover, TNF-α-induced IL-8 secretion was polarized in the physiological apical-to-basolateral direction. Of the total amount of IL-8 secreted after TNF-α stimulation, 94% was secreted into the basolateral reservoir and 6% was secreted into the apical reservoir (data not shown). Further experiments demonstrated that during differentiation of T84 monolayers TNF-α stimulation from the apical side decreased. Thus, after 6 to 8 days it was no longer possible to induce significant IL-8 secretion by applying TNF-α to the apical surface (Fig. 3b). However, at these time points the cells still secreted IL-8 in response to TNF-α stimulation from the basolateral side, suggesting that the lack of apical stimulation was due to a polarized distribution of TNF-α receptors on the basolateral surfaces. In agreement with previous work (15), stimulation of T84 cells with lipopolysaccharide from E. coli did not induce IL-8 secretion (data not shown).
FIG. 1.
TER of T84 monolayers after seeding on permeable supports. TER was measured at different time points after seeding either on 24-mm-diameter supports with 0.4-μm pores or on 6.5-mm-diameter supports with 3.0-μm pores, as described in Materials and Methods, using a voltage clamp interfaced with an equilibrated pair of calomel electrodes along with a pair of Ag-AgCl electrodes. The values represent means ± standard deviations from three independent experiments with triplicate samples.
FIG. 2.
T84 cells, 10 days after seeding on permeable supports, analyzed by confocal laser scan microscopy. The green signal in the left panel represents immunolabeling with an antibody specific for β1 integrins. The red signal in the right panel shows F-actin staining with tetramethyl rhodamine isocyanate-conjugated phalloidine. Vertical (x-z) optical sections are shown.
FIG. 3.
(a) Basolateral IL-8 secretion by T84 cells grown on permeable supports (24-mm diameter, 0.4-μm pore size) at different time points after TNF-α stimulation. IL-8 concentrations in the basolateral compartments were determined by ELISA. (b) IL-8 secretion after TNF-α stimulation. T84 cells grown on permeable supports (6.5-mm diameter, 3.0-μm pore size) were stimulated with 100 ng of TNF-α from either the apical or basolateral surfaces. IL-8 concentrations in untreated T84 cells were not significantly different from those of cells stimulated with TNF-α from the apical surfaces. The values are means ± standard deviations of triplicate samples. The data are from a representative experiment. Comparable results were obtained in two additional experiments.
Role of epithelial cell differentiation in entry of Y. enterocolitica into T84 cells.
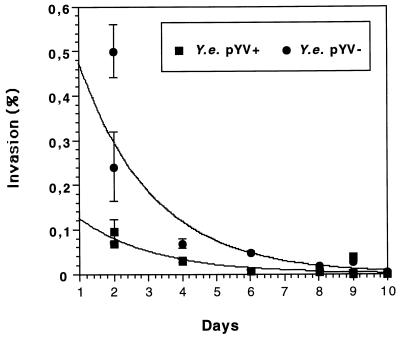
Previous experiments showed that plasmid-carrying (pYV+) and plasmid-cured (pYV−) Y. enterocolitica strains of serotypes O:9 and O:8 are able to invade nondifferentiated T84 cells (52). To analyze the influence of epithelial cell differentiation on invasion by Y. enterocolitica, gentamicin killing assays were performed. At different time points after being seeded on permeable supports, T84 cells were infected from the apical side with Y. enterocolitica pYV+ and pYV− strains at a multiplicity of infection (MOI) of 500. After 1 h of incubation, the monolayers were washed with PBS and further incubated for 4 h in the presence of gentamicin to kill residual extracellular bacteria. Finally, the number of viable intracellular bacteria was determined as described in Materials and Methods. The results indicate that Y. enterocolitica is able to invade nondifferentiated T84 cells. The pYV− strain invaded twice as extensively as the pYV+ strain (1.6 versus 0.9%, respectively). However, as early as day 2 after the cells were seeded on permeable supports, invasion was reduced, and it further declined to background levels of 0.001 or 0.006% invasion on day 10 for pYV+ and pYV− strains, respectively (Fig. 4).
FIG. 4.
Apical invasion of cultured human intestinal T84 cells by the Y. enterocolitica pYV+ or pYV− strain. T84 cells on permeable supports (24-mm diameter, 0.4-μm pore size) were infected for 1 h with Y. enterocolitica (Y.e.) strains cultured at 27°C at a bacterium-to-cell ratio of 500:1. After being washed with PBS, the cultures were incubated for 4 h with gentamicin. Finally, the number of intracellular bacteria was determined. Invasion is expressed as percent of intracellular Yersinia compared to the starting inoculum. The values are means ± standard deviations of triplicate samples. The data shown are from two independent experiments; the graphs were obtained by regression analysis. Comparable results were obtained in additional experiments.
In addition to Inv, the major Yersinia invasin, there are two additional Yersinia adherence and/or invasion factors: Ail and YadA. In contrast to Inv, Ail and YadA are maximally expressed at 37°C (30, 38). However, the experiments described above were performed with Y. enterocolitica cultured at 27°C. In order to investigate the roles of Ail and YadA in invasion of polarized T84 cells, experiments were also performed with Y. enterocolitica cultured at 37°C. The results of these experiments did not reveal significantly greater invasion by Y. enterocolitica grown at 37°C (data not shown).
In further experiments the influences of the infectious dose and infection time were analyzed. However, increasing the infectious dose to MOIs of up to 104 did not significantly increase invasion by Y. enterocolitica of polarized T84 cells (data not shown). Extending the period for adherence and invasion to 20 h (initial MOI = 20) did not reveal significant apical invasion by Y. enterocolitica of polarized T84 cells. Numbers of intracellular Yersinia were in the same order of magnitude as those of a noninvasive E. coli strain (data not shown). In contrast, Salmonella typhimurium, which was used as a positive control in these experiments, was able to invade T84 monolayers.
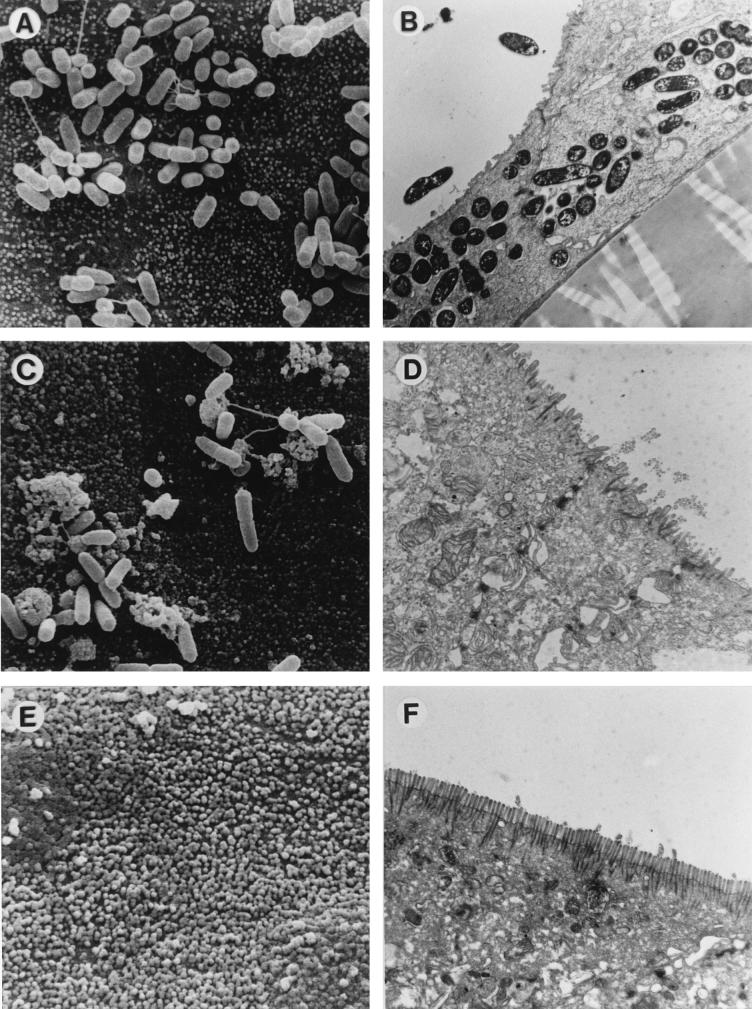
To confirm the above results, electron microscopy studies were performed. T84 monolayers were infected on day 2, 5, or 9 postseeding with the Y. enterocolitica pYV+ or pYV− strain. SEM of permeable supports infected on day 2 revealed confluent layers of T84 cells. Only a few cells established cellular protrusions resembling microvilli (Fig. 5A). Y. enterocolitica bacteria frequently adhered to the surface of T84 cells on day 2 (Fig. 5A). TEM revealed the presence of intracellular Y. enterocolitica cells in phagocytic vacuoles in T84 cells (Fig. 5B). On day 5 almost all T84 cells (approximately 95%) showed irregular brush borders (Fig. 5C). Adhering Y. enterocolitica cells were less frequent. When bacteria were present, they were most often observed close to the region of contact between adjacent cells (Fig. 5C). TEM revealed that on day 5 T84 cells exhibited tall columnar shapes, more or less irregular brush borders, and tight junctions (Fig. 5D). Intracellular Y. enterocolitica cells were not detected (Fig. 5D). On day 9 postseeding regular brush borders of microvilli were found on T84 cells (Fig. 5E). Adhering Y. enterocolitica cells were hardly detectable at this time point. As on day 5, intracellular Yersinia cells were not found on day 9.
FIG. 5.
Interaction of the Y. enterocolitica (Y.e.) pYV+ strain with T84 cells after apical infection. T84 cells on permeable supports were infected for 1 h with the Y. enterocolitica pYV+ strain cultured at 27°C. (A) SEM of the apical surfaces of T84 cells 2 days after culture on permeable supports (24-mm diameter, 0.4-μm pore size). The T84 cells form a confluent monolayer, and single cells exhibit microvilli. Several yersiniae closely adhere to the cells. (B) TEM corresponding to panel A. Multiple intracellular yersiniae in nondifferentiated T84 cells are located in intracellular vacuoles. (C) SEM of the apical surfaces of T84 cells 5 days after culture. Adherent bacteria are located in a region of contact between adjacent T84 cells. (D) TEM on day 5. The T84 cells have adopted columnar shapes and irregular brush borders. Intracellular bacteria were not observed. (E) SEM of the apical surfaces of T84 cells 9 days after culture. The T84 cells have acquired regular brush borders. Adhering Y. enterocolitica cells were hardly detectable. (F) TEM of T84 cells on day 9. Almost all cells exhibit a polarized architecture, including regular apical brush borders and tight junctions. Intracellular Yersinia cells were not detectable at this time point.
Taken together, the above results show that invasion of T84 cells by Y. enterocolitica decreases with cell differentiation, suggesting that the receptor(s) involved in cell entry by Y. enterocolitica disappears from the apical surfaces during differentiation into polarized T84 cells.
Role of intestinal epithelial cell differentiation in Yersinia-induced IL-8 secretion.
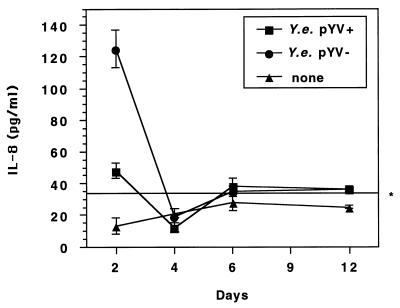
Previous studies revealed that invasion of nonpolarized T84 cells by Y. enterocolitica induces IL-8 secretion (52). In the present study we investigated Yersinia-induced IL-8 secretion as a function of cell differentiation. At different time points after being seeded on permeable supports (24-mm diameter, 0.4-μm pore size) T84 cells were infected with pYV+ or pYV− Y. enterocolitica cells and the TER, the number of intracellular bacteria, and the amount of secreted IL-8 were measured. Apical infection of T84 cells on day 2 resulted in significant IL-8 secretion into the basolateral compartment. The Y. enterocolitica pYV+ strain induced less secreted IL-8 than the nonvirulent pYV− strain (47 ± 5 versus 125 ± 12 pg/ml) (Fig. 6). Apical infection with the Y. enterocolitica pYV+ or pYV− strain at time points when T84 monolayers had reached confluency did not induce significant IL-8 secretion compared with that of noninfected controls. Thus, apical infection of polarized T84 epithelial cells with Y. enterocolitica does not cause significant IL-8 secretion.
FIG. 6.
IL-8 secretion by T84 cells after apical Y. enterocolitica (Y.e.) infection. T84 cells on permeable supports (24-mm diameter, 0.4-μm pore size) were infected with the Y. enterocolitica pYV+ or pYV− strain cultured at 27°C at a bacterium-to-cell ratio of 500:1. The bacteria were allowed to enter the cells for 1 h. Four hours after the addition of gentamicin, IL-8 concentrations in the basolateral compartments were determined by ELISA. The values are means ± standard deviations (SD) of triplicate samples. The data are from a representative experiment. Comparable results were obtained in two additional experiments. The asterisk indicates the cutoff value (i.e., the IL-8 concentration of noninfected controls + 2 SD).
Invasion by Y. enterocolitica of polarized T84 cells from the basolateral surfaces.
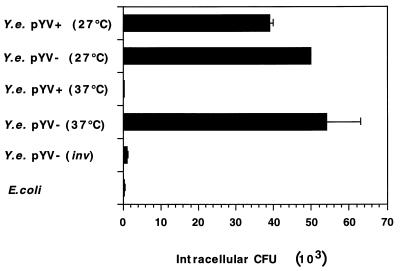
Polarized T84 cells grown on permeable supports showed a basolateral distribution of β1 integrins (Fig. 2). In order to evaluate the effect of the segregation of β1 integrins between the apical and basolateral surfaces of differentiated cells on Y. enterocolitica entry, infection of T84 monolayers from the basolateral surface was investigated. Inverted T84 monolayers on collagen-coated permeable supports were infected from the apical or basolateral side with the pYV+ or pYV− Y. enterocolitica strain. Monitoring the number of viable intracellular bacteria by the gentamicin killing assay, we observed a significant invasion of polarized T84 cells by Y. enterocolitica from the basolateral but not from the apical surfaces. As depicted in Fig. 7, the Yersinia pYV+ and pYV− strains grown at 27°C invaded T84 cells to the same extent. When the strains were precultured at 37°C, we observed a reduction of invasion by the virulent plasmid-harboring strain. Thus, only small numbers of intracellular pYV+ Y. enterocolitica bacteria precultured at 37°C were found. Likewise, a noninvasive E. coli strain did not significantly invade T84 cells. Infection with a Y. enterocolitica pYV− strain defective in the inv gene revealed numbers of intracellular bacteria similar to those of E. coli and Y. enterocolitica pYV+ strains precultured at 37°C (Fig. 7). These results suggest that invasion by Y. enterocolitica of T84 monolayers from the basolateral surface involves binding of invasin protein to β1 integrin receptors localized on the basolateral membranes of T84 cells.
FIG. 7.
Number of viable intracellular Y. enterocolitica (Y.e.) cells after basolateral infection of T84 cells. T84 cells were grown on collagen-coated 6.5-mm-diameter permeable supports (3.0-μm pore size) until they reached steady-state transepithelial resistance. Cells were infected for 1 h at a bacterium-to-cell ratio of 1,000:1 with different strains cultured at 27 or 37°C. The bacteria were allowed to enter the cells for 1 h before they were removed and gentamicin was added. After 4 h, the cells were lysed and intracellular CFU were counted. The values are means ± standard deviations of triplicate samples. The data are from a representative experiment. Comparable results were obtained in two additional experiments.
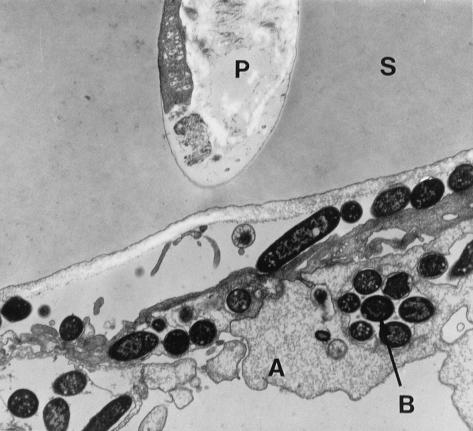
Invasion by Y. enterocolitica of polarized T84 cells by the basolateral route was confirmed by TEM examinations. Figure 8 shows T84 cells infected with the Y. enterocolitica pYV+ strain from the basolateral side. Cellular extensions of T84 cells, grown through the 3.0-μm pores of the permeable supports, on the basolateral side are invaded by Y. enterocolitica bacteria which reside intracellularly in phagocytic vacuoles (Fig. 8).
FIG. 8.
Interaction of the Y. enterocolitica pYV+ strain with T84 cells on permeable supports after basolateral infection. The TEM shows T84 cells on collagen-coated permeable supports (6.5-mm diameter, 3.0-μm pore size) infected for 1 h with Y. enterocolitica cells cultured at 27°C. The T84 cells form a confluent monolayer of polarized cells on the upper surface of permeable supports (S). Cellular appendices (A) grow through the filter pores (P) and appear on the lower surface. Multiple intracellular Y. enterocolitica cells (B) can be seen in the appendices.
IL-8 secretion by polarized T84 cells after basolateral infection with Y. enterocolitica.
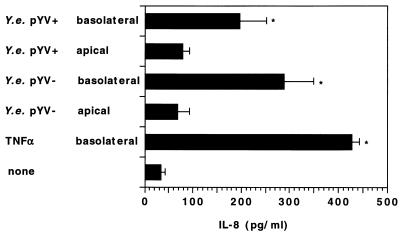
The experiments described above revealed that the pYV− or pYV+ Y. enterocolitica strain is able to invade polarized T84 cells from the basolateral side. We further investigated whether this interaction of Y. enterocolitica with T84 cells is able to induce IL-8 secretion. Therefore, polarized T84 monolayers grown on collagen-coated permeable supports were infected with Y. enterocolitica strains grown at 37°C from the basolateral side and the amount of IL-8 secretion was determined. Noninfected controls showed concentrations of IL-8 in the basolateral compartments of approximately 60 pg/ml. These concentrations were higher than those found in T84 cells seeded on permeable supports with a 0.4-μm pore size. Thus, the amount of IL-8 constitutively secreted by T84 cells depends on the pore size of the permeable supports. After infection from the basolateral surface IL-8 concentrations were 196 ± 56 and 289 ± 60 pg/ml for pYV+ and pYV− Yersinia strains, respectively. In contrast, infection of T84 monolayers from the apical surface did not significantly induce IL-8 secretion (Fig. 9). Although the pYV+ Y. enterocolitica strain grown at 37°C did not significantly invade polarized T84 monolayers from the basolateral surface (Fig. 7), the amount of IL-8 secreted was comparable to that induced by infection with the pYV− strain. Thus, induction of IL-8 secretion by basolateral infection of polarized T84 cells with Y. enterocolitica did not strictly depend on cell invasion.
FIG. 9.
Comparison of IL-8 secretion by T84 cells after apical or basolateral infection with Y. enterocolitica (Y.e.) T84 cells on collagen-coated permeable supports (6.5-mm diameter, 3.0-μm pore size) were infected for 1 h at a bacterium-to-cell ratio of 1,000:1 with the Y. enterocolitica pYV+ or pYV− strain cultured at 37°C. The bacteria were allowed to enter the cells for 1 h before they were removed and gentamicin was added. After 4 h the concentration of IL-8 in the basolateral reservoir was determined by ELISA. TNF-α (50 ng/ml) was used as a positive control. Asterisks indicate significant differences (P < 0.01) from noninfected controls. The values are means ± standard deviations of triplicate samples from two independent experiments.
DISCUSSION
Most of the data for Y. enterocolitica invasion of cultured host cells and IL-8 induction were acquired in experiments with nonintestinal, or at least nonpolarized, cell types. Interestingly, it was demonstrated that intestinal epithelial cell differentiation inhibits apical invasion by Y. pseudotuberculosis of the intestinal epithelial cell line Caco-2 (8). The goal of this study was to examine Y. enterocolitica invasion and Y. enterocolitica-induced IL-8 secretion in a more relevant in vitro cell culture system, employing monolayers of polarized human intestinal epithelial cells (T84).
The experiments reported here show that invasion of T84 cells by pYV+ and pYV− Y. enterocolitica strains from the apical surfaces is significantly diminished during polarization of the cells. The residual number of so-called intracellular bacteria in apically infected polarized T84 cells detected by the gentamicin killing assay could be due to microdiscontinuities in T84 monolayers or to extracellular bacteria that survived the gentamicin killing rather than to actual invasion. Electron microscopic examinations of T84 monolayers infected at different time points after culture on permeable supports confirmed this assumption.
Y. enterocolitica, like Y. pseudotuberculosis, expresses three different adhesion and/or invasion factors. The invasin Inv, the major invasion factor, is maximally expressed at 27°C (27, 44, 46). In contrast, the additional factors Ail and YadA are expressed at 37°C (30, 38). Since the contribution of each of these factors to the invasion of intestinal epithelial cells is unclear, we also investigated invasion by bacteria that were cultured at 37°C. However, Y. enterocolitica cultured at Ail- and YadA-inducing temperatures did not enter T84 monolayers to a significant extent (data not shown).
During polarization intestinal epithelial cells acquire two distinguishable domains, apical and basolateral. Interestingly, a redistribution of β1 integrins during differentiation of Caco-2 cells in culture to the basolateral domains of the cells has been documented (56). We have also observed a restricted localization of β1 integrins in the basolateral domains of differentiated T84 cells. It has already been suggested that this redistribution of β1 integrins accounts for the diminishment of epithelial cell invasion by Y. pseudotuberculosis (8). A recent investigation reported by McCormick et al. (37) showed that Y. pseudotuberculosis was not able to invade polarized T84 cells. However, T84 cells became susceptible to Y. pseudotuberculosis invasion in regions of microdiscontinuity induced by transepithelial migration of PMNs. The authors state that neutrophil transepithelial migration forces neighboring epithelial cells to separate, thereby giving yersiniae access to unmasked basolateral β1 integrins.
The presence of β1 integrins on the basolateral membranes of polarized cells indicated the possibility of invasion via the basolateral route. In subsequent experiments monolayers were infected from the basolateral surface. The results showed basolateral invasion of polarized T84 cells by Y. enterocolitica. The inability of a Y. enterocolitica inv mutant to invade suggests that the Inv protein serves as a major invasion factor in this system. Furthermore, reduced invasion by the pYV+ strain was observed after it was cultured at 37°C. Similar observations have been reported after infection of HeLa and HEp-2 cells with Y. enterocolitica and Y. pseudotuberculosis. This was interpreted as a consequence of the antiphagocytic effect of YopE and YopH (18, 20, 49, 50).
Apical infection of polarized T84 cells with Y. enterocolitica for 1 h or over a period of 20 h did not induce IL-8 secretion, whereas apical infection with S. typhimurium induced substantial IL-8 secretion (data not shown). Previous reports indicated that invasion is an essential signal for induction by invasive bacteria of IL-8 secretion (15). The fact that differentiation of epithelial cells diminishes invasion by Y. enterocolitica could explain the lack of IL-8 secretion after infection from the apical surfaces. Consistently, infection from the basolateral surfaces not only led to invasion but also to IL-8 secretion in the physiological apical-to-basolateral direction.
Comparison of invasion and IL-8 secretion after infection with the Y. enterocolitica pYV+ strain cultured at 27 or 37°C revealed that the pYV+ strain grown at 37°C did not significantly invade but induced secretion of IL-8. This finding suggests that Yersinia invasion is not required to induce IL-8 secretion by polarized T84 cells after basolateral infection. Whether adherence-triggered IL-8 secretion is mediated by Ail or YadA is not yet clear.
Previous investigations revealed a suppressive effect of virulent pVY+ Y. enterocolitica bacteria on infection-triggered IL-8 secretion by nonpolarized HeLa or T84 cells. This effect required the presence of an active type III secretion-translocation mechanism and at least YopB and -D (52). However, in the present study we could not detect a suppression of IL-8 secretion after basolateral infection of polarized T84 cells. Thus, although the pYV+ Y. enterocolitica strain cultured at 37°C did not significantly invade polarized T84 cells from the basolateral side, IL-8 secretion was comparable to that induced by the pYV− strain, which invaded to a greater extent. This finding could be explained by the distinct differentiation of the cells employed in the two studies. However, it is more likely that this discrepancy is due to the special features of the in vitro system used in the present study. Thus, the absence of a certain host cell receptor(s) from the basolateral surfaces of polarized T84 cells may account for the lack of translocation of Yop protein(s), some of which suppresses IL-8 secretion. On the other hand, it might well be that prior to a Yop-mediated suppression residual amounts of IL-8 or other cytokines secreted by infected cells induce IL-8 secretion by a paracrine-autocrine pathway in neighboring cells (47) which are not in contact with Y. enterocolitica. Consequently, IL-8 production in these cells would not be suppressed by Y. enterocolitica.
Data from experiments in the mouse model suggest that yersiniae cross the intestinal epithelium by transcytosis via M cells (1, 21). Subsequently, Y. enterocolitica might gain access to neighboring epithelial cells of the follicle-associated epithelium and trigger IL-8 secretion. Whether basolateral IL-8 secretion by epithelial cells and recruitment of PMNs are involved in the pathogenesis of Yersinia-induced abscess formation in Peyer’s patch tissue remains to be shown.
ACKNOWLEDGMENTS
We thank H. Rüssmann for a critical reading of the manuscript. We are indebted to C. Gehring and G. Krohne (BIOZENTRUM, Abt. Elektronenmikroskopie, Universität Würzburg) for expert electron microscopy.
R.S. is the recipient of a fellowship from BMBF (Bundesministerium für Bildung und Forschung).
REFERENCES
- 1.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balk S P, Burke S, Polischuk J E, Frantz M E, Yang L, Porcelli S, Colgan S P, Blumberg R S. Beta 2-microtubulin-independent MHC class Ib molecules expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 4.Beuscher H U, Rödel F, Forsberg A, Röllinghoff M. Bacterial evasion of host immune response: Yersinia enterocolitica encodes a suppressor of tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuscher H U, Rausch U P, Otterness I G, Röllinghoff M. Transition from interleukin-1 beta to IL-1 alpha production during maturation of inflammatory macrophages in vivo. J Exp Med. 1992;175:1793–1797. doi: 10.1084/jem.175.6.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliska J B, Copass M C, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottone E J. Yersinia enterocolitica. A panoramic view of a charismatic microorganism. Crit Rev Microbiol. 1977;5:211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- 8.Coconnier M-H, Bernet-Camard M-F, Servin A L. How intestinal epithelial cell differentiation inhibits the cell-entry of Yersinia pseudotuberculosis in colon carcinoma Caco-2 cell line in culture. Differentiation. 1994;58:87–94. doi: 10.1046/j.1432-0436.1994.5810087.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis G R. Yersinia pathogenicity factors. Curr Top Microbiol Immunol. 1994;192:243–263. doi: 10.1007/978-3-642-78624-2_11. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis G R. Yersiniae, finely tuned pathogens. In: Hormaeche C E, Penn C W, Smyth C J, editors. Molecular biology of bacterial infection. Current status and future perspectives. Society for General Microbiology symposium no. 49. Cambridge, England: Cambridge University Press; 1992. pp. 231–265. [Google Scholar]
- 11.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;6:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 12.Delor I, Kaeckenbeeck A, Wauters G, Cornelis G R. Nucleotide sequence of yst, the Yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic yersiniae. Infect Immun. 1990;58:2983–2988. doi: 10.1128/iai.58.9.2983-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmsathaphorn K, Madara J L. Established intestinal epithelial cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann L, Jung H C, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 15.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckmann L, Kagnoff M F, Fierer J. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 1995;3:118–120. doi: 10.1016/s0966-842x(00)88894-0. [DOI] [PubMed] [Google Scholar]
- 17.Emödy L, Heesemann J, Wolf-Watz H, Skurnik M, Kapperud G, O’Toole P, Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989;171:6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fällman M, Andersson K, Håkansson S, Magnusson K-E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierer J, Eckmann L, Kagnoff M. IL-8 secreted by epithelial cells invaded by bacteria. Infect Agents Dis. 1994;2:255–258. [PubMed] [Google Scholar]
- 20.Forsberg A, Rosqvist R, Wolf-Watz H. Regulation and polarized transfer of the Yersinia outer proteins (Yops) involved in antiphagocytosis. Trends Microbiol. 1994;2:14–19. doi: 10.1016/0966-842x(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 21.Grützkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O:8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heesemann J, Grüter L. Genetic evidence that the outer membrane protein Yop1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol Lett. 1987;40:37–41. [Google Scholar]
- 24.Heesemann J, Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983;155:761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 26.Isberg R R, Voorhis D L, Falkow S. Mammalian cell adhesion functions and the cellular penetration of enteropathogenic Yersinia species. Mol Microbiol. 1989;3:1449–1453. doi: 10.1111/j.1365-2958.1989.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 27.Isberg R R, Swain A, Falkow S. Analysis of expression and regulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect Immun. 1988;56:2133–2138. doi: 10.1128/iai.56.8.2133-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapperud G, Namork E, Skurnik M, Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987;55:2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karunasagar I, Senghaas B, Krohne G, Goebel W. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect Immun. 1994;62:3554–3558. doi: 10.1128/iai.62.8.3554-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karunasagar I, Krohne G, Goebel W. Listeria ivanovii is capable of cell-to-cell spread involving actin polymerization. Infect Immun. 1993;61:162–169. doi: 10.1128/iai.61.1.162-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W H, McGrath P P, Carter P H, Eide E L. The ability of some Yersinia enterocolitica to invade HeLa cells. Can J Microbiol. 1977;23:1714–1722. doi: 10.1139/m77-247. [DOI] [PubMed] [Google Scholar]
- 34.Madara J L, Colgan S P, Nusrat A, Delp C, Parkos C A. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial interactions. J Tissue Cult Methods. 1992;14:209–216. [Google Scholar]
- 35.Madara J L, Parkos C A, Colgan S P, MacLeod R J, Nash S, Matthews J, Delp C, Lencer W S. Cl− secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer L, Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166:1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick B A, Nusrat A, Parkos C A, D’Andrea L, Hofman P M, Carnes D, Liang T W, Madara J L. Unmasking of intestinal epithelial lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller V L, Bliska J B, Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990;172:1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller V L, Farmer III J J, Hill W E, Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989;57:121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller V L, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller V L, Finlay B B, Falkow S. Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol. 1988;138:15–34. [PubMed] [Google Scholar]
- 42.Pai C H, Mors V. Production of enterotoxin by Yersinia enterocolitica. Infect Immun. 1978;19:908–911. doi: 10.1128/iai.19.3.908-911.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in the physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepe C J, Badger J L, Miller V. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol. 1994;11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 45.Pepe C J, Miller V. The Yersinia enterocolitica inv gene product is an outer membrane protein that shares epitopes with Yersinia pseudotuberculosis invasin. J Bacteriol. 1990;172:3780–3789. doi: 10.1128/jb.172.7.3780-3789.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierson D E, Falkow S. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect Immun. 1990;58:1059–1064. doi: 10.1128/iai.58.4.1059-1064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role of epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rausch U P, Jordan M, Rodel F, Aigner T, Otterness I G, Beuscher N, Röllinghoff M, Beuscher H U. Transcriptional and translational regulation of IL-1 alpha and IL-1 beta account for the control of IL-1 in experimental yersiniosis. Cytokine. 1994;6:504–511. doi: 10.1016/1043-4666(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 49.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosqvist R, Forsberg A, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 51.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulte R, Wattiau P, Hartland E L, Robins-Browne R M, Cornelis G R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulze-Koops H, Burkhardt H, Heesemann J, von der Mark K, Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992;60:2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skurnik M, Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989;3:517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 55.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vachon P H, Durand J, Beaulieu J-F. Basement membrane formation and redistribution of β1 integrins in a human intestinal coculture system. Anat Rec. 1993;236:567–576. doi: 10.1002/ar.1092350409. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Isberg R. Cellular internalization in the absence of invasin expression is promoted by the Yersinia pseudotuberculosis yadA product. Infect Immun. 1993;61:3907–3913. doi: 10.1128/iai.61.9.3907-3913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young V B, Falkow S, Schoolnik G K. The invasin product of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;116:197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]