Abstract
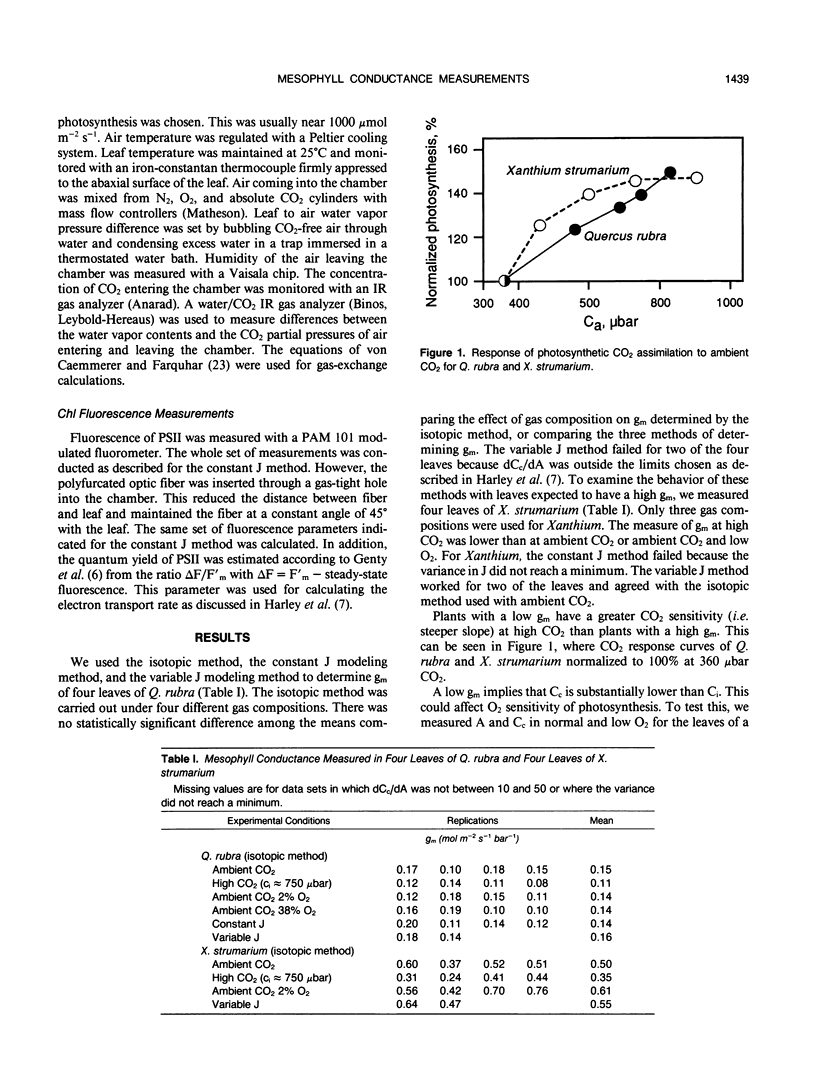
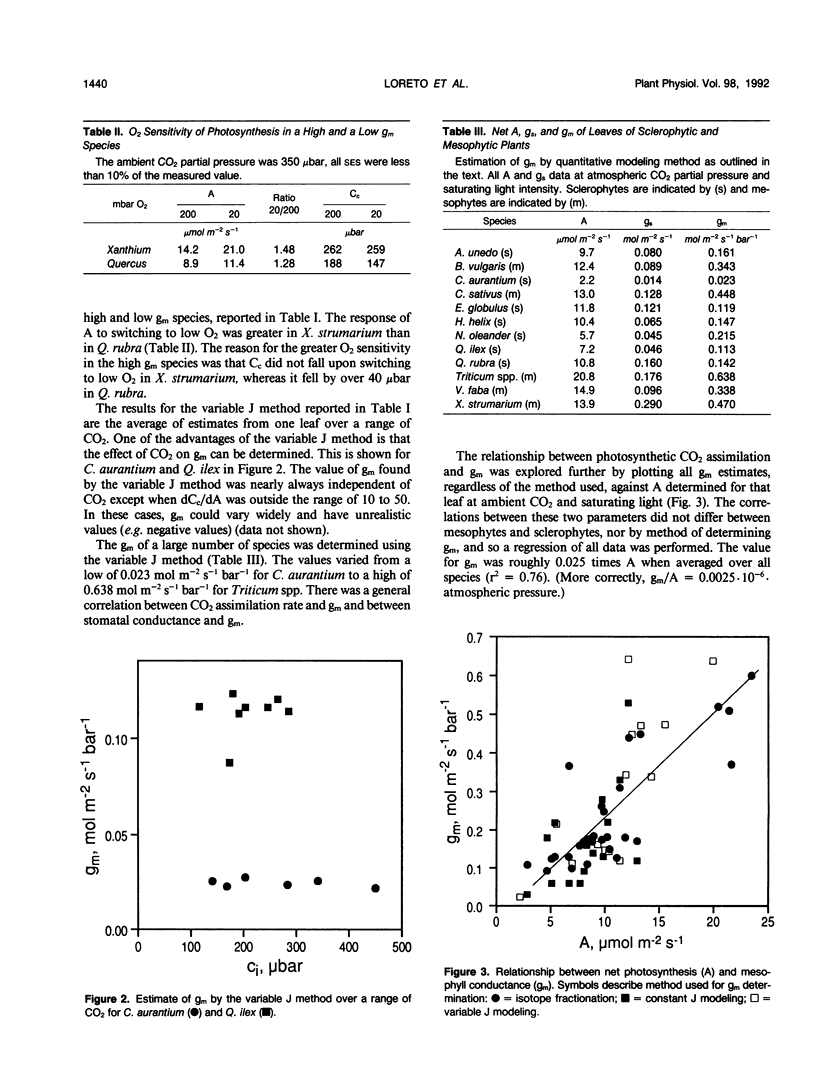
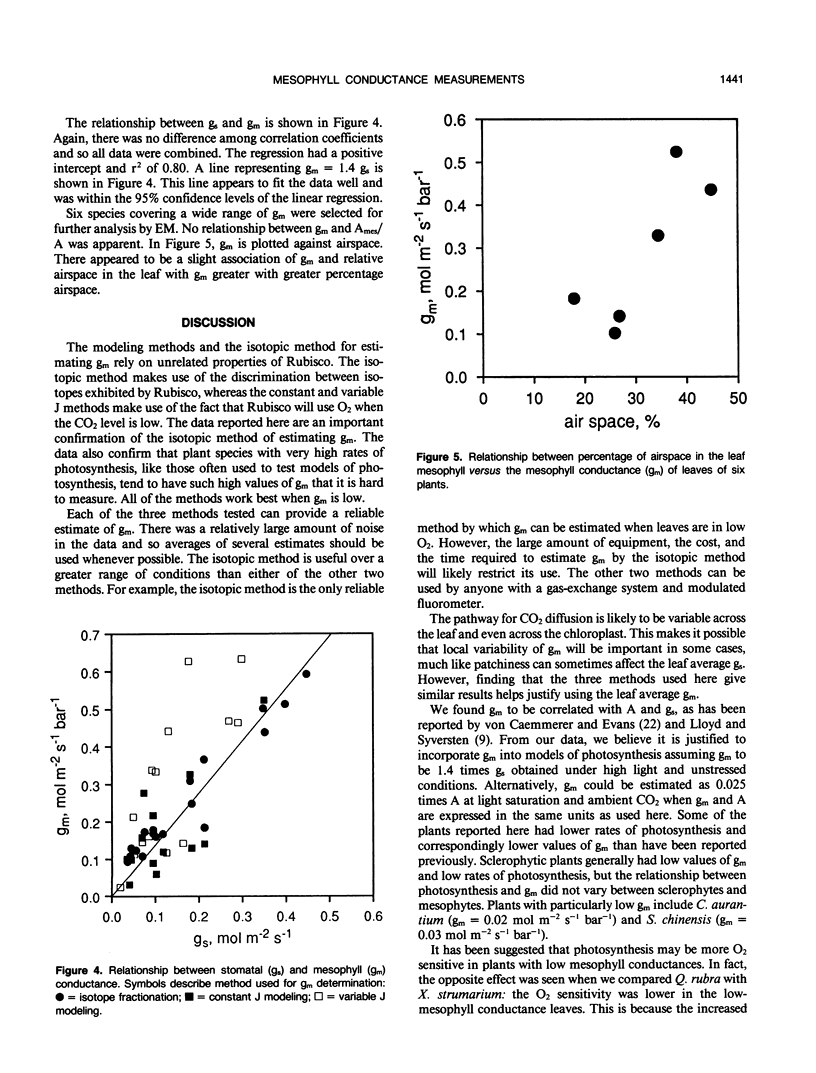
The resistance to diffusion of CO2 from the intercellular airspaces within the leaf through the mesophyll to the sites of carboxylation during photosynthesis was measured using three different techniques. The three techniques include a method based on discrimination against the heavy stable isotope of carbon, 13C, and two modeling methods. The methods rely upon different assumptions, but the estimates of mesophyll conductance were similar with all three methods. The mesophyll conductance of leaves from a number of species was about 1.4 times the stomatal conductance for CO2 diffusion determined in unstressed plants at high light. The relatively low CO2 partial pressure inside chloroplasts of plants with a low mesophyll conductance did not lead to enhanced O2 sensitivity of photosynthesis because the low conductance caused a significant drop in the chloroplast CO2 partial pressure upon switching to low O2. We found no correlation between mesophyll conductance and the ratio of internal leaf area to leaf surface area and only a weak correlation between mesophyll conductance and the proportion of leaf volume occupied by air. Mesophyll conductance was independent of CO2 and O2 partial pressure during the measurement, indicating that a true physical parameter, independent of biochemical effects, was being measured. No evidence for CO2-accumulating mechanisms was found. Some plants, notably Citrus aurantium and Simmondsia chinensis, had very low conductances that limit the rate of photosynthesis these plants can attain at atmospheric CO2 level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bongi G., Loreto F. Gas-Exchange Properties of Salt-Stressed Olive (Olea europea L.) Leaves. Plant Physiol. 1989 Aug;90(4):1408–1416. doi: 10.1104/pp.90.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley P. C., Loreto F., Di Marco G., Sharkey T. D. Theoretical Considerations when Estimating the Mesophyll Conductance to CO(2) Flux by Analysis of the Response of Photosynthesis to CO(2). Plant Physiol. 1992 Apr;98(4):1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idso S. B., Kimball B. A. Downward Regulation of Photosynthesis and Growth at High CO(2) Levels : No Evidence for Either Phenomenon in Three-Year Study of Sour Orange Trees. Plant Physiol. 1991 Jul;96(3):990–992. doi: 10.1104/pp.96.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst D. F., Mott K. A. Intercellular Diffusion Limits to CO(2) Uptake in Leaves : Studies in Air and Helox. Plant Physiol. 1990 Nov;94(3):1024–1032. doi: 10.1104/pp.94.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]


