Abstract
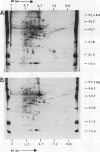
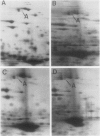
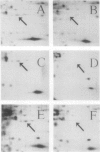
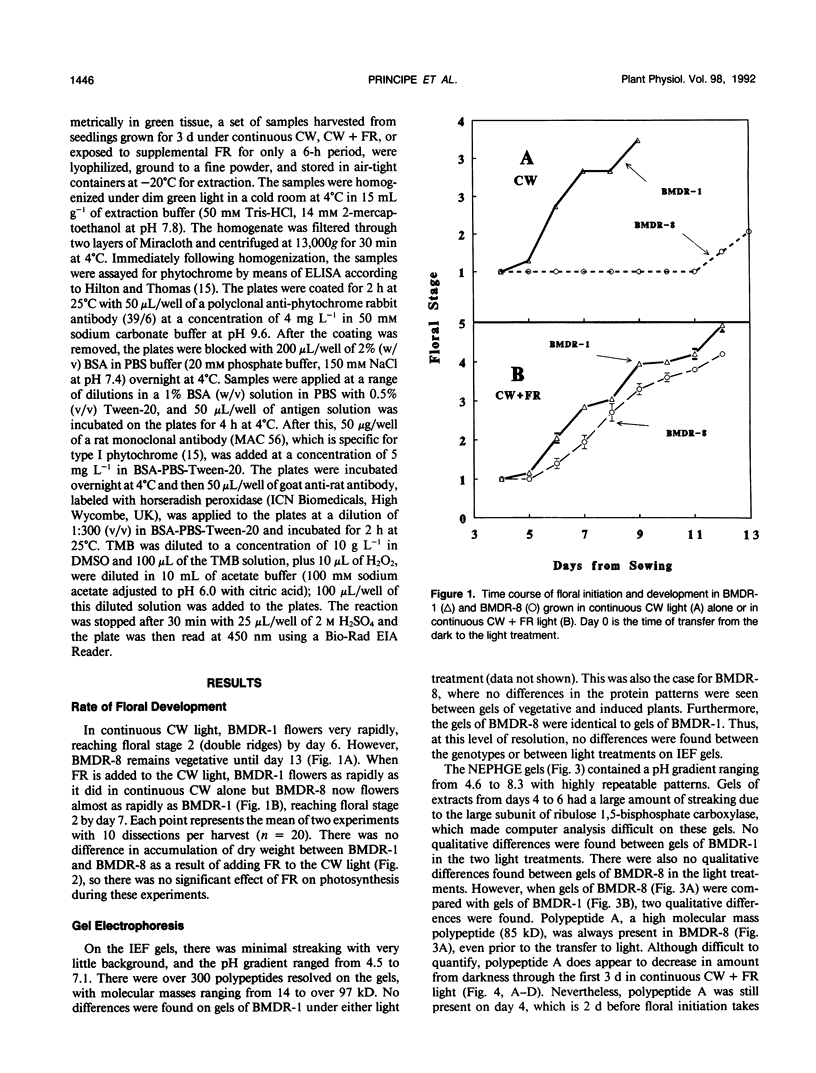
A photoperiodically sensitive cultivar of barley (Hordeum vulgare L. Shabet) (BMDR-8) and an isogenic, single-gene recessive mutant of this genotype that is insensitive to photoperiod (BMDR-1) were grown under continuous cool white light with or without supplemental far-red fluorescent light. BMDR-1 initiates flowers 6 days after germination, irrespective of light treatment, whereas BMDR-8 remains vegetative for at least a week longer, even in continuous light. When far-red light is added, the delay of flowering in BMDR-8 is overcome and both genotypes initiate floral primordia at the same time. Total phenol extracted proteins of seedlings of both genotypes were resolved by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. No protein differences were found between the genotypes when isoelectric focusing gels were run in the first dimension. Two qualitative genotypic differences were found when nonequilibrium pH gradient gel electrophoresis was run in the first dimension. An 85-kilodalton polypeptide (A) and a 26-kilodalton polypeptide (B) were always present in BMDR-8 but never found in BMDR-1. The levels of A appeared to decrease from the BMDR-8 during the first 3 days of far-red treatment but did not disappear completely even after 6 days of growth in the presence of farred. Polypeptide B decreases rapidly in continuous cool white light but is stabilized by far-red. The phytochrome content of BMDR-1 was found to be greater than that for BMDR-8. This increase appears to be caused by the type I (etiolated-tissue abundant) phytochrome pool, even in plants grown in continuous light.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER H. N., PATON D. M. A gene controlled flowering inhibitor in Pisum. Nature. 1952 Apr 5;169(4301):592–592. doi: 10.1038/169592a0. [DOI] [PubMed] [Google Scholar]
- Cleland C. F. Isolation of Flower-inducing and Flower-inhibitory Factors from Aphid Honeydew. Plant Physiol. 1974 Dec;54(6):899–903. doi: 10.1104/pp.54.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitzer G. F., Hayes R., Jabben M. Kinetics and time dependence of the effect of far red light on the photoperiodic induction of flowering in wintex barley. Plant Physiol. 1979 Dec;64(6):1015–1021. doi: 10.1104/pp.64.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormling I., Gustafsson A., Ekman G. Growth disorders and phenotype variability in phytotron-cultivated barley. Hereditas. 1975;79(2):255–272. [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]