Abstract
The neuroprotective effects of umbelliferone (UMB) were visualized in three-dimensional (3D) images on vesicle density changes of organotypic hippocampal slice tissues (OHSCs) induced by scopolamine by high voltage electron microscopy. Observations revealed that the number of vesicles decreased in OHSCs induced by scopolamine, and UMB was found to inhibit scopolamine-induced reduction in vesicles, resulting in an increase in vesicle count. These 3D models provide valuable insight for understanding the increase of synapse vesicles in hippocampal tissues treated with UMB.
Keywords: Umbelliferone, Scopolamine, Synaptic vesicle, Hippocampus, Electron tomography, High voltage electron microscopy
Description
Umbelliferone (UMB) is a 7-hydroxycoumarin, which is a pharmacologically active component derived from the Apiaceae family. Anti-inflammatory, antioxidant (Althunibat et al. 2022), anti-glycation, antidiabetic (Jin and Chen 2022), anticancer (Aslanturk and Askin Celik 2023), and anti-hepatic (Hassanein et al. 2021) properties have been reported from UMB. However, the neuroprotective effects of UMB have not been confirmed by ultrastructural morphological approaches. In previous studies, the neuroprotective effects of UMB were investigated in an animal model where learning and memory were impaired using scopolamine (SCOP), a non-selective muscarinic acetylcholine receptor antagonist (Wan et al. 2019). This investigation involved behavioral experiments, molecular biology analysis, and electrophysiological analysis (Choi et al. 2023). In this study, we visualized and analyzed the neuroprotective effects of UMB treatment on hippocampal synaptic ultrastructures in organotypic hippocampal slice cultures using a biological high voltage electron microscopy (Bio-HVEM) system.
The organotypic hippocampal slice tissues used in this study followed the protocol described in the previous study (Stoppini et al. 1991). The hippocampus was extracted from the rat’s brain and immersed in HBSS containing 20 mM HEPES. The hippocampus was chopped into 350 μm-thick slices using a chopper (Cavey Laboratory Engineering Co., Guildford, Surrey, UK) and placed on a 0.4 µm membrane insert (Millicell-CM; Millipore®, Merck KGaA, Darmstadt, Germany) in a six-well tissue culture plate with a medium. The tissues were treated with control, SCOP (300 μM), or UMB (10 μM) + SCOP (300 μΜ) and postfixed in 2% OsO4/1.5% potassium ferrocyanide for 1 h. The tissues were submerged in 1% thiocarbohydrazide (Ted Pella Inc., Redding, CA, USA) for 20 min, followed by 2% OsO4 for 30 min. Next, the tissues were incubated overnight in 1% uranyl acetate at 4 °C, followed by a lead aspartate solution at 60 °C for 30 min.
For high voltage electron tomography, the tissues were dehydrated in a series of ethanol and embedded in Epon 812 resin, and relatively 300 nm-thick sections were obtained on the grids. The grids were placed in a double-tilt specimen holder (EM-Z19103TDTH; JEOL Ltd., Tokyo, Japan) and imaged in the KBSI Bio-HVEM System (JEM-1000BEF; JEOL Ltd., Tokyo, Japan) operating at 1 MeV. A total of 121 tilt images were captured by transmission electron microscopy (TEM) recorder software (JEOL Ltd., Tokyo, Japan). The digitized tilting images were aligned and tomographically reconstructed using Composer and Visualizer-Kai software (TEMography.com, System In Frontier Inc., Tokyo, Japan). Selected tomograms were manually segmented, and AMIRA software (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for volume rendering and 3D modeling (Kim et al. 2012).
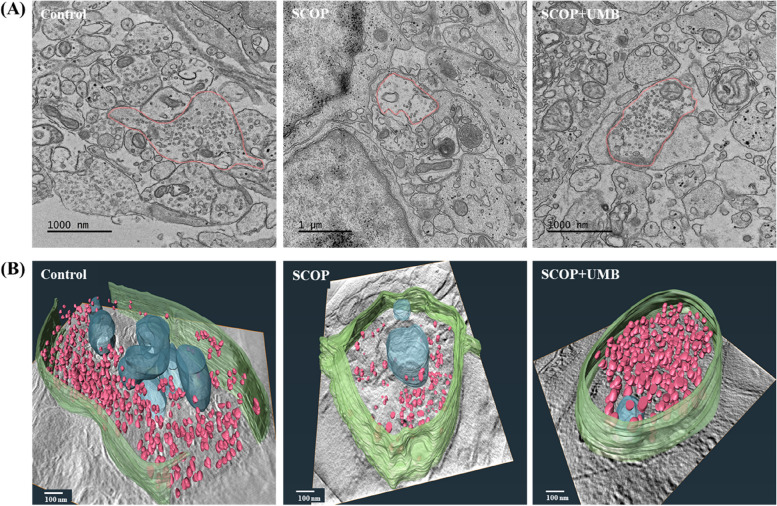
As shown in Fig. 1, the synaptic vesicle density decreased in the SCOP group compared to the control group. The SCOP + UMB group, in contrast, reversed the reduction in synaptic vesicle density observed in the SCOP group. In particular, the number of synaptic vesicles within 300 nm of the active zone increased dramatically in the SCOP + UMB group compared to the SCOP group. Increased synaptic vesicle density is strongly associated with learning and memory improvement due to changes in synaptic morphology and structure (Sousa et al. 2000; Lee et al. 2018). Therefore, our results demonstrate that UMB mitigates SCOP-induced learning and memory impairments. In conclusion, through the 3D models which make volumetric analysis easier than 2D, we can clearly suggest that UMB may be a crucial neuroprotective compound for enhancing learning and memory in neurodegenerative diseases.
Fig. 1.
Hippocampal synaptic ultrastructure of the UMB-treated OHSC tissues. A Representative 2D electron micrograph images of synapses in the hippocampus of each group (control, SCOP, SCOP + UMB). Scale bar = 1000 nm. B 3D models of the reconstructed synapse in each group showing synaptic vesicles (red) and mitochondria (blue) on synaptic ultrastructure of the hippocampus using the KBSI Bio-HVEM System. Scale bar = 100 nm
Acknowledgements
Not applicable.
Authors’ contributions
Hee-Seok Kweon supervised the project. Ga-young Choi wrote the manuscript and prepared the organotypic hippocampal tissues. Eunyoung Moon and Hyosung Choi conducted the electron tomography. All the authors discussed the results and approved the final manuscript.
Funding
This work was supported by the National Research Foundation (NRF) funded by the Ministry of Science and ICT [2022M3H9A2083956].
Availability of data and materials
Not applicable. “Please contact the corresponding author for data requests.”
Declarations
Competing interests
The author declares no competing interests relevant to the article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- O.Y. Althunibat, M.S. Abduh, M.H. Abukhalil, S.H. Aladaileh, H. Hanieh, A.M. Mahmoud, Umbelliferone prevents isoproterenol-induced myocardial injury by upregulating Nrf2/HO-1 signaling, and attenuating oxidative stress, inflammation, and cell death in rats. Biomed. Pharmacother. 149(2022) [DOI] [PubMed]
- O.S. Aslanturk, T. Askin Celik, Anticancer effect of umbelliferone on MKN-45 and MIA PaCa-2 cell lines. Toxicol In Vitro 93, 105694 (2023) [DOI] [PubMed]
- G.Y. Choi, H.B. Kim, J.M. Cho, I. Sreelatha, I.S. Lee, H.S. Kweon, S. Sul, S.A. Kim, S. Maeng, J.H. Park, Umbelliferone ameliorates memory impairment and enhances hippocampal synaptic plasticity in scopolamine-induced rat model. Nutrients. 15(10), 2351 (2023) [DOI] [PMC free article] [PubMed]
- Hassanein EHM, Khader HF, Elmansy RA, Seleem HS, Elfiky M, Mohammedsaleh ZM, Ali FEM, Abd-Elhamid TH. Umbelliferone alleviates hepatic ischemia/reperfusion-induced oxidative stress injury via targeting Keap-1/Nrf-2/ARE and TLR4/NF-kappaB-p65 signaling pathway. Environ. Sci. Pollut. Res. Int. 2021;28(47):67863–67879. doi: 10.1007/s11356-021-15184-8. [DOI] [PubMed] [Google Scholar]
- T. Jin, C. Chen, Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem. Toxicol. 163, 112892 (2022) [DOI] [PubMed]
- M.J. Kim, Y.H. Huh, K.J. Choi, S. Jun, A.R. Je, H. Chae, C. Lee, H.-S. Kweon, Ultrastructural abnormalities in APP/PSEN1 transgenic mouse brain as the Alzheimer’s disease model. Appl Microsc. 42(4), 179–185 (2012)
- S.J. Lee, H.-W. Kim, J.E. Na, D. Kim, D.H. Kim, J.R. Ryu, W. Sun, I.J. Rhyu, Role of Actin Filament on Synaptic Vesicle Pooling in Cultured Hippocampal Neuron. Appl Microsc. 48(3), 55–61 (2018)
- N. Sousa, N.V. Lukoyanov, M.D. Madeira, O.F. Almeida, M.M. Paula-Barbosa, Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97(2), 253–266 (2000) [DOI] [PubMed]
- L. Stoppini, P.-A. Buchs, D. Muller, A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37(2), 173–182 (1991) [DOI] [PubMed]
- T. Wan, Z. Wang, Y. Luo, Y. Zhang, W. He, Y. Mei, J. Xue, M. Li, H. Pan, W. Li, Q. Wang, Y. Huang, FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxid. Med. Cell. Longev. 2019, 8239642 (2019) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. “Please contact the corresponding author for data requests.”