Abstract
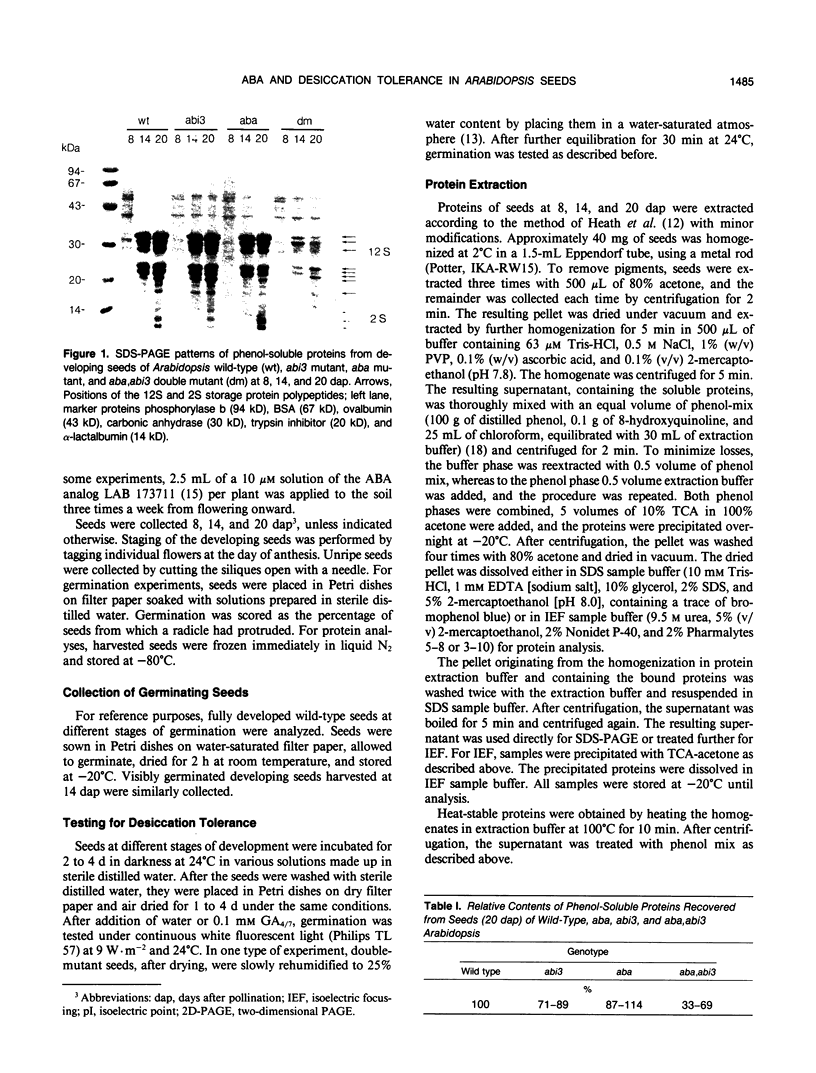
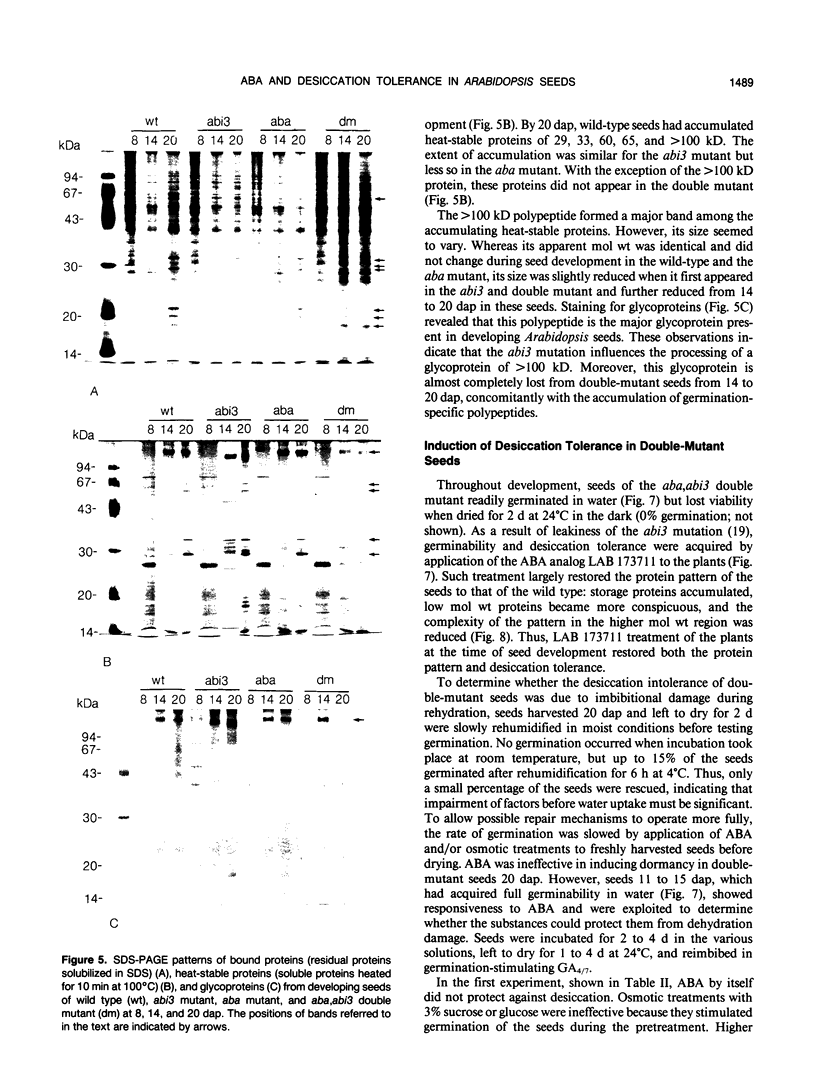
In contrast to wild-type seeds of Arabidopsis thaliana and to seeds deficient in (aba) or insensitive to (abi3) abscisic acid (ABA), maturing seeds of recombinant (aba,abi3) plants fail to desiccate, remain green, and lose viability upon drying. These double-mutant seeds acquire only low levels of the major storage proteins and are deficient in several low mol wt polypeptides, both soluble and bound, and some of which are heat stable. A major heat-stable glycoprotein of more than 100 kilodaltons behaves similarly; during seed development, it shows a decrease in size associated with the abi3 mutation. In seeds of the double mutant from 14 to 20 days after pollination, the low amounts of various maturation-specific proteins disappear and many higher mol wt proteins similar to those occurring during germination are induced, but no visible germination is apparent. It appears that in the aba,abi3 double mutant seed development is not completed and the program for seed germination is initiated prematurely in the absence of substances protective against dehydration. Seeds may be made desiccation tolerant by watering the plants with the ABA analog LAB 173711 or by imbibition of isolated immature seeds, 11 to 15 days after pollination, with ABA and sucrose. Whereas sucrose stimulates germination and may protect dehydration-sensitive structures from desiccation damage, ABA inhibits precocious germination and is required to complete the program for seed maturation and the associated development of desiccation tolerance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Hoekstra F. A., Crowe L. M. Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Natl Acad Sci U S A. 1989 Jan;86(2):520–523. doi: 10.1073/pnas.86.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Somerville C. R. Three Classes of Abscisic Acid (ABA)-Insensitive Mutations of Arabidopsis Define Genes that Control Overlapping Subsets of ABA Responses. Plant Physiol. 1990 Nov;94(3):1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Shaw D. C. Heat-stable proteins and abscisic Acid action in barley aleurone cells. Plant Physiol. 1989 Dec;91(4):1520–1526. doi: 10.1104/pp.91.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C. J., Hilhorst H. W., Karssen C. M. In Vivo Inhibition of Seed Development and Reserve Protein Accumulation in Recombinants of Abscisic Acid Biosynthesis and Responsiveness Mutants in Arabidopsis thaliana. Plant Physiol. 1989 Jun;90(2):463–469. doi: 10.1104/pp.90.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ried J. L., Walker-Simmons M. K. Synthesis of abscisic Acid-responsive, heat-stable proteins in embryonic axes of dormant wheat grain. Plant Physiol. 1990 Jun;93(2):662–667. doi: 10.1104/pp.93.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]